Abstract
Background
Atrial fibrillation (AF) patterns and their relations with long‐term prognosis are uncertain, partly because pattern definitions are challenging to implement in longitudinal data sets. We developed a novel AF classification algorithm and examined AF patterns and outcomes in the community.
Methods and Results
We characterized AF patterns between 1980 and 2005 among Framingham Heart Study participants who survived ≥1 year after diagnosis. We classified participants based on their pattern within the first 2 years after detection as having AF without recurrence, recurrent AF, or sustained AF. We examined associations between AF patterns and 10‐year survival using proportional hazards regression. Among 612 individuals with AF, mean age was 72.5±10.8 years, and 53% were men. Of these, 478 participants had ≥2 electrocardiograms (median, 3; limits 2 to 23) within 2 years after initial AF and were classified as having AF without 2‐year recurrence (n=63, 10%), recurrent AF (n=162, 26%) or sustained AF (n=207, 34%), although some (n=46, 8%) were indeterminate. Of 432 classified participants, 363 died, 75 had strokes, and 110 were diagnosed with heart failure during the next 10 years. Relative to individuals without AF recurrence, the multivariable‐adjusted mortality was higher among people with recurrent AF (hazard ratio [HR], 2.04; 95% confidence interval [CI], 1.26 to 3.29) and sustained AF (HR, 2.36; 95% CI, 1.49 to 3.75).
Conclusions
In our community‐based AF sample, only 10% had AF without early‐term (2‐year) recurrence. Compared with individuals without 2‐year AF recurrences, the 10‐year prognosis was worse for individuals with either sustained or recurrent AF. Our proposed AF classification algorithm may be applicable in longitudinal datas ets.
Keywords: atrial fibrillation, heart failure, mortality, pattern, risk, stroke
Introduction
Atrial fibrillation (AF) is associated with substantial risks of stroke, heart failure, and death.1 AF may be episodic and may recur.2–8 The AF classification scheme endorsed by major cardiovascular societies classifies individuals according to temporal patterns.1 A single AF episode is described as first detected. Recurrent AF is described as paroxysmal if an episode lasts ≤7 days and is self‐terminating or as persistent if it lasts >7 days or is not self‐terminating. AF is considered permanent if efforts to restore sinus rhythm are either abandoned or are regarded as unsuccessful.
The existing classification scheme provides standardized nomenclature for describing AF patterns.1,9 However, this scheme is limited because it is based on consensus opinion rather than pathophysiological or experimental information. Application of the current AF classification scheme in observational studies has been challenging because of several factors. First, distinction between paroxysmal and persistent AF is predicated on knowledge of episode duration. In observational studies, rhythm status may be ascertained at a single encounter, and therefore it may not be possible to determine if spontaneous cardioversion has occurred within 7 days. Second, classification of persistent versus permanent AF may depend on patient‐ or provider‐level factors rather than the intrinsic pattern of AF. Specifically, patient symptoms and provider willingness to perform cardioversion or use an antiarrhythmic agent to restore sinus rhythm affect the distinction between persistent and permanent AF. Third, AF classification is ambiguous when individuals have episodes that both do and do not self‐terminate within 7 days.
The descriptors paroxysmal, persistent, and permanent have been widely used in studies describing the longitudinal course of AF. However, definitions have deviated substantially from the guidelines and differ between studies.2–7,2–18 As a result of these and other limitations,9 ambiguity exists regarding AF pattern frequency in the community and relations between AF type and long‐term prognosis.
We sought to develop a simplified AF pattern classification scheme that would minimize subjectivity in pattern classification and be applicable in longitudinal community‐based study settings. Given the aforementioned challenges of imposing conventional AF pattern definitions, we characterized AF patterns as either first detected without recurrence, recurrent, or sustained during the 2 years following detection among community‐dwelling participants enrolled in the Framingham Heart Study. Using this classification scheme we further aimed to examine AF pattern frequencies in the community and determine relations between specific patterns and long‐term clinical outcomes.
Methods
Participants
We identified participants from the Framingham Heart Study Original19 and Offspring20 cohorts with first‐detected AF between January 1, 1980, and December 31, 2005. The Boston University Medical Center Institutional Review Board approved study protocols, and all participants signed consent forms at each examination cycle.
Assessment of AF
At each Framingham Heart Study clinic examination, participants' medical histories, physical examinations, and electrocardiograms were obtained to ascertain symptoms and findings suggestive of cardiovascular disease. Each participant's primary physician was notified of the Framingham electrocardiographic findings, including AF. Records of all interim hospitalizations for cardiovascular disease were sought for review. Participants were classified as having AF if either atrial flutter or fibrillation was present on an electrocardiogram obtained at a Framingham Heart Study clinic visit or encounter with an external clinician, was present on Holter monitoring, or was noted in hospital records. After first‐detected AF, all subsequent Framingham Heart Study clinic visits and acquired external healthcare encounters (outpatient and hospital) were reviewed for evidence of AF. If the rhythm remained stable during a hospitalization, only 1 electrocardiogram was coded. If the rhythm varied during a hospitalization, the first episode of AF, the first episode of sinus rhythm, and the final electrocardiogram rhythm were recorded. Cardioversion attempts and successes were systematically ascertained (see Data S1). Two physicians adjudicated first‐detected AF events and ≥1 adjudicated subsequent encounter.
Definition of AF Patterns
We included participants with ≥2 recorded electrocardiograms and ≥1 year between the electrocardiogram with first‐detected AF and last electrocardiogram to avoid AF associated with early mortality (ie, within 1 year). Follow‐up began after first‐detected AF. We classified patterns as AF without recurrence, recurrent, or sustained on the basis of the cardiac rhythm pattern during the 2‐year period following first‐detected AF (Figure 1). We did not reclassify individuals during follow‐up after the initial classification period. We performed classification using 1‐, 2‐, and 4‐year windows but used 2‐year classification windows in our analysis to minimize the number of unclassified individuals while accounting for intercurrent stroke, heart failure, or death events during the classification period (Figure 2). If participants died during the classification period, classifications were made on the basis of available data that occurred until that point. Based on the first 2 years after initial AF detection, participants were classified as follows:
AF without 2‐year recurrence was defined as sinus rhythm without any subsequent AF episodes observed after first‐detected AF.
Recurrent AF was defined as AF with sinus rhythm of any duration between 2 AF episodes, including a successful cardioversion.
Sustained AF was defined as the absence of sinus rhythm or successful cardioversion after first‐detected AF.
Indeterminate patterns included rhythms other than sinus or AF or those in which a sequence of electrocardiograms included consecutive AF followed by sinus rhythm at the end of the classification window (because AF recurrence could not be verified without examining beyond the classification window).
Inadequate data refers to individuals with only 1 electrocardiogram during the classification window.
Figure 1.
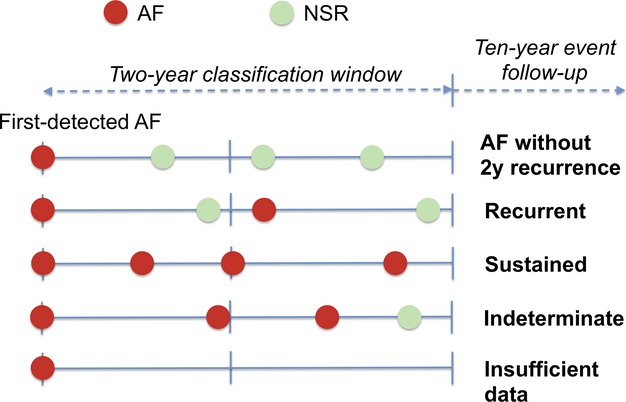
Illustrative example of atrial fibrillation pattern classification. Individuals were classified as having various atrial fibrillation patterns on the basis of the cardiac rhythm pattern during the 2 years following first‐detected atrial fibrillation. After pattern classification, the occurrence of clinical events during the subsequent 10 years was assessed. See text for detailed description of pattern definitions. AF indicates atrial fibrillation; NSR, normal sinus rhythm.
Figure 2.
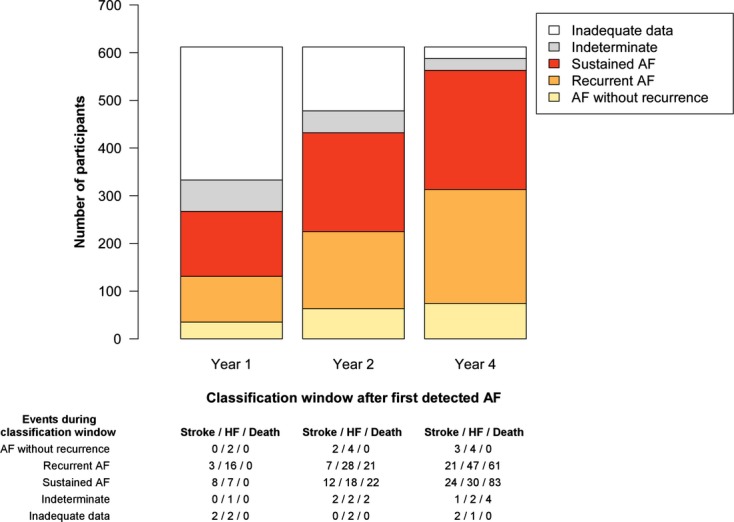
Number of participants classified and number of interim events according to duration of classification window after first‐detected atrial fibrillation. The number of participants in the sample who were classified as having atrial fibrillation without recurrence, recurrent atrial fibrillation, or sustained atrial fibrillation differed according to the length of the classification window because of differing numbers of electrocardiograms available for review. Classification windows of 1, 2, and 4 years are displayed. Below the bar graph are the numbers of incident strokes, heart failure events, and deaths that occurred before the end of each classification period. Individuals with such events during the classification period were excluded from analyses examining relations between patterns and that respective clinical event. AF indicates atrial fibrillation; HF, heart failure.
Ascertainment of Covariates, Stroke, Heart Failure, and Death
We ascertained participant characteristics at Framingham Heart Study clinic examinations (see Data S1). We included characteristics from the closest examination before and within 8.5 years of first‐detected AF. Framingham Heart Study participants are prospectively followed for the occurrence of cardiovascular events. A panel of 3 Framingham Heart Study investigators adjudicated strokes and heart failure events on the basis of review of acquired medical records and Framingham data based on published criteria.21 For strokes (including transient ischemic attacks), the panel included a neurologist.
Statistical Analysis
We estimated the 10‐year cumulative incidence of stroke, heart failure, or death using the Kaplan‐Meier method. Person‐time began with classification 2 years following first‐detected AF. We examined differences in the unadjusted cumulative incidence of clinical outcomes according to AF pattern using the log‐rank statistic. We examined adjusted associations between 2‐year AF patterns and outcomes using multivariable Cox proportional hazards regression.22 In the primary analysis, we examined associations between AF patterns classified in the first 2 years and subsequent 10‐year risk of death. We contrasted associations between death and each pattern with one another. In the secondary analyses, we studied relations between AF patterns and 10‐year risks of stroke and heart failure.
Models considered established risk factors for each respective outcome on the basis of published prediction algorithms, which were entered directly (covariates are listed in the legend for Table 2).21,23 Analyses of stroke and heart failure excluded individuals with prevalent stroke and heart failure, respectively. Individuals with clinical events that occurred during the classification window were excluded from analyses of the respective modeled clinical events. All the multivariable models included terms for each of the comparator patterns — recurrent, sustained, indeterminate, and inadequate — for comparison to the referent pattern, adjusted for important clinical risk factors. Participants in our analysis were followed through December 31, 2009, to their last exam, health history update, or death. Follow‐up was censored at 10 years, last follow‐up, loss to follow‐up, or death for analyses of stroke and heart failure. Proportional hazards assumptions were tested with multiplicative interaction terms between covariates and survival time.
Table 2.
Association Between Early AF Patterns and Death, Heart Failure, and Stroke Among Individuals With Incident AF
| AF Without 2‐Year Recurrence | Recurrent AF | Sustained AF | |||
|---|---|---|---|---|---|
| Total number with specific pattern | 63 | 162 | 207 | ||
| Primary outcome | |||||
| Death | |||||
| No. of events/person‐years | 29/431 | 87/678 | 141/852 | ||
| Adjustment | HR (95% CI) | P | HR (95%) CI | P | |
| Age and sex | Referent | 1.91 (1.25 to 2.90) | 0.003 | 1.99 (1.33 to 2.97) | <0.001 |
| Multivariable* | Referent | 2.04 (1.27 to 3.29) | 0.003 | 2.36 (1.49 to 3.75) | <0.001 |
| Secondary outcomes | |||||
| Heart failure | |||||
| No. of events/person‐years | 12/360 | 29/401 | 29/595 | ||
| Adjustment | HR (95% CI) | P | HR (95% CI) | P | |
| Age and sex | Referent | 2.08 (1.06 to 4.07) | 0.03 | 1.08 (0.54 to 2.13) | 0.84 |
| Multivariable* | Referent | 2.53 (1.19 to 5.38) | 0.02 | 1.23 (0.56 to 2.67) | 0.61 |
| Stroke | |||||
| No. of events/person‐years | 9/352 | 24/570 | 22/621 | ||
| Adjustment | HR (95% CI) | P | HR (95% CI) | P | |
| Age and sex | Referent | 1.52 (0.70 to 3.27) | 0.29 | 1.15 (0.53 to 2.50) | 0.73 |
| Multivariable* | Referent | 1.84 (0.77 to 4.38) | 0.17 | 1.32 (0.55 to 3.18) | 0.54 |
All models were adjusted for participants with indeterminate patterns as well as those with inadequate data for classification. AF indicates atrial fibrillation; HR, hazard ratio; CI, confidence interval.
Adjusted for age, sex, smoking status, systolic blood pressure, diabetes mellitus, history of heart failure, history of myocardial infarction, clinically significant murmur, and electrocardiographic left ventricular hypertrophy.
Adjusted for age, sex, systolic blood pressure, heart rate, electrocardiographic left ventricular hypertrophy, clinically significant murmur, body mass index, diabetes mellitus, and history of coronary heart disease.
The a priori significance threshold was P<0.05 using 2‐sided tests. We used the log‐rank test with the observed numbers of participants in the comparison groups and the observed 10‐year event rates to estimate our power to detect associations between patterns and clinical outcomes.24 We estimated we would have 80% power to detect associations between recurrent AF and AF without 2‐year recurrence with hazard ratios of at least 1.73 for death, 2.22 for stroke, and 2.47 for heart failure. We estimated we would have 80% power to detect associations between sustained AF and AF without 2‐year recurrence of at least 1.70 for death, 2.16 for stroke, and 2.40 for heart failure. Analyses were performed using SAS version 9.2 for Windows.22
Results
Of 1821 participants with AF and available electrocardiographic data, 1758 were from the Original and Offspring cohorts. Of these, 1338 had first‐detected AF between 1980 and 2005. Among the 612 participants who survived ≥1 year after AF detection, the mean age was 72.5±10.8 years, and 327 (53%) were men (Table 1). Using the initial 2‐year period after first‐detected AF to classify patterns, 478 individuals were classified using a median of 3 (limits, 2 to 23) electrocardiograms. Sixty‐three (10%) had AF without 2‐year recurrence, 162 (26%) had recurrent AF, and 207 (34%) had sustained AF, whereas 46 (8%) had indeterminate patterns. Among those with AF without 2‐year recurrence, 4 (6%) had a history of heart failure, 17 (27%) had a history of myocardial infarction, and 9 (14%) had prior coronary artery bypass surgery. Table 1 displays the baseline characteristics, clinical settings where first‐detected AF was identified, and average numbers of electrocardiograms on which classifications were based. Details regarding cardioversion attempts and antiarrhythmic and anticoagulation use are detailed in Table S1.
Table 1.
Characteristics of the 612 Participants Included in the Analysis by Early AF Pattern
| Characteristic | Overall | AF Without 2‐Year Recurrence | Recurrent AF | Sustained AF | Indeterminate | Inadequate Data |
|---|---|---|---|---|---|---|
| No. of participants | 612 | 63 (10) | 162 (26) | 207 (34) | 46 (8) | 134 (22) |
| No. of electrocardiograms | 3.2±2.3 | 2.9±1.3 | 5.1±2.9 | 3.2±1.6 | 3.3±1.5 | 1±0 |
| First‐detected AF identified at FHS* | 132 (22) | 6 (10) | 6 (4) | 64 (31) | 3 (7) | 53 (40) |
| Age, y | 73±11 | 71±11 | 72±11 | 75±10 | 71±12 | 70±11 |
| Men | 327 (53) | 37 (59) | 86 (53) | 105 (51) | 26 (57) | 73 (54) |
| Body mass index, kg/m2 | 28±5 | 28.1±4.5 | 28.2±5.1 | 28.0±5.5 | 28.4±5.4 | 27.2±5.0 |
| Systolic blood pressure, mm Hg | 141±22 | 143±22 | 142±22 | 144±21 | 137±19 | 138±24 |
| Antihypertensive therapy | 290 (54) | 36 (63) | 76 (52) | 97 (54) | 26 (59) | 55 (47) |
| Smoker | 84 (16) | 7 (12) | 17 (12) | 22 (12) | 7 (16) | 31 (27) |
| Diabetes mellitus | 87 (16) | 9 (16) | 22 (15) | 36 (20) | 10 (23) | 10 (9) |
| Heart rate, bpm | 68±13 | 66±12 | 66±12 | 70±15 | 65±12 | 68±13 |
| Left ventricular hypertrophy | 28 (5) | 5 (9) | 9 (6) | 11 (6) | 0 | 3 (3) |
| Clinically significant heart murmur | 77 (15) | 7 (12) | 16 (12) | 39 (22) | 3 (7) | 12 (11) |
| History of heart failure | 79 (13) | 4 (6) | 26 (16) | 34 (16) | 5 (11) | 11 (8) |
| History of myocardial infarction | 116 (19) | 17 (27) | 42 (26) | 23 (11) | 11 (24) | 23 (17) |
| History of coronary artery bypass surgery | 36 (6) | 9 (14) | 17 (10) | 6 (3) | 4 (9) | 0 (0) |
| Within 30 days of incident AF | 14 (2) | 5 (8) | 5 (3) | 2 (1) | 2 (4) | 0 (0) |
| History of stroke | 74 (12) | 5 (8) | 24 (15) | 24 (12) | 8 (17) | 13 (10) |
Data are shown as mean±standard deviation or number (%). AF indicates atrial fibrillation.
Compared with detection in a hospital or emergency department, by an outside clinician, on an outside electrocardiogram or Holter monitor, or by history alone. Detection at FHS refers to detection on a Framingham Heart Study electrocardiogram or Holter monitor.
Shorter classification periods (1 year) resulted in the inability to classify 279 (46%) of 612 participants owing to the infrequency of acquired electrocardiograms during the period (Tables S2 and S3). In contrast, longer classification periods (4 years) were accompanied by the inability to classify only 24 (4%) of individuals at the expense of many intercurrent strokes, heart failure diagnoses, or deaths, thereby precluding inclusion of these individuals in analyses examining associations between patterns and respective clinical outcomes (Figure 2).
Regardless of the length of the classification window, AF without recurrence was rare. The proportion of participants with AF without recurrence during the classification window was 6% using 1‐year classification windows and slightly higher, at 12%, using 4‐year windows (because of the greater number of classifiable participants when using the longer classification window).
Of 432 participants with AF classified in the first 2 years, 363 died, 75 had a stroke, and 110 were diagnosed with heart failure during the next 10 years. Figure 3 displays the 10‐year cumulative incidence of stroke, heart failure, or death following classification. Cumulative incidence curves for the composite of clinical events are provided in Figure S3. The median time to the first of the clinical events was 3.1 years following the classification window (range, 0.007 to 9.96 years). Among individuals without a clinical event, the median follow‐up time was 10 years (range, 0.760 to 10 years).
Figure 3.
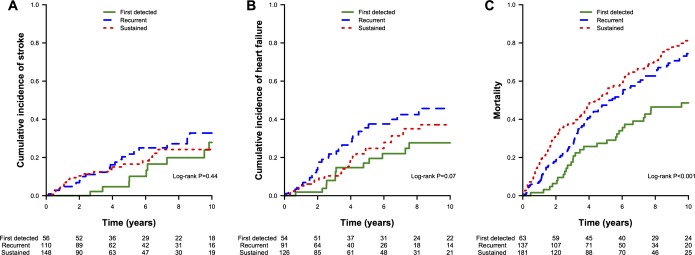
Cumulative incidence of stroke, heart failure, and death by atrial fibrillation pattern. The cumulative incidence of (A) stroke, (B) heart failure, and (C) death is displayed stratified by atrial fibrillation pattern over the 10 years of follow‐up after atrial fibrillation pattern classification.
In the primary analysis, mortality was higher among individuals with either recurrent AF (multivariable‐adjusted hazard ratio [HR], 2.04; 95% confidence interval [CI], 1.27 to 3.29; P=0.003) or sustained AF (HR, 2.36; 95% CI, 1.49 to 3.75, P=0.003), compared with those with AF without 2‐year recurrence (Table 2). The magnitude of risk associated with both recurrent and sustained AF was not attenuated by adjustment for comorbid conditions. We did not observe a significant difference in mortality between groups with recurrent and sustained AF (P=0.33).
In secondary analyses (Table 2), incident heart failure risk was higher among participants with recurrent AF compared with those without recurrence and was not attenuated by adjustment for heart failure risk factors (HR, 2.53; 95% CI, 1.19 to 5.38; P=0.02). We did not observe a significant association between sustained AF and heart failure relative to participants with AF without 2‐year recurrence (HR, 1.23; 95% CI, 0.56 to 2.67; P=0.61). Heart failure risk differed significantly between recurrent and sustained AF (P=0.02).
Stroke risk did not differ significantly among individuals with recurrent (HR, 1.84; 95% CI, 0.77 to 4.38; P=0.17) or sustained AF (HR, 1.32; 95% CI, 0.55 to 3.18; P=0.54), relative to those without a 2‐year AF recurrence. Results of primary and secondary analyses for individuals with indeterminate patterns or with insufficient data for classification are displayed in Table S4.
Discussion
In our community‐based sample of individuals with incident AF, we applied a simplified classification algorithm on the basis of cardiac rhythm at multiple times to characterize AF patterns and their associations with clinical outcomes. Few individuals (10%) presented without AF on a subsequent electrocardiogram within 2 years. Recurrent (26%) or sustained (34%) AF was common and was associated with about a 2‐fold increased risk of mortality compared with individuals without a 2‐year recurrence of AF. Overall, individuals with AF were at substantial risk of morbidity, with greater than two thirds experiencing incident stroke, heart failure, or death within about a decade of their first‐detected AF episode. Our findings implicate heterogeneity in both the frequency of AF patterns and the clinical implications of particular patterns.
Comparison of results between studies examining the longitudinal course of AF is challenging, in part because AF pattern definitions have been applied differently.2–7,2–18,25 Previous definitions have relied on heterogeneous AF episode durations for pattern classification and have variably attempted to distinguish AF patterns other than paroxysmal from one another. Our approach to classifying AF patterns on the basis of the rhythm over a preceding time interval is applicable to the clinical setting and similar to that suggested by the American College of Cardiology and American Heart Association clinical data standards committee.26 We used a 2‐year window for classification rather than the suggested 1‐year period to ensure sufficient density of encounters with electrocardiograms for classification in our observational data set.
Our findings extend previous observations regarding AF pattern frequency. In the Euro Heart Survey of AF, comprised primarily of individuals with AF enrolled in the hospital setting, 46% of 621 individuals with first‐detected AF did not have a recurrent episode within 1 year.15 In contrast, our findings suggest that AF without recurrence is rare in the community, even when restricting our follow‐up to 1 year (6%). In the prospective Etude en Activité Libérale de la Fibrillation Auriculaire study of patients from cardiology practices in France, 22% presented with paroxysmal AF.6 In an analysis from the General Practice Research Database from the UK from 1996 that included 1888 individuals with first‐detected AF, AF was classified as paroxysmal in 28% and chronic in the remainder.17 In individuals with first‐detected lone AF referred to a single cardiovascular practice in Italy between 1966 and 1995, 55% were identified as having paroxysmal AF and the remainder with chronic AF at the time of AF diagnosis.5 These data support our observation that AF frequently recurs early after detection.
Our results also extend previous data regarding the relations between AF patterns and long‐term prognosis. In our cohort, mortality was increased about 2‐fold among participants with recurrent or sustained AF relative to those without recurrence 2 years after detection. This finding contrasts an analysis of 270 patients with AF from the Mayo Clinic, in which permanent AF was not associated with increased mortality after multivariable adjustment.16
Two primary hazards of AF are heart failure and stroke, both of which serve as proximate risk factors for death. We observed an estimated 2‐fold increased heart failure risk among individuals with recurrent AF compared with those with AF without early recurrence. It is possible that the association between heart failure and recurrent AF was attributable to particular characteristics of recurrent AF. Alternatively, the association may reflect reverse causation; in other words, providers may have been more inclined to restore sinus rhythm in patients with heart failure. The reason that sustained AF was not associated with increased heart failure risk may reflect exclusion of participants most likely to develop heart failure during the 2‐year classification window or lack of power to detect an association. We did not observe differences in stroke risk among different AF patterns, findings that are generally consistent with previous reports.27 However, we had limited power to detect small differences (ie, HR<2) in stroke risk.
Strengths of our study include standardized ascertainment of clinical outcomes and potential confounding factors in a community‐based cohort. Framingham Heart Study participants were followed longitudinally through routine study visits and acquisition of intercurrent medical records from healthcare encounters.
Our study should be interpreted in the context of the observational study design. First, in the absence of continuous electrocardiographic monitoring, it is possible that AF patterns were misclassified in some participants. Participants who had only 1 electrocardiogram during the 2‐year classification period (22%) could not be classified using our algorithm, whereas 8% were indeterminate. A greater number of participants had classifiable AF patterns as the duration of the classification window was extended. However, longer classification windows may have less direct clinical relevance than 2‐year windows. Furthermore, longer classification periods were associated with a greater number of intercurrent stroke, heart failure, or death events during the classification window, thereby limiting an examination of the associations between patterns and subsequent clinical outcomes. Nevertheless, we adjusted for participants with inadequate data or indeterminate patterns in our analyses. Second, there is potential for biased electrocardiogram sampling having occurred in our cohort. Electrocardiograms between routine Framingham Heart Study clinic research examinations were not performed at standardized intervals, which may have affected associations with clinical outcomes. Participants who did not develop heart failure or strokes may have had fewer healthcare encounters and therefore may have had fewer electrocardiograms than others. Infrequent electrocardiogram ascertainment is a limitation of most observational studies and might be overcome in studies with frequent standardized cardiac rhythm assessment such as clinical trials. Although an analysis including only electrocardiograms from Framingham Heart Study visits would offer standardized assessment of the cardiac rhythm status, Framingham Heart Study visits were spaced ≥2 years apart, limiting the sample size available for evaluation, and may have less direct clinical relevance than the analysis that we performed, which included assessments during routine clinical care. In addition, limiting cardiac rhythm assessment to Framingham visits alone would likely bias the sample toward those with sustained AF. Third, residual confounding remains possible despite adjustment for established risk factors derived from published risk prediction models.21,23 Fourth, although we did not attempt to distinguish between AF episodes that do and do not self‐terminate, there may be clinical differences associated with such AF episodes. Fifth, our secondary analyses examining associations between AF patterns and heart failure and stroke had limited power to detect small effect sizes. More events may be necessary to determine whether the poorer prognosis among those with recurrent or sustained AF is mediated by such clinical events. Sixth, we studied middle‐aged to older adults of European ancestry; the generalizability to younger individuals or other races/ethnicities remains to be determined.
Our observations have 2 main implications. First, our results confirm the heterogeneity of AF patterns in the community and demonstrate that AF without early recurrence rarely occurs. AF without recurrence is associated with a better prognosis than either recurrent or sustained AF, both of which are associated with similar mortality. That so many individuals had sustained AF at presentation raises questions of whether such individuals had a period of unrecognized and progressive AF prior to arrhythmia detection or instead developed sustained AF at the outset.
Second, our findings imply that a simplified classification system that minimizes subjectivity may be applicable in the context of observational data sets, electronic health records, or clinical trials when the cardiac rhythm is ascertained at multiple points over time. In these contexts, knowledge of episode duration, self‐termination of episodes, and provider intent to restore sinus rhythm are frequently unknown. Application of such an algorithm may facilitate examination of the effectiveness of interventions for AF, cost‐effectiveness analyses, and quality‐of‐life assessments. Furthermore, application of such an algorithm may enhance future efforts to determine the influence of AF progression on health outcomes. Our classification system is only applicable to other studies in which sufficient density of rhythm assessment is available.
Conclusions
In our community‐based cohort, few individuals with newly detected AF presented without an early‐term recurrence, yet their long‐term prognosis appeared better than that for individuals with either recurrent or sustained AF. No substantial difference in prognosis was observed between individuals with either recurrent or sustained AF. A simplified AF classification algorithm that minimizes subjectivity may be applicable in the context of large data sets, yet requires validation. Identification of the specific causes of increased mortality associated with recurrent or sustained AF needs further investigation.
Sources of Funding
This work was funded by N01‐HC 25195, 6R01‐NS 17950, grants to Dr Benjamin (1RC1HL101056; 1R01HL102214, 1R01AG028321), Drs Benjamin and Ellinor (1RO1HL092577), Dr Ellinor (5R21DA027021, 1RO1HL104156, 1K24HL105780), Dr Lubitz (1K23HL114724), and Dr McManus (U01HL105268, KL2RR031981), and in part by the Evans Center for Interdisciplinary Biomedical Research ARC on Atrial Fibrillation at Boston University (http://www.bumc.bu.edu/evanscenteribr/). Dr Lubitz (12FTF11350014), Dr Magnani (09FTF2190028), and Dr Ellinor (13EIA14220013) were supported by AHA awards.
Disclosures
None.
Acknowledgments
Dr Lubitz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS, Smith SC, Jr, Priori SG, Estes NA, 3rd, Ezekowitz MD, Jackman WM, January CT, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Hochman JS, Kushner FG, Ohman EM, Tarkington LG, Yancy CW. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011; 123:e269-e367 [DOI] [PubMed] [Google Scholar]
- 2.Takahashi N, Seki A, Imataka K, Fujii J. Clinical features of paroxysmal atrial fibrillation. An observation of 94 patients. Jpn Heart J. 1981; 22:143-149 [DOI] [PubMed] [Google Scholar]
- 3.Kopecky SL, Gersh BJ, McGoon MD, Whisnant JP, Holmes DR, Jr, Ilstrup DM, Frye RL. The natural history of lone atrial fibrillation. A population‐based study over three decades. N Engl J Med. 1987; 317:669-674 [DOI] [PubMed] [Google Scholar]
- 4.Davidson E, Rotenberg Z, Weinberger I, Fuchs J, Agmon J. Diagnosis and characteristics of lone atrial fibrillation. Chest. 1989; 95:1048-1050 [DOI] [PubMed] [Google Scholar]
- 5.Scardi S, Mazzone C, Pandullo C, Goldstein D, Poletti A, Humar F. Lone atrial fibrillation: prognostic differences between paroxysmal and chronic forms after 10 years of follow‐up. Am Heart J. 1999; 137:686-691 [DOI] [PubMed] [Google Scholar]
- 6.Levy S, Maarek M, Coumel P, Guize L, Lekieffre J, Medvedowsky JL, Sebaoun A. Characterization of different subsets of atrial fibrillation in general practice in France: the ALFA study. The College of French Cardiologists. Circulation. 1999; 99:3028-3035 [DOI] [PubMed] [Google Scholar]
- 7.Kerr CR, Humphries KH, Talajic M, Klein GJ, Connolly SJ, Green M, Boone J, Sheldon R, Dorian P, Newman D. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J. 2005; 149:489-496 [DOI] [PubMed] [Google Scholar]
- 8.Flaker GC, Fletcher KA, Rothbart RM, Halperin JL, Hart RG. Clinical and echocardiographic features of intermittent atrial fibrillation that predict recurrent atrial fibrillation. Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Am J Cardiol. 1995; 76:355-358 [DOI] [PubMed] [Google Scholar]
- 9.Lubitz SA, Benjamin EJ, Ruskin JN, Fuster V, Ellinor PT. Challenges in the classification of atrial fibrillation. Nat Rev Cardiol. 2010; 7:451-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen P, Godtfredsen J. Embolic complications in paroxysmal atrial fibrillation. Stroke. 1986; 17:622-626 [DOI] [PubMed] [Google Scholar]
- 11.Kato T, Yamashita T, Sagara K, Iinuma H, Fu LT. Progressive nature of paroxysmal atrial fibrillation. Observations from a 14‐year follow‐up study. Circ J. 2004; 68:568-572 [DOI] [PubMed] [Google Scholar]
- 12.Jahangir A, Lee V, Friedman PA, Trusty JM, Hodge DO, Kopecky SL, Packer DL, Hammill SC, Shen WK, Gersh BJ. Long‐term progression and outcomes with aging in patients with lone atrial fibrillation: a 30‐year follow‐up study. Circulation. 2007; 115:3050-3056 [DOI] [PubMed] [Google Scholar]
- 13.Al‐Khatib SM, Wilkinson WE, Sanders LL, McCarthy EA, Pritchett EL. Observations on the transition from intermittent to permanent atrial fibrillation. Am Heart J. 2000; 140:142-145 [DOI] [PubMed] [Google Scholar]
- 14.Pappone C, Radinovic A, Manguso F, Vicedomini G, Ciconte G, Sacchi S, Mazzone P, Paglino G, Gulletta S, Sala S, Santinelli V. Atrial fibrillation progression and management: a 5‐year prospective follow‐up study. Heart Rhythm. 2008; 5:1501-1507 [DOI] [PubMed] [Google Scholar]
- 15.Nieuwlaat R, Prins MH, Le Heuzey JY, Vardas PE, Aliot E, Santini M, Cobbe SM, Widdershoven JW, Baur LH, Levy S, Crijns HJ. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow‐up of the Euro Heart Survey on atrial fibrillation. Eur Heart J. 2008; 29:1181-1189 [DOI] [PubMed] [Google Scholar]
- 16.Keating RJ, Gersh BJ, Hodge DO, Weivoda PL, Patel PJ, Hammill SC, Shen WK. Effect of atrial fibrillation pattern on survival in a community‐based cohort. Am J Cardiol. 2005; 96:1420-1424 [DOI] [PubMed] [Google Scholar]
- 17.Ruigomez A, Johansson S, Wallander MA, Garcia Rodriguez LA. Predictors and prognosis of paroxysmal atrial fibrillation in general practice in the UK. BMC Cardiovasc Disord. 2005; 5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, van den Heijkant AC, Allessie MA, Crijns HJ. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010; 55:725-731 [DOI] [PubMed] [Google Scholar]
- 19.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951; 41:279-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975; 4:518-525 [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999; 159:1197-1204 [DOI] [PubMed] [Google Scholar]
- 22. Phreg procedure. Sas© 9.2 user's guide. 2008Cary, NC: Sas institute inc; 4517-4721 [Google Scholar]
- 23.Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D'Agostino RB, Larson MG, Kannel WB, Benjamin EJ. A risk score for predicting stroke or death in individuals with new‐onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003; 290:1049-1056 [DOI] [PubMed] [Google Scholar]
- 24.Machin D, Campbell M, Fayers P, Pinol A. Sample size tables for clinical studies. 1997Malden, MA: Blackwell Science [Google Scholar]
- 25.Tsang TS, Barnes ME, Miyasaka Y, Cha SS, Bailey KR, Verzosa GC, Seward JB, Gersh BJ. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008; 29:2227-2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNamara RL, Brass LM, Drozda JP, Jr, Go AS, Halperin JL, Kerr CR, Levy S, Malenka DJ, Mittal S, Pelosi F, Jr, Rosenberg Y, Stryer D, Wyse DG, Radford MJ, Goff DC, Jr, Grover FL, Heidenreich PA, Peterson ED, Redberg RF. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Data Standards on Atrial Fibrillation). Circulation. 2004; 109:3223-3243 [DOI] [PubMed] [Google Scholar]
- 27.Lubitz SA, Rosen AB, Ellinor PT, Benjamin EJ. Stroke risk in AF: do AF patterns matter? Eur Heart J. 2010; 31:908-910 [DOI] [PMC free article] [PubMed] [Google Scholar]


