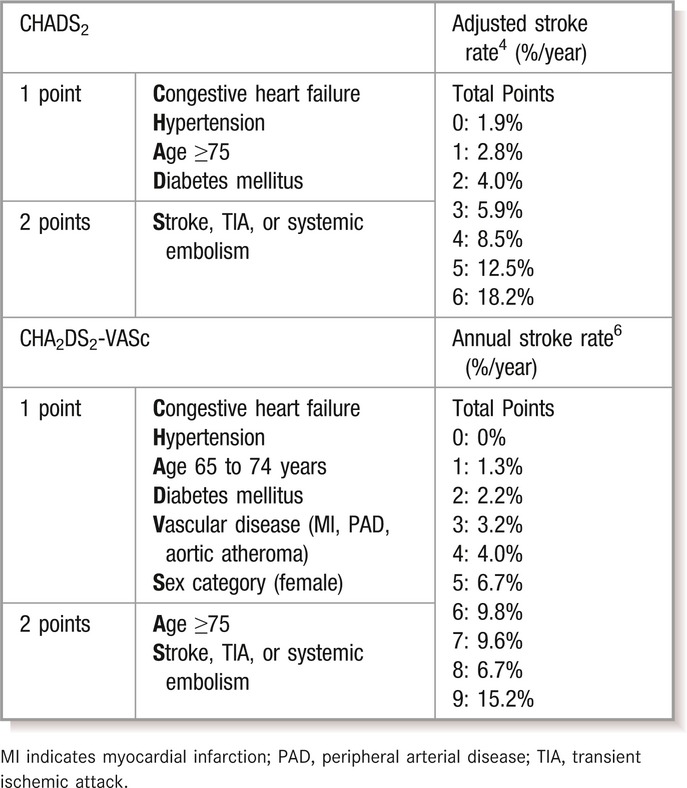
Stroke Risk Stratification
Atrial fibrillation (AF) is a common arrhythmia in the adult population. It is associated with up to a 5‐fold increased risk of stroke and contributes to a higher morbidity and mortality compared with non–AF‐related strokes.1 The current prevalence of AF in the adult population, estimated at 1% to 2%, is expected to increase by up to 5 times by 2050, which will in turn increase the overall burden of ischemic stroke in the aging population.2–3 Various stroke risk stratification schemes have been developed to quantify stroke risk in patients with AF and guide preventive treatment decisions for the clinician. The most widely used has been the CHADS2 score, which estimates risk based on the presence of congestive heart failure, hypertension, age 75 years or greater, diabetes mellitus, and prior stroke or transient ischemic attack (TIA).4
A revision of the CHADS2 scheme has been developed for use in stroke risk assessment in AF. It dichotomizes age and incorporates vascular disease and female sex, to create the CHA2DS2‐VASc (VA, vascular disease; Sc, sex category) score5 (Table 1). Compared with CHADS2, this stroke risk stratification scheme is better able to discriminate among individuals at lowest risk.6–8 For example, in patients with a CHADS2 score of 0, the 1‐year stroke and embolic event rates range from 0.84% (CHA2DS2‐VASc score of 0) to 3.2% (CHA2DS2‐VASc score of 3).8 By both risk stratification schemes, those patients who score ≥1 are recommended to receive oral anticoagulant therapy unless major contraindications are present. Only those who are <65 years of age and have lone AF are truly considered “low risk” and may not need antithrombotic treatment.9–10
Table 1.
Comparison of CHADS2 and CHA2DS2VASc Risk Stratification Schemes
In patients appropriate for oral anticoagulant therapy for stroke prevention in AF, the mainstay of treatment for decades has been a vitamin K antagonist (VKA). Compared with placebo or no treatment, adjusted‐dose warfarin was found in the early AF trials to reduce stroke by about 64% (95% CI 49% to 74%) without a significant increase in major bleeding. Antiplatelet therapy was also effective, but to a lesser extent, with a relative risk reduction of 22% compared with placebo (95% CI 2% to 39%).11 Although these early AF trials concluded a favorable safety profile for warfarin,12 there are some notable limitations that challenge extrapolation of bleeding risk from trial to general populations. The early trials assessing efficacy and safety of adjusted‐dose warfarin (ie, Second Copenhagen Atrial Fibrillation, Aspirin and Anticoagulant Therapy Study [AFASAK], Stroke Prevention in Atrial Fibrillation [SPAF], Boston Area Anticoagulation Trial for Atrial Fibrillation [BAATAF], Canadian Atrial Fibrillation Anticoagulation [CAFA], Stroke Prevention in Nonrheumatic Atrial Fibrillation [SPINAF], and European Atrial Fibrillation Trial [EAFT]) had relatively small study populations composed of participants with few risk factors for bleeding compared with the “real world” AF populations on anticoagulant therapy.13
Assessment of Bleeding Risk on Anticoagulant Therapy in Patients With AF
Risk for hemorrhage in patients on anticoagulant therapy has been studied extensively. The HAS‐BLED risk stratification scheme is one of several that has been validated to estimate baseline risk of major hemorrhage (defined as hemorrhage involving a critical anatomic site, for example, intracranial, or a bleed requiring hospitalization, transfusion of ≥2 units of packed cells, or associated with a decrease in hemoglobin level of ≥2 g/L). One point is assigned to each of the following risk factors for bleeding: uncontrolled Hypertension, Abnormal renal function, Abnormal liver function, Stroke, history of Bleeding, Labile international normalized ratio (INR), Elderly status (>65 years old), and alcohol or Drugs, such as nonsteroidal anti‐inflammatory or antiplatelet therapy. The presence of ≥3 risk factors is indicative of high risk for bleeding.14 The use of these risk stratification schemes has been a useful tool in clinical practice in determining those patients who need more aggressive risk modification and monitoring on anticoagulant therapy.10
Warfarin and Time in Therapeutic Range
Time in the therapeutic range (TTR) is a significant risk factor for stroke, major bleeding, and mortality. Data from the warfarin arms of the Stroke Prophylaxis using an ORal Thrombin Inhibitor in atrial Fibrillation (SPORTIF) III and V trials concluded significantly higher rates of major hemorrhage in the poor INR control group (TTR<60%) compared with the moderate INR control group (TTR 60% to 75%) and good INR control group (TTR>75%) (major bleeding rates 3.85% versus 1.96% versus 1.58%, respectively; P<0.01).15 It has therefore been difficult to translate safety outcomes from clinical trials into general practice, when outcomes vary by TTR, and TTR varies by population. In a meta‐analysis investigating a study setting's influence on TTR, trial study populations had a significantly higher mean TTR compared with community‐based cohort study populations (66.4% versus 56.7%; P<0.001).16 Because there is conflicting evidence on the safety of warfarin, clinicians have been shown to underuse anticoagulant therapy, especially in the elderly—a population typically with greater risk for stroke in AF.17
The challenges to optimal warfarin use and achieving adequate INR control are complex and multifactorial. Genetic mutations have been described that alter the pharmacodynamics of warfarin, and environmental factors such as various drugs (through the cytochrome P450 [CYP] system), herbal supplements, dietary changes in vitamin K, and certain disease states (ie, liver dysfunction) can affect warfarin's pharmacokinetics. Due to the variable dose response of warfarin, frequent monitoring is required, which creates a significant barrier to its use.18 There has subsequently been a demand for oral anticoagulant drugs with a wider therapeutic window, that do not require frequent monitoring, that are easier to administer for the patient, and that exhibit fewer drug–drug and diet–drug interactions.
Target‐Specific Oral Anticoagulants in the Prevention of Stroke in AF
Background
The challenges associated with managing warfarin in clinical practice have prompted the extensive research and development of target‐specific oral anticoagulants (TSOAs) that are now available for use. This group of drugs exerts its anticoagulant effects through inhibition of factor Xa or thrombin (Figure). TSOAs are promising as safe and efficacious alternatives to warfarin with some favorable pharmacological qualities (Table 2). Not only do they offer quick time‐to‐peak effects, fixed dosing regimens, and no monitoring, but they also have fewer drug–drug and dietary interactions compared with warfarin. Disadvantages of these TSOAs include a current lack of accurate monitoring in cases of suspected toxicity, lack of antidote in cases of life‐threatening bleeds or urgent surgery, and the inability to administer to those individuals with stage V chronic kidney disease or a prosthetic heart valve.19 Safety and efficacy data of the TSOAs have been derived from multiple phase III randomized clinical trials, the largest of them being those in stroke prevention in nonvalvular AF (Table 3).
Figure 1.
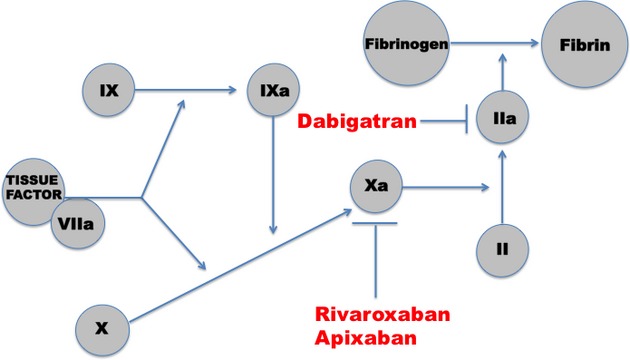
Targets in anticoagulation cascade for novel anticoagulants.
Table 2.
Comparison of Warfarin With Target‐Specific Oral Anticoagulants
| Drug Feature | Warfarin19 | Dabigatran20 | Rivaroxaban21 | Apixaban22 |
|---|---|---|---|---|
| Target | Vitamin K | Thrombin | Factor Xa | Factor Xa |
| Dose frequency | Daily | Once or twice daily | Once or twice daily | Twice daily |
| Onset | Slow | Rapid | Rapid | Rapid |
| Peak effect | 4 to 5 days | 1 to 2 hours | 2 to 3 hours | 1 to 2 hours |
| Offset | Long | Short | Short | Short |
| Half‐life | 40 hours | 12 to 17 hours | 7 to 11 hours | 12 hours |
| Renal clearance | None | 80% | 33% | 25% |
| Interactions | Many | P‐gp | CYP3A4; P‐gp | CYP3A4 |
| Monitoring | Yes | No | No | No |
| Dialyzable | No | Yes | No | No |
| Antidote | Vitamin K | No | No | No |
b.i.d. indicates twice daily; CYP3A4, cytochrome P450 3A4 enzyme; P‐gp, P‐glycoprotein; q.d., once daily.
Table 3.
Efficacy and Safety Outcomes in Stroke Prevention in Atrial Fibrillation (AF) Trials
| Novel Drug (Dose) | Stroke Prevention | Myocardial Infarction | Major Bleeding |
|---|---|---|---|
| Dabigatran 110 mg23 | 0.91 (0.74 to 1.11) | 1.35 (0.98 to 1.87) | 0.8 (0.69 to 0.93) |
| Dabigatran 150 mg23 | 0.66 (0.53 to 0.82) | 1.38 (1.00 to 1.91) | 0.93 (0.81 to 1.07) |
| Rivaroxaban 20 mg24 | 0.88 (0.75 to 1.03) | 0.81 (0.63 to 1.06) | 1.04 (0.9 to 1.2) |
| Apixaban 5 mg25 | 0.79 (0.66 to 0.95) | 0.88 (0.66 to 1.17) | 0.69 (0.6 to 0.8) |
Results from stroke prevention in AF trials presented as relative risk or hazard ratios with 95% CIs.
Pharmacological Properties and Efficacy of Dabigatran in Stroke Prevention
Dabigatran is a direct thrombin inhibitor that has been approved by the US Food and Drug Administration (FDA) for the prevention of stroke in nonvalvular AF. It requires twice‐daily dosing, reaches peak effect within 1 to 2 hours, and has a half‐life of 12 to 17 hours. It is cleared by the kidneys (≈80%) and requires no routine coagulation monitoring. Dabigatran is a P‐glycoprotein (P‐gp) substrate. Concomitant use with the potent P‐gp inhibitor rifampin should be avoided. Individuals with moderate renal impairment (creatinine clearance 30 to 50 mL/min) may experience increased concentrations of dabigatran when coadminstered with the P‐gp inducers ketoconazole and dronedarone. In this setting, a reduced dose of dabigatran is recommended, and use of these medications should be avoided if the creatinine clearance is <30 mL/min.19–20
Dabigatran is presently the only FDA‐approved direct thrombin inhibitor for stroke prevention in patients with nonvalvular AF. The Randomized Evaluation of Long‐term anticoagulant therapY (RE‐LY) trial compared 2 different dosages of dabigatran (110 mg twice daily or 150 mg twice daily) to adjusted‐dose warfarin (target INR of 2.0 to 3.0). The study population had a mean CHADS2 score of 2.1. The TTR for the warfarin arm was 64%. The lower dose of dabigatran was found to be noninferior to warfarin for the reduction of stroke or systemic embolism (relative risk [RR] 0.91, 95% CI 0.74 to 1.11; P<0.001 for noninferiority). However, the higher dose was found to be superior to warfarin (RR 0.66, 95% CI 0.53 to 0.82; P<0.001 for superiority) and ultimately gained FDA approval for stroke prevention in the United States. There has also been a small and statistically insignificant increase in myocardial infarction seen with both doses of dabigatran, but overall cardiovascular mortality was reduced with dabigatran compared with warfarin.23
Dabigatran and Bleeding Risk
Risk of hemorrhagic stroke was lower with both the 110‐mg and 150‐mg doses of dabigatran compared with warfarin; however, only the 110‐mg dose of dabigatran had fewer major bleeding events compared with warfarin (2.71% versus 3.76%; P=0.003).23 In a subset analysis of bleeding events by age group in the RE‐LY trial, it was found that patients >75 years receiving the 110‐mg dose had similar rates of bleeding compared with warfarin. Moreover, the higher dose of dabigatran was associated with an increase in major bleeding events, especially gastrointestinal hemorrhage, in the elderly population.26 Despite these data, the FDA concluded the overall risk‐benefit still weighed in favor of the higher 150‐mg dose.27 A total of 153 patients suffered an intracranial hemorrhage (either intracerebral, subdural, or subarachnoid) in the RE‐LY trial. The yearly rates of intracranial hemorrhage were 0.76%, 0.31%, and 0.23% for the participants in the warfarin, dabigatran 150‐mg dose, and dabigatran 110‐mg dose arms, respectively (P<0.001 for each comparison versus warfarin). For both dabigatran arms, the events were less likely to be fatal compared with the intracranial hemorrhagic events in the warfarin arm (P<0.05). Independent risk factors for intracranial hemorrhage in all participants in the RE‐LY trial were age, aspirin use, assignment to the warfarin arm, and prior stroke or TIA.28
The RE‐LY and other AF trials excluded patients with valvular AF or prosthetic heart valves. The phase II trial of dabigatran (Randomized, Phase II Study to Evaluate the Safety and Pharmacokinetics of Oral Dabigatran Etexilate in Patients after Heart Valve Replacement [RE‐ALIGN]), which studied patients with mechanical heart valves, was stopped early due to an increase in thrombotic events and an increase in bleeding postsurgery with dabigatran compared with warfarin. The FDA has since issued a contraindication for dabigatran use with mechanical heart valves (both with and without AF).29
Pharmacological Properties of Rivaroxaban and Efficacy in Stroke Prevention
Rivaroxaban is a factor Xa inhibitor that is also FDA approved for stroke prevention treatment in nonvalvular AF. As a once‐daily dose, it reaches its peak effect in ≈2 to 3 hours, with a half‐life between 7 and 11 hours. About 30% of the drug remains unchanged while excreted by the kidneys, whereas the remainder of the drug is metabolized via the CYP3A4 system in the liver. In addition to avoiding use with other drugs that are potent inhibitors of CYP3A4, rivaroxaban use should also be avoided (as with dabigatran) with drugs that inhibit P‐gp.19
ROCKET AF (Rivaroxaban Once‐Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) was a noninferiority trial that compared a fixed 20‐mg daily dosage of rivaroxaban with adjusted‐dose warfarin. The study population recruited into the trial was one of higher risk with a mean CHADS2 score of 3.5. Mean TTR was 55% for the participants randomized to warfarin. Compared with adjusted‐dose warfarin, rivaroxaban was noninferior in reducing the rate of stroke and non–central nervous system embolism in patients with nonvalvular AF (hazard ratio [HR] 0.79, 95% CI 0.74 to 1.03; P<0.001 for noninferiority).24 In a subgroup analysis, rivaroxaban was noninferior to warfarin for both primary and secondary stroke prevention.30
Rivaroxaban and Bleeding Risk
Rivaroxaban was associated with a lower risk of intracranial bleeding (0.5% versus 0.7%; P=0.02) and fatal bleeding (0.2% versus 0.5%; P=0.003) compared with warfarin.24 Rates of major and nonmajor clinically relevant bleeding were similar for rivaroxaban and warfarin (14.9% versus 14.5%, respectively; P=0.44); however, gastrointestinal bleeding was more common in the rivaroxaban group (3.2% versus 2.2%, respectively; P<0.001). Risk differences in major bleeding between rivaroxaban and warfarin remained consistent when stratified according to underlying history of stroke or TIA in a subgroup analysis.30
Pharmacological Properties of Apixaban and Efficacy in Stroke Prevention
Apixaban, like rivaroxaban, is an oral factor Xa inhibitor that has recently been approved by the FDA for stroke prevention in nonvalvular AF. Given as a twice‐daily dose, apixaban reaches peak plasma concentration within 3 to 4 hours and has a half‐life of 8 to 15 hours. Apixaban is both metabolized by the liver through the CYP3A4 pathway and cleared by the kidneys (≈25%). Similar to rivaroxaban, coadministration with potent CYP3A4 inhibitors and P‐gp inhibitors should be avoided.19
In the A Phase III Study of Apixaban in Patients With Atrial Fibrillation (AVERROES) trial, apixaban 5 mg twice daily was compared with aspirin (81 to 324 mg) among individuals deemed to be unsuitable for a VKA. The trial enrolled 5598 participants with a mean CHADS2 score of 2.0 (25% ≥3). At the first interim analysis, apixaban was found to be superior to aspirin and thus the trial was terminated early for efficacy. After 1.1 years of follow‐up, compared with aspirin, apixaban was associated with a 55% stroke risk reduction (HR 0.45, 95% CI 0.32 to 0.62; P<0.001).31 These results were independent of baseline stroke risk according to CHADS2 score.32
Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation (ARISTOTLE) was a noninferiority trial of apixaban 5 mg twice daily (2.5 mg twice daily for a select subset) compared with adjusted‐dose warfarin. The mean CHADS2 score for the study population was 2.1, and mean TTR for those randomized to warfarin was 62.2%. Apixaban was shown to be superior to warfarin for the reduction of stroke and systemic embolism (HR 0.79, 95% CI 0.66 to 0.95; P=0.01).25 In subgroup analyses, apixaban was favorable compared with warfarin independent of baseline stroke risk (CHADS2 of 1, 2, or ≥3) and history of stroke or TIA.33–34
Apixaban and Bleeding Risk
Apixaban showed a favorable profile compared with warfarin in overall safety outcomes. The rate of intracranial hemorrhage was reduced in the apixaban group compared with warfarin (HR 0.42, 95% CI 0.30 to 0.58; P<0.001). Major bleeding, defined by International Society of Thrombosis and Haemostasis (ISTH) criteria, occurred less frequently in the apixaban group (HR 0.69, 95% CI 0.60 to 0.80; P<0.001). This trend was consistent when using other definitions of major bleeding, including Global Utilization of Streptokinase and Tissue plasminogen activator for Occluded coronary arteries (GUSTO) and Thrombolysis In Myocardial Infarction (TIMI). Gastrointestinal bleeding was numerically lower with apixaban compared with warfarin but did not achieve statistical significance (HR 0.89, 95% CI 0.70 to 1.15; P<0.37).25
The AVERROES investigators found the apixaban group to have similar major bleeding rates compared with the aspirin group (HR 1.13, 95% CI 0.74 to 1.75; P=0.57).30 Major bleeding was associated with higher CHADS2 scores, but the relative risk was similar between the apixaban and aspirin arms.31 In a subset analysis from the AVERROES trial, apixaban was well tolerated and still efficacious in stroke risk reduction in a population with chronic kidney disease stage III, without a significant increase in major bleeding rates compared with aspirin.35
TSOAs in Venous Thromboembolic Disease
Background
Risk factors for venous thromboembolism (VTE) are well described and include older age, obesity, cancer, prior VTE, hereditary thrombophilia, hormonal therapy, chronic venous insufficiency, prolonged bed rest or immobility, and major surgery, especially total knee and hip arthroplasty (TKA/THA).36
VTE is a significant but preventable cause of death in hospitalized and postsurgical patients.37 Implementing evidence‐based guidelines into clinical practice for VTE prophylaxis can reduce the incidence of symptomatic VTE by up to 6‐fold.38 However, often patients at high risk for VTE are not treated with the appropriate prophylaxis. The ENDORSE (Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting) study reported guideline‐based prophylaxis was used in only 64.4% and 41.5% of at‐risk surgical and medical patients, respectively.39
The mainstay of VTE prophylaxis for patients undergoing major orthopedic surgery (ie, THA, TKA, and hip fracture surgery) has been extended low‐molecular‐weight heparin (LMWH). Multiple randomized trials found LMWH to be associated with a relative risk reduction of ≈50% compared with no prophylaxis in symptomatic DVT following THA, TKA, and hip fracture surgery, with minimal hemorrhagic events.40 The appropriate duration of therapy in the earlier trials was between 10 and 14 days but recent data have shown a possible benefit of anticoagulant therapy >30 days postoperatively, especially with THA.41 As an alternative to LMWH, other pharmacological treatments recommended in prevention of VTE in this patient population include fondaparinux, low‐dose unfractionated heparin, aspirin, or adjusted‐dose VKA to a goal INR of 2.0 to 3.0 with appropriate bridging treatment.40
VTE Prevention in THA and TKA Patients
At present, rivaroxaban, dabigatran, and apixaban have demonstrated efficacy in the prevention of VTE in orthopedic surgical patients (Table 4). Multiple randomized trials from the RECORD (Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism) study group have evaluated rivaroxaban's efficacy in prevention of VTE compared with LMWH.42–49 In a pooled analysis of these trials, rivaroxaban was associated with a relative risk reduction of >50% compared with enoxaparin (RR 0.41, 95% CI 0.20 to 0.83). However, there was a trend (though statistically insignificant) toward increased bleeding events (major bleeding and bleeding leading to reoperation) with rivaroxaban (combined RR 1.73, 95% CI 0.94 to 3.17).50 Of the 3 anticoagulants, rivaroxaban is at present the only one that is FDA approved for use in the United States for VTE prophylaxis in TKA and THA patients.
Table 4.
Trials for Prevention of VTE in Total Hip and Knee Replacement
| TSOA and Randomized Clinical Trial | Enoxaparin Treatment Dose | Days of TSOA Treatment | VTE Risk Difference, % | Bleeding Risk Difference, % |
|---|---|---|---|---|
| Dabigatran | ||||
| RE‐MOBILIZE51 | 30 mg twice daily | 12 to 15 | 5.8* | 0.8* |
| RE‐MODEL52 | 40 mg once daily | 6 to 10 | 1.3 | 0.2 |
| RE‐NOVATE53 | 40 mg once daily | 28 to 35 | 0.7 | 0.4 |
| RE‐NOVATE II54 | 40 mg once daily | 28 to 35 | 1.1 | 0.5 |
| Rivaroxaban | ||||
| RECORD 144 | 40 mg once daily | 31 to 39 | 2.6 | 0.7 |
| RECORD 245 | 40 mg once daily | 31 to 39 | 7.3 | 0.6 |
| RECORD 348 | 40 mg once daily | 10 to 14 | 9.1 | 0.6 |
| RECORD 449 | 30 mg twice daily | 10 to 14 | 3.2 | 0.7 |
| Apixaban | ||||
| ADVANCE‐155 | 30 mg twice daily | 10 to 14 | 0.2 | 1.5* |
| ADVANCE‐256 | 40 mg once daily | 10 to 14 | 9.3 | 1.2* |
| ADVANCE‐357 | 40 mg once daily | 35 | 2.5 | 0.2* |
TSOA indicates target‐specific oral anticoagulant; VTE, venous thromboembolism.
Significant risk reduction in favor of enoxaparin in prevention of VTE or death.
Reduction in major and clinically relevant nonmajor bleeding rates in favor of TSOA.
Although the 220‐mg dose of dabigatran is available for prophylactic use in Europe and Canada, it has not been approved by the FDA for use in orthopedic patients in the United States. This decision was based on results from the RE‐MODEL (Dabigatran Etexilate 150 or 220 mg Once Daily vs. Enoxaparin 40 mg Once Daily in Prevention of Venous Thromboembolism Post Total Knee Replacement), RE‐MOBILIZE (Dabigatran Etexilate 220 mg vs. Enoxaparin 30 mg Twice Daily in Prevention of Venous Thromboembolism Post Total Knee Replacement), and RE‐NOVATE (Dabigatran Etexilate Compared With Enoxaparin in Prevention of Venous Thromboembolism Following Total Hip Arthroplasty) I and II trials, all of which analyzed the difference in incidence of VTE and VTE‐related death after TKA or THA between dabigatran and enoxaparin. Of the 4 trials, the RE‐MOBILIZE investigators used the standard North American regimen of enoxaparin (30 mg SC twice daily given postoperatively) in comparison with dabigatran in TKA patients. Incidence of major VTE and VTE‐related death after TKA was found to be higher in both high‐ and low‐dose dabigatran arms compared with enoxaparin (3.0% and 3.4% versus 2.2%, respectively) with overall similar bleeding rates.51
The RE‐MODEL (TKA) and RE‐NOVATE I (THA) trials also analyzed both the high and low doses of dabigatran but compared them with the European dosing regimen of enoxaparin (40 mg daily started the night before surgery). Both the RE‐MODEL and RE‐NOVATE I trials showed that both doses of dabigatran were noninferior to enoxaparin without a significant increase in bleeding.52–53 A subsequent meta‐analysis of RE‐MOBILIZE, RE‐MODEL, and RE‐NOVATE I trial data reported the outcome event rates at 3.8%, 3.0%, and 3.3% for the dabigatran 150 mg, dabigatran 220 mg, and enoxaparin groups, respectively,58 resulting in the approval of the higher dose in Europe and Canada. The RE‐NOVATE II trial randomized THA patients to either extended prophylaxis with high‐dose dabigatran or 40 mg SC daily enoxaparin and found similar efficacy and safety results as the higher dose dabigatran arms of RE‐MODEL and RE‐NOVATE I.54
Apixaban's efficacy and safety in the prevention of VTE in TKA and THA patients has been tested in the Apixaban Versus Enoxaparin for Thromboprophylaxis After Hip or Knee Replacement (ADVANCE) trials. The ADVANCE‐I trial included the standard North American postoperative 30‐mg twice‐daily dosing of enoxaparin compared with apixaban in patients who underwent TKA. Apixaban did not meet the prespecified margin to claim noninferiority, but it did show significant reduction in major bleeding (2.9% versus 4.2%; P=0.03).55 In the subsequent ADVANCE‐2 trial, the preoperative 40‐mg daily dose of enoxaparin (European standard of care) was compared with apixaban in patients who underwent TKA.56 The ADVANCE‐3 trial analyzed the same dosing regimens as ADVANCE‐2 but studied the effects and safety in patients following THA.57 In a pooled analysis of the ADVANCE‐2 and ADVANCE‐3 trials, apixaban was found to be superior to enoxaparin in the reduction of major VTE incidence (risk difference of −0.8%, 95% CI −1.2 to −0.3; P=0.001 for superiority). Superiority was achieved by apixaban without an increase in major bleeding.59 Based on the results from the ADVANCE‐2 and ADVANCE‐3 trials, apixaban has been approved for use in VTE prophylaxis in TKA and THA in Europe and Canada.
In summarizing these trials, rivaroxaban, dabigatran, and apixaban have all demonstrated some efficacy in the prevention of major VTE following orthopedic surgery without a significant increase in bleeding. In a pooled analysis of 16 randomized clinical trials comparing the 3 novel anticoagulants with enoxaparin, the drugs were generally as effective and safe. In the meta‐analysis, rivaroxaban had the greatest risk reduction in VTE but the highest risk of clinically relevant bleeding. While dabigatran and apixaban had similar VTE risk reductions as enoxaparin, apixaban was the only drug of the 3 to demonstrate a decrease in bleeding events.60
VTE Prevention in Medically Ill Patients
Prospective data from the national DVT Free Registry illustrate that a vast number of acute perihospitalization–related VTE occurs in nonsurgical patients.61 Risk factors include age, obesity, prolonged immobility, chronic obstructive pulmonary disease, advanced congestive heart failure, stroke, paralysis, cancer, prior VTE, acute infection, central venous catheter use, and rheumatologic disease, among others. Prior guidelines support the use of pharmacological VTE prophylaxis with LMWH or low‐dose unfractionated subcutaneous heparin in those deemed moderate‐high risk.62
Several phase III randomized trials have analyzed the use of the novel anticoagulants in prophylaxis of VTE in medically ill patients. In the ADOPT (Apixaban Dosing to Optimize Protection from Thrombosis) trial, participants were randomized to receive either apixaban 2.5 mg twice daily for 30 days or enoxaparin 40 mg once daily for 6 to 14 days. The rates of VTE or VTE‐related mortality with apixaban versus enoxaparin were 2.7% and 3.06%, respectively (RR 0.87, 95% CI 0.62 to 1.23). In addition, at day 30, apixaban was associated with a significantly greater risk of bleeding compared with enoxaparin (RR 2.58, 95% CI 1.02 to 7.24).63 The MAGELLAN (Multicenter, Randomized, Parallel Group Efficacy and Safety Study for the Prevention of Venous Thromboembolism in Hospitalized Acutely Ill Medical Patients Comparing Rivaroxaban with Enoxaparin) trial with similar aims compared extended‐duration rivaroxaban with enoxaparin.64 Rivaroxaban was associated with a reduction in VTE events (RR 0.77, 95% CI 0.62 to 0.96; P=0.02), but it was associated with a significantly higher risk of bleeding (RR 2.46, 95% CI 1.9 to 3.3).65 Data for dabigatran use in this population are not yet available.
Oral Anticoagulants and Treatment of VTD
Before the emergence of new oral anticoagulants, the approved initial anticoagulant therapy for DVT and pulmonary embolism included intravenous unfractionated heparin, LMWH, or fondaparinux with a transition to warfarin or other VKA.62 Multiple trials have assessed the efficacy of the new anticoagulants in the acute treatment of VTE (Table 5). At present, rivaroxaban is the only drug that is approved for treatment in acute DVT and pulmonary embolism based on results from the EINSTEIN DVT (Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients With Acute Symptomatic Deep Vein Thrombosis) and EINSTEIN PE (Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients With Acute Symptomatic Deep Vein Thrombosis) randomized clinical trials. With the primary end point being recurrence of VTE at 3, 6, and 12 months, investigators from EINSTEIN DVT report event rates for rivaroxaban and VKA of 2.1% versus 3.0%, respectively (HR 0.68, 95% CI 0.44 to 1.04; P<0.001 for noninferiority). Similarly, in EINSTEIN PE, rivaroxaban also met noninferiority compared with VKA (HR 1.12, 95% CI 0.75 to 1.68; P=0.003 for noninferiority). Rivaroxaban was not associated with an increased risk for major bleeding in either trial. It is important to highlight the treatment doses of rivaroxaban for acute treatment of VTE: 15 mg twice daily for the initial 3 weeks followed by a 20‐mg once‐daily maintenance dose. The extension study of EINSTEIN DVT also reported noninferiority of rivaroxaban, but versus placebo, when assessed for efficacy and safety outcomes at 12‐month follow‐up.66–67
Table 5.
Risk of Recurrent Venous Thromboembolism With Target‐Specific Anticoagulants Versus Conventional Therapy
| Randomized Clinical Trial | Number of Events/Total | Risk Ratio (95% CI) | |
|---|---|---|---|
| TSOA | LMWH+VKA | ||
| Dabigatran | |||
| RECOVER I68 | 30/1274 | 27/1265 | 1.10 (0.66 to 1.84) |
| RECOVER II69 | 30/1279 | 28/1289 | 1.08 (0.65 to 1.8) |
| Rivaroxaban | |||
| EINSTEIN‐DVT66 | 36/1731 | 51/1718 | 0.70 (0.46 to 1.07) |
| EINSTEIN‐PE67 | 50/2419 | 44/2413 | 1.13 (0.76 to 1.69) |
| Apixaban | |||
| AMPLIFY70 | 59/2609 | 71/2635 | 0.84 (0.60 to 1.18) |
LMWH indicates low‐molecular‐weight heparin; TSOA, target‐specific oral anticoagulant; VKA, vitamin K antagonist.
Dabigatran, at a fixed dosage of 150 mg twice daily for acute VTE, was also found to be noninferior in the prevention of recurrent VTE compared with dose‐adjusted warfarin in the double‐blind RE‐COVER (Efficacy and Safety of Dabigatran Compared to Warfarin for 6 Month Treatment of Acute Symptomatic Venous Thromboembolism) trial. The study end point was assessed at 6 months. Event rates for dabigatran and warfarin were 2.4% and 2.1%, respectively (HR 1.10, 95% CI, 0.65 to 1.84; P<0.001 for noninferiority). Although the risk of dyspepsia and adverse events leading to study drug discontinuation was increased with dabigatran, the risk of bleeding was similar between the 2 arms.68 In the duplicate RE‐COVER II trial, results were similar.69 Individuals in this study were randomized to dabigatran after receiving at least 5 days of a parenteral anticoagulant. The RE‐MEDY (a Phase III, Randomised, Multicenter, Double‐blind, Parallel‐group, Active Controlled Study to Evaluate the Efficacy and Safety of Oral Dabigatran Etexilate [150 mg Bid] Compared to Warfarin [INR 2.0–3.0] for the Secondary Prevention of Venous Thromboembolism) investigators looked at extended anticoagulation for unprovoked VTE following appropriate initial treatment. Dabigatran was noninferior to warfarin in the reduction of VTE and VTE‐related deaths (HR 1.05, 95% CI 0.65 to 1.70; P<0.0001 for noninferiority).71
The AMPLIFY‐EXT (a Safety and Efficacy Trial Evaluating the Use of Apixaban for the Extended Treatment of Deep Vein Thrombosis and Pulmonary Embolism) trial compared apixaban with placebo in patients who had already completed at least 6 months of standard anticoagulant therapy for VTE. Rate of recurrent VTE or death at 12‐month follow‐up was significantly reduced in both the 2.5‐ and 5‐mg doses of apixaban compared with placebo (RR 0.33 and 0.36, respectively; P<0.001).72 The AMPLIFY trial, which compares apixaban with the conventional acute VTE treatment with LMWH or heparin and VKA, has just been completed. Apixaban at a dosage of 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months, compared with conventional therapy, was found to be noninferior for the treatment of acute VTE (RR 0.84, 95% CI 0.60 to 1.18; P<0.001 for noninferiority). It was also associated with significantly less bleeding (RR 0.44, 95% CI 0.36 to 0.55; P<0.001).70
TSOA Use in Acute Coronary Syndromes
Acute coronary syndromes (ACS) (ie, unstable angina, non–ST‐elevation myocardial infarction, and ST‐elevation myocardial infarction) are often life‐threatening events. Importantly, patients remain at significant risk for subsequent events such as recurrent myocardial infarction or ischemia, stroke, or death during the subsequent months. The current standard of care includes extended therapy often with dual antiplatelet agents. The role of the new anticoagulants in the management of secondary prevention following ACS is less clear.
The ATLAS ACS‐TIMI 46 (a Randomized, Double‐Blind, Placebo‐Controlled, Multicenter, Dose‐Escalation and Dose‐Confirmation Study to Evaluate the Safety and Efficacy of Rivaroxaban in Combination With Aspirin Alone or With Aspirin and a Thienopyridine in Subjects With Acute Coronary Syndromes) was a phase II dose‐escalation study assessing the safety and efficacy of rivaroxaban (5 to 20 mg daily dosing) versus placebo in patients already on mono‐antiplatement or dual‐antiplatelet therapy for ACS. The primary end point was the composite of death, myocardial infarction, stroke, or recurrent ischemia requiring revascularization. The study found that rivaroxaban was not associated with a reduction in the primary efficacy end point versus placebo over the 6‐month follow‐up period (HR 0.79, 95% CI 0.6 to 1.05; P=0.10); however, it did reduce the main secondary end point (death, myocardial infarction, or stroke) compared with placebo (HR 0.69, 95% CI 0.50 to 0.96; P=0.027). The study also found a dose‐dependent increased risk of bleeding.73 The subsequent ATLAS‐ACS 2 TIMI 51 trial randomized patients with a recent ACS to receive twice‐daily 2.5 mg or 5 mg of rivaroxaban versus placebo. The primary end point, which was a reduction in the composite of death from cardiovascular disease, myocardial infarction, or stroke, was achieved with rivaroxaban (HR 0.84, 95% CI 0.74 to 0.96). Compared with placebo, rivaroxaban was associated with an increased risk of nonfatal major bleeding (2.1% versus 0.6%; P<0.001) without a significant increase in fatal bleeding (0.3% versus 0.2%; P=0.66).74 Although rivaroxaban has been approved in Europe (2.5 mg twice daily) for secondary prevention in biomarker‐confirmed ACS, the FDA has not granted approval for this indication due to concerns over incomplete follow‐up and missing data (FDA briefing document, May 23, 2012, Cardiovascular and Renal Drugs Advisory Committee [CRDAC]).75
Apixaban was studied in the APPRAISE (Apixaban for Prevention of Acute Ischemic Events)‐I and APPRAISE‐II trials. Results from the initial phase II trial demonstrated a trend (although nonsignificant) toward a dose‐dependent reduction in recurrent ischemic events with apixaban compared with placebo (HR 0.61, 95% CI 0.35 to1.04; P=0.07 for apixaban 10 mg total daily dose). There was also a dose‐dependent increased risk of bleeding.76 Given the results from the phase II trial, the phase III APPRAISE‐II assessed lower‐dose apixaban (5 mg twice daily) versus placebo and was terminated early due to a significant increase in major bleeding events without a significant reduction in recurrent ischemic events.77 Of note, the 5‐mg twice‐daily dose is the dosage approved for stroke prevention in AF.
The phase II RE‐DEEM (Randomized Dabigatran Etexilate Dose Finding Study in Patients With Acute Coronary Syndromes Post Index Event With Additional Risk Factors for Cardiovascular Complications Also Receiving Aspirin and Clopidogrel: Multicenter, Prospective, Placebo Controlled, Cohort Dose Escalation Study) trial, which randomized post‐ACS patients to placebo versus 4 incremental twice‐daily doses of dabigatran (50, 75, 110, and 150 mg), showed a dose‐dependent increased risk of bleeding with dabigatran. Although dabigatran lowered coagulation activity (measured with D‐dimer levels), there was no significant reduction in death, myocardial infarction, or stroke.78 With increased bleeding with new agents while on antiplatelet therapy, there currently is no FDA‐approved use for the TSOAs in ACS until further evidence becomes available.
Potential Challenges in the Translation of Trial Results Into Clinical Practice
Results from the randomized clinical trials have demonstrated some promising advantages of novel anticoagulants over standard VKAs, thus leading to FDA approval and an increase in choice for the practicing physician (Table 6). Despite these results, however, TSOA use in certain patient subgroups not represented in trials remains unclear until further evidence emerges. Physicians should consider carefully each patient's concomitant medications and comorbid disorders, while also considering baseline characteristics and key exclusionary criteria of the trial populations, before TSOA transition79 (Table 7). Further data are needed especially for elderly patients, those with moderate‐severe kidney disease (both chronic and acute), those on antiplatelet agents, and those undergoing emergent surgery or endovascular procedures. In addition, the optimal management of patients who have either very low TTR due to nonadherence, or very high TTR due to well‐controlled warfarin, warrants further study.79
Table 6.
Target‐Specific Anticoagulants Approved for Use
| Indication | Dabigatran | Rivaroxaban | Apixaban |
|---|---|---|---|
| Stroke prevention in AF | 150 mg twice daily | 20 mg once daily | 5 mg twice daily |
| Acute VTE treatment | Not approved | 20 mg once daily | Not approved |
| VTE prevention in TKA or THA | 220 mg once daily* | 10 mg once daily | 2.5 mg twice daily* |
AF indicates atrial fibrillation; THA, total hip arthroplasty; TKA, total knee arthroplasty; VTE, venous thromboembolism.
Only approved in Europe and Canada for this indication.
Table 7.
Stroke Prevention in Atrial Fibrillation Trial Populations
| Trial Characteristics | Dabigatran (RE‐LY)23 | Rivaroxaban (ROCKET AF)24 | Apixaban (ARISTOTLE)25 |
|---|---|---|---|
| Participants, n | 18 113 | 14 264 | 18 201 |
| Median age | 71 (mean) | 73 | 70 |
| Mean CHADS2 | 2.1 | 3.5 | 2.1 |
| Mean TTR | 64% | 55% | 62% |
| Median CrCl | 68 | 67 | N/A* |
CrCl indicates creatinine clearance; CHADS2, congestive heart failure, hypertension, age, diabetes, stroke; TTR, time in therapeutic range.
Data on renal function presented as 83% with CrCl ≥50 mL/min.
Currently, rivaroxaban is the only available TSOA approved for use in VTE disease in the United States. However, dabigatran, rivaroxaban, and apixaban are all viable options in managing patients with AF, as their efficacy and safety profiles met FDA requirements for approval. Given the fact that randomized trials rarely perform new drug–drug comparisons, it becomes crucial before transitioning to TSOAs that the clinician considers the above limitations and applies such to their patients' disease profile and concomitant medications. For example, as mean TTR never exceeded 65% in the trial populations, it becomes difficult to conclude comparative efficacy of the TSOAs in patients who are on a very stable warfarin regimen.
In addition, careful monitoring and dose adjustments should be considered in those patient populations that were less likely to be included in the phase III trial populations, that is, subjects 65 years or older and those with a creatinine clearance <30 mL/min resulting from chronic or acute kidney disease. TSOAs are contraindicated in patients with end‐stage chronic kidney disease or those on dialysis. Dose reduction is required for those with severe renal insufficiency, that is, creatinine clearance 15 to 30 mL/min on dabigatran or creatinine clearance 15 to 50 mL/min on rivaroxaban. The presence of ≥2 of the following mandate the reduced dose of 2.5 mg twice daily for apixaban: aged ≥80 years, weight <60 kg, or creatinine level of ≥1.5 mg/dL. Regular monitoring of kidney function at least once per year is recommended for patients on these drugs. More frequent monitoring is prudent for certain populations at risk for a decline in kidney function.20–22
Although concern has been raised about excessive thrombotic risk with cessation of these agents, there is no evidence to support a drug‐specific rebound phenomenon. As with warfarin, periods off of drug should be minimized to the extent that is safely possible given the underlying hypercoagulable states present with AF and VTE.
Monitoring and Reversibility With Novel Anticoagulants
Whereas VKAs are highly variable in pharmacokinetics necessitating frequent monitoring, the TSOAs have a more predictable pharmacokinetic profile and routine monitoring is not needed. However, there exist potential clinical scenarios for which monitoring and reversal of the anticoagulant effect would be highly desirable, such as with intracranial hemorrhage, life‐threatening gastrointestinal bleeding, urgent surgery, or trauma. These clinical situations may be especially hazardous in those patients with a baseline increased risk of bleeding, such as patients 65 years or older, and those with kidney failure or on antiplatelet therapy.
Although beneficial to have a test measuring the level of anticoagulation in these urgent situations and high‐risk patients, there is a lack of readily available accurate laboratory testing in most clinical settings. Dabigatran prolongs not only the INR but also the activated partial thromboplastin time, thrombin clotting time, and, to some extent, prothrombin time. The thrombin clotting time assay is the most accurate test in detecting the presence of dabigatran and increases with increasing drug concentration. This test, however, is not routinely available in most institution laboratories.19,80–81
The factor Xa inhibitors rivaroxaban and apixaban can prolong the INR and prothrombin time, but the degree of prothrombin time prolongation does not reliably predict drug concentrations. There is a potential for drug monitoring with anti–factor Xa assays, but these assays may not be amply available to ensure a fast turnaround, which is critical in these emergent situations.82
Currently, there are no available drug‐specific antidotes for the new oral anticoagulants, but several are under development. One such antidote is the monoclonal antibody directed against dabigatran, which has shown positive results in inhibiting its anticoagulant activity in both human and animal studies. There also exist potential factor Xa inhibitors (pd–factor Xa and r–factor Xa) that may be used to reverse rivaroxaban and apixaban. Studies showed these factors had the ability to neutralize anticoagulation test abnormalities associated with the Xa inhibitors. Four‐ and 3‐factor prothrombin complex concentrates are available for use, but additional study is ongoing to fully understand their efficacy in TSOA‐induced life‐threatening bleeds.83
Conclusions
For several decades, warfarin has been the standard of care in oral anticoagulation therapy for stroke prevention in AF and management of VTE. Despite a vast understanding on food and drug interactions and available algorithms for dose adjustments, adequate TTR is still difficult for many patients to achieve. Recent pharmacological advancements have enabled several TSOAs to be available for use in clinical practice. Based on recent data from randomized clinical trials involving AF and VTE, these drugs have demonstrated significant efficacy compared with warfarin and are associated with a significant reduction in intracranial hemorrhage. In addition, these agents are advantageous to both the prescribing clinician and the patient, as they are available in fixed doses, and no drug‐level monitoring is required. As further data emerge on safety and efficacy of these agents, clinicians should be aware of the present limitations in clinical trials as they apply to their patients, paying special attention to those populations that may exhibit higher risk. In the meantime, there are emerging data on target‐specific reversal agents and use of PCCs that could prove to be useful in life‐threatening situations.
Sources of Funding
This work was supported by the National Institutes of Health (NIH)–sponsored Boston University Medical Center Leadership Program in Vascular Medicine K12 HL083781 grant support to Drs Cove and Hylek. Dr Hylek also receives funding from the NIH: 1RO1NS070307.
Disclosures
Dr Cove has no conflicts of interest to disclose. Dr Hylek has served as an advisor to Bayer, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Johnson & Johnson, Merck, and Pfizer and has received research funding from Bristol‐Myers Squibb and Ortho McNeil.
References
- 1.Gattellari M, Goumas C, Aitken R, Worthington JM. Outcomes for patients with ischaemic stroke and atrial fibrillation: the PRISM study (A Program of Research Informing Stroke Management). Cerebrovasc Dis. 2011; 32:370-382 [DOI] [PubMed] [Google Scholar]
- 2.DeWilde S, Carey IM, Emmas C, Richards N, Cook DG. Trends in the prevalence of diagnosed atrial fibrillation, its treatment with anticoagulation and predictors of such treatment in UK primary care. Heart. 2006; 92:1064-1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001; 285:2370-2375 [DOI] [PubMed] [Google Scholar]
- 4.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001; 285:2864-2870 [DOI] [PubMed] [Google Scholar]
- 5.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010; 137:263-272 [DOI] [PubMed] [Google Scholar]
- 6.Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010; 41:2731-2738 [DOI] [PubMed] [Google Scholar]
- 7.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halparin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011; 57:e101-e198 [DOI] [PubMed] [Google Scholar]
- 8.Olesen JB, Torp‐Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2‐VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0‐1: a nationwide cohort study. Thromb Haemost. 2012; 107:1172-1179 [DOI] [PubMed] [Google Scholar]
- 9.Potpara TS, Polovina MM, Licina MM, Marinkovic JM, Prostran MS, Lip GY. Reliable identification of “truly low” thromboembolic risk in patients initially diagnosed with “lone” atrial fibrillation: the Belgrade atrial fibrillation study. Circ Arrhythm Electrophysiol. 2012; 5:319-326 [DOI] [PubMed] [Google Scholar]
- 10.Lane DA, Lip GY. Use of the CHA2DS2‐VASc and HAS‐BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012; 126:860-865 [DOI] [PubMed] [Google Scholar]
- 11.Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007; 146:857-867 [DOI] [PubMed] [Google Scholar]
- 12.Saxena R, Koudstaal P. Anticoagulants versus antiplatelet therapy for preventing stroke in patients with nonrheumatic atrial fibrillation and a history of stroke or transient ischemic attack. Cochrane Database Syst Rev. 2004; 4:CD000187. [DOI] [PubMed] [Google Scholar]
- 13.Alberts MJ, Eikelboom JW, Hankey GJ. Antithrombotic therapy for stroke prevention in non‐valvular atrial fibrillation. Lancet Neurol. 2012; 11:1066-1081 [DOI] [PubMed] [Google Scholar]
- 14.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010; 138:1093-1100 [DOI] [PubMed] [Google Scholar]
- 15.White HD, Gruber M, Feyzi J, Kaatz S, Tse HF, Husted S, Albers GW. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med. 2007; 167:239-245 [DOI] [PubMed] [Google Scholar]
- 16.Van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ. Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest. 2006; 129:1155-1166 [DOI] [PubMed] [Google Scholar]
- 17.Gage BF, Boechler M, Doggette AL, Fortune G, Flaker GC, Rich MW, Radford MJ. Adverse outcomes and predictors of underuse of antithrombotic therapy in medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000; 31:822-827 [DOI] [PubMed] [Google Scholar]
- 18.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2008; 133:160S-198S [DOI] [PubMed] [Google Scholar]
- 19.Weitz JI. New oral anticoagulants: a view from the laboratory. Am J Hematol. 2012; 87:S133-S136 [DOI] [PubMed] [Google Scholar]
- 20.Package Insert Pradaxa (Dabigatran Etexilate). 2010Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc [Google Scholar]
- 21.Package Insert Xaralto (Rivaroxaban). 2012Titusville, NJ: Janssen Pharmaceuticals, Inc [Google Scholar]
- 22.Package Insert Eliquis (Apixaban). 2012Princeton, NJ: Bristol‐Myers Squibb, Inc [Google Scholar]
- 23.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin LRE‐LY Steering Committee and Investigators Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139-1151 [DOI] [PubMed] [Google Scholar]
- 24.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RMROCKET AF Steering Committee for the ROCKET AF Investigators Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365:883-891 [DOI] [PubMed] [Google Scholar]
- 25.Granger CB, Alexander JH, McMurray JJV, Lopes R, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit C, Diaz R, Easton D, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopz‐Sendon JL, Pais P, Parkhomenko A, Verheugt FWA, Zhu J, Wallentin LARISTOTLE Committees and Investigators Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365:981-992 [DOI] [PubMed] [Google Scholar]
- 26.Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, Yang S, Alings M, Kaatz S, Hohnloser SH, Diener HC, Franzosi MG, Huber K, Reilly P, Varrone J, Yusuf S. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long‐term anticoagulant therapy (RE‐LY) trial. Circulation. 2011; 123:2363-2372 [DOI] [PubMed] [Google Scholar]
- 27.Beasley BN, Unger EF, Temple R. Anticoagulant options: why the FDA approved a higher but not a lower dose of dabigatran. N Engl J Med. 2011; 364:1788-1790 [DOI] [PubMed] [Google Scholar]
- 28.Hart RG, Diener HC, Yang S, Connolly SJ, Wallentin L, Reilly PA, Ezekowitz MD, Yusuf S. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE‐LY trial. Stroke. 2012; 43:1511-1517 [DOI] [PubMed] [Google Scholar]
- 29.Van de Werf F, Brueckmann M, Connolly SJ, Friedman J, Granger CB, Härtter S, Harper R, Kappetein AP, Lehr T, Mack MJ, Noack H, Eikelboom JW. A comparison of dabigatran etexilate with warfarin in patients with mechanical heart valves: the randomized, phase II study to evaluate the safety and pharmacokinetics of oral dabigatran etexilate in patients after heart valve replacement (RE‐ALIGN). Am Heart J. 2012; 163:931.e1-937.e1 [DOI] [PubMed] [Google Scholar]
- 30.Hankey GJ, Patel MR, Stevens SR, Becker RC, Breithardt G, Carolei A, Diener HC, Donnan GA, Halperin JL, Mahaffey KW, Mas JL, Massaro A, Norrving B, Nessel CC, Paolini JF, Roine RO, Singer DE, Wong L, Califf RM, Fox KA, Hacke WROCKET AF Steering Committee Investigators Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol. 2012; 11:315-322 [DOI] [PubMed] [Google Scholar]
- 31.Connolly SJ, Eikelboom J, Joyner C, Diener H, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Iansky P, Commerford P, Tan RS, Sim K, Van Mieghem W, Lip G, Hyung J, Kim JH, Lanas‐Zanetti F, Gonzalez‐Hermosillo A, Dans AL, Munawar M, O'Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf SAVERROES Steering Committee and Investigators Apixaban in patients with atrial fibrillation. N Engl J Med. 2011; 364:806-817 [DOI] [PubMed] [Google Scholar]
- 32.Flaker GC, Eikelboom J, Shstakovska O, Connoly SJ, Kaatz S, Budaj A, Husted S, Yusuf S, Lip G, Hart RG. Bleeding during treatment with aspirin versus apixaban in patients with atrial fibrillation unsuitable for warfarin: the apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment (AVERROES) trial. Stroke. 2012; 43:3291-3297 [DOI] [PubMed] [Google Scholar]
- 33.Lopes RD, Al‐Khatib SM, Wallentin L, Yang H, Ansell J, Bahit MC, De Caterina R, Dorian P, Easton JD, Erol C, Ezekowitz JA, Gersh BJ, Granger CB, Hohnloser SH, Horowitz J, Hylek EM, McMurray JJ, Mohan P, Vinereanu D, Alexander JH. Efficacy and safety of apixaban compared with warfarin according to patient risk of stroke and of bleeding in atrial fibrillation: a secondary analysis of a randomised controlled trial. Lancet. 2012; 380:1749-1758 [DOI] [PubMed] [Google Scholar]
- 34.Easton JD, Lopes RD, Bahit MC, Wojdyla DM, Granger CB, Wallentin L, Alings M, Goto S, Lewis BS, Rosenqvist M, Hanna M, Mohan P, Alexander JH, Diener HC, Diener HC. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol. 2012; 11:503-511 [DOI] [PubMed] [Google Scholar]
- 35.Eikelboom JW, Connolly SJ, Gao P, Paolasso E, De Caterina R, Husted S, O'Donnell M, Yusuf S, Hart RG. Stroke risk and efficacy of apixaban in atrial fibrillation patients with moderate chronic kidney disease. J Stroke Cerebrovasc Dis. 2012; 21:429-435 [DOI] [PubMed] [Google Scholar]
- 36.Hirsh J, Guyatt G, Albers GW, Harrington R, Schunemann HJ. Antithrombotic and thrombolytic therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2008; 133:110S-112S [DOI] [PubMed] [Google Scholar]
- 37.Alikhan R, Peters F, Wilmott R, Cohen AT. Fatal pulmonary embolism in hospitalised patients: a necropsy review. J Clin Pathol. 2004; 57:1254-1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayer RS, Streiff MB, Hobson DB, Halpert DE, Berenholtz SM. Evidence‐based venous thromboembolism prophylaxis is associated with a six‐fold decrease in numbers of symptomatic venous thromboembolisms in rehabilitation inpatients. PM&R. 2011; 3:1111-1115 [DOI] [PubMed] [Google Scholar]
- 39.Cohen AT, Tapson VF, Bergmann J, Goldhaber SZ, Kakkar AK, Deslandes B, Huang W, Zayaruzny M, Emery L, Anderson FA. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2002; 371:387-394 [DOI] [PubMed] [Google Scholar]
- 40.Falck‐Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012; 141:e278S-e325S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hull RD, Pineo GF, Francis C, Bergqvist D, Fellenius C, Soderberg K, Holmqvist A, Mant M, Dear R, Baylis B, Mah A, Brant R. Low‐molecular‐weight heparin prophylaxis using dalteparin extended out‐of‐hospital vs in‐hospital warfarin/out‐of‐hospital placebo in hip arthroplasty patients: a double‐blind, randomized comparison. Arch Intern Med. 2000; 160:2208-2215 [DOI] [PubMed] [Google Scholar]
- 42.Eriksson BI, Kakkar AK, Turpie AG, Gent M, Bandel TJ, Homering M, Misselwitz F, Lassen MR. Oral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacement. J Bone Joint Surg Br. 2009; 91:636-644 [DOI] [PubMed] [Google Scholar]
- 43.Eriksson BI, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, Muehlhofer E, Dierig C, Misselwitz F, Kälebo PODIXa‐HIP Study Investigators A once‐daily, oral, direct factor Xa inhibitor, rivaroxaban (BAY 59‐7939), for thromboprophylaxis after total hip replacement. Circulation. 2006; 114:2374-2381 [DOI] [PubMed] [Google Scholar]
- 44.Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kaakar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts WRECORD1 Study Group Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008; 358:2765-2775 [DOI] [PubMed] [Google Scholar]
- 45.Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, Soglian AG, Pap AF, Misselwitz F, Haas SRECORD2 Investigators Extended duration rivaroxaban versus short‐term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double‐blind, randomized controlled trial. Lancet. 2008; 373:31-39 [DOI] [PubMed] [Google Scholar]
- 46.Eriksson BI, Borris L, Dahl OE, Haas S, Huisman MV, Kakka AK, Misselwitz F, Kälebo PODIXa‐HIP Study Investigators Oral, direct factor Xa inhibition with BAY 59‐7939 for the prevention of venous thromboembolism after total hip replacement. J Thromb Haemost. 2006; 4:121-128 [DOI] [PubMed] [Google Scholar]
- 47.Turpie AG, Fisher WD, Bauer KA, Kwong LM, Irwin MW, Kälebo P, Misselwitz F, Gent MODIXa‐HIP Study Investigators BAY 59‐7939: an oral, direct factor Xa inhibitor for the prevention of venous thromboembolism in patients after total knee replacement. A phase II dose‐ranging study. J Thromb Haemost. 2005; 3:2479-2486 [DOI] [PubMed] [Google Scholar]
- 48.Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, Misselwitz F, Turpie AGRECORD3 Investigators Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008; 358:2776-2786 [DOI] [PubMed] [Google Scholar]
- 49.Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, Cushner FD, Lotke PA, Berkowitz SD, Bandel TJ, Benson A, Misselwitz F, Fisher WDRECORD4 Investigators Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009; 373:1673-1680 [DOI] [PubMed] [Google Scholar]
- 50.Dahl OE, Quinlan DJ, Bergqvist D, Eikelboom JW. A critical appraisal of bleeding events reported in venous thromboembolism prevention trials of patients undergoing hip and knee arthroplasty. J Thromb Haemost. 2010; 8:1966-1975 [DOI] [PubMed] [Google Scholar]
- 51.Ginsberg JS, Davidson BL, Comp P, Francis CW, Friedman RJ, Huo MH, Lieberman JR, Muntz JE, Raskob GE, Clements ML, Hantal S, Schnee JM, Caprini JA. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009; 24:1-9 [DOI] [PubMed] [Google Scholar]
- 52.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Kälebo P, Christiansen AV, Hantel S, Hettiarachchi R, Schnee J, Büller HRRE‐MODEL Study Group Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE‐MODEL randomized trial. J Thromb Haemost. 2007; 5:2178-2185 [DOI] [PubMed] [Google Scholar]
- 53.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Prins MH, Hettiarachchi R, Hantel S, Schnee J, Büller HRRE‐NOVATE Study Group Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double‐blind, non‐inferiority trial. Lancet. 2007; 370:949-956 [DOI] [PubMed] [Google Scholar]
- 54.Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K, Schnee JM, Friedman RJRE‐NOVATE Study Group Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE‐NOVATE II): a randomised, double‐blind, non‐inferiority trial. Thromb Haemost. 2011; 105:721-729 [DOI] [PubMed] [Google Scholar]
- 55.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009; 361:594-604 [DOI] [PubMed] [Google Scholar]
- 56.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE‐2): a randomised double‐blind trial. Lancet. 2006; 375:807-815 [DOI] [PubMed] [Google Scholar]
- 57.Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LMADVANCE‐3 Investigators Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010; 363:2487-2498 [DOI] [PubMed] [Google Scholar]
- 58.Friedman RJ, Dahl OE, Rosencher N, Caprini JA, Kurth AA, Francis CW, Clemens A, Hantel S, Schnee JM, Eriksson BIRE‐MOBILIZE, RE‐MODEL, and RE‐NOVATE Steering Committees Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: a pooled analysis of three trials. Thromb Res. 2010; 126:175-182 [DOI] [PubMed] [Google Scholar]
- 59.Raskob GE, Gallus AS, Pineo GF, Chen D, Ramirez LM, Wright RT, Lassen MR. Apixaban versus enoxaparin for thromboprophylaxis after hip or knee replacement: pooled analysis of major venous thromboembolism and bleeding in 8464 patients from the ADVANCE‐2 and ADVANCE‐3 trials. J Bone Joint Surg Br. 2012; 94B:257-264 [DOI] [PubMed] [Google Scholar]
- 60.Gómez‐Outes A, Terleira‐Fernández AI, Suárez‐Gea ML, Vargas‐Castrillón E. Dabigatran, rivaroxaban, or apixaban versus enoxaparin for thromboprophylaxis after total hip or knee replacement: systematic review, meta‐analysis, and indirect treatment comparisons. BMJ. 2012; 344:e3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goldhaber SZ, Tapson VF. A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis. Am J Cardiol. 2004; 93:259-262 [DOI] [PubMed] [Google Scholar]
- 62.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012; 141:e419S-e494S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldhaber SZ, Leizorovicz A, Kakkar AK, Haas SK, Merli G, Knabb RM, Weitz JIADOPT Trial Investigators Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011; 365:2167-2177 [DOI] [PubMed] [Google Scholar]
- 64.Cohen AT, Spiro TE, Büller HR, Haskell L, Hu D, Hull R, Mebazaa A, Merli G, Schellong S, Spyropoulos A, Tapson V. Extended‐duration rivaroxaban thromboprophylaxis in acutely ill medical patients: MAGELLAN study protocol. J Thromb Thrombolysis. 2011; 31:407-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen AT, Spiro TE, Büller HR, Haskell L, Hu D, Hull R, Mebazaa A, Merli G, Schellong S, Spyropoulos AC, Tapson VMAGELLAN Investigators Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013; 368:513-523 [DOI] [PubMed] [Google Scholar]
- 66.Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong SEINSTEIN Investigators Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010; 363:2499-2510 [DOI] [PubMed] [Google Scholar]
- 67.Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, Chlumsky J, Verhamme P, Wells P, Agnelli G, Cohen A, Berkowitz SD, Bounameaux H, Davidson BL, Misselwitz F, Gallus AS, Raskob GE, Schellong S, Segers AEINSTEIN‐PE Investigators Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012; 366:1287-1297 [DOI] [PubMed] [Google Scholar]
- 68.Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, Baanstra D, Schnee J, Goldhaber SZRE‐COVER Study Group Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009; 361:2342-2352 [DOI] [PubMed] [Google Scholar]
- 69.Schulman S, Kakkar AK, Schellong SM, Goldhaber SZ, Eriksson H, Mismetti P, Christiansen AV, Schnee J, Kearson C. A randomized trial of dabigatran versus warfarin in the treatment of acute venous thromboembolism (RE‐COVER II). American Society of Hematology 2011 Annual Meeting, December 12, 2011, San Diego, CA; 2011. Abstract 205. [Google Scholar]
- 70.Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Masiukiewicz U, Pak R, Thompson J, Raskob GE, Weitz JIAMPLIFY Investigators Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013; 369:799-808 [DOI] [PubMed] [Google Scholar]
- 71.Schulman S, Kearson C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, Kvamme AM, Friedman J, Mismetti P, Goldhaber SZRE‐MEDY Trial Investigators, RE‐SONATE Trial Investigators Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013; 368:709-718 [DOI] [PubMed] [Google Scholar]
- 72.Agnelli G, Büller HR, Cohen A, Curto M, Gallus AS, Johnson M, Porcari A, Raskob GE, Weitz JIAMPLIFY‐EXT Investigators Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013; 368:699-708 [DOI] [PubMed] [Google Scholar]
- 73.Mega JL, Braunwald E, Mohanavelu S, Burton P, Poulter R, Misselwitz F, Hricak V, Barnathan ES, Bordes P, Witkowski A, Markov V, Oppenheimer L, Gibson CM. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS‐TIMI 46): a randomised, double‐blind, phase II trial. Lancet. 2004; 374:29-38 [DOI] [PubMed] [Google Scholar]
- 74.Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook‐Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CMATLAS ACS 2–TIMI 51 Investigators Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012; 366:9-19 [DOI] [PubMed] [Google Scholar]
- 75.FDA FDA Advisory Committee Briefing Document: Rivaroxaban; 2012. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM304755.pdf Accessed February 1, 2012.
- 76.Alexander JH, Becker RC, Bhatt DL, Cools F, Crea F, Dellborg M, Fox KA, Goodman SG, Harrington RA, Huber K, Husted S, Lewis BS, Lopez‐Sendon J, Mohan P, Montalescot G, Ruda M, Ruzyllo W, Verheugt F, Wallentin LAPPRAISE Steering Committee and Investigators Apixaban, an oral, direct, selective factor Xa inhibitor, in combination with antiplatelet therapy after acute coronary syndrome: results of the apixaban for prevention of acute ischemic and safety events (APPRAISE) trial. Circulation. 2009; 119:2877-2885 [DOI] [PubMed] [Google Scholar]
- 77.Alexander JH, Lopes RD, James S, Kilaru R, He Y, Mohan P, Bhatt DL, Goodman S, Verheugt FW, Flather M, Huber K, Liaw D, Husted SE, Lopez‐Sendon J, De Caterina R, Jansky P, Darius H, Vinereanu D, Cornel JH, Cools F, Atar D, Leiva‐Pons JL, Keltai M, Ogawa H, Pais P, Parkhomenko A, Ruzyllo W, Diaz R, White H, Ruda M, Geraldes M, Lawrence J, Harrington RA, Wallentin LAPPRAISE‐2 Investigators Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011; 365:699-708 [DOI] [PubMed] [Google Scholar]
- 78.Oldgren J, Budaj A, Granger CB, Khder Y, Roberts J, Siegbahn A, Tijssen JG, Van de Werf F, Wallentin LRE‐DEEM Investigators Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double‐blind, phase II trial. Eur Heart J. 2011; 32:2781-2789 [DOI] [PubMed] [Google Scholar]
- 79.Connolly G, Spyropoulos AC. Practical issues, limitations, and periprocedural management of the NOAC's. J Thromb Thrombolysis. 2013; 36:212-222 [DOI] [PubMed] [Google Scholar]
- 80.Van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M, Clemens A. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010; 103:1116-1127 [DOI] [PubMed] [Google Scholar]
- 81.Helin TA, Pakkanen A, Lassila R, Joutsi‐Korhonen L. Laboratory assessment of novel anticoagulants: method suitability and variability between coagulation laboratories. Clin Chem. 2013; 59:807-814 [DOI] [PubMed] [Google Scholar]
- 82.Samama MM, Contant G, Spiro TE, Perzborn E, Guinet C, Gourmelin Y, Le Flem L, Rohde G, Martinoli JL. Evaluation of the anti‐factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost. 2012; 107:379-387 [DOI] [PubMed] [Google Scholar]
- 83.Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J. 2013; 34:489-498b [DOI] [PubMed] [Google Scholar]


