Abstract
Background
Oxidative stress is implicated in the etiology of coronary heart disease (CHD). New measures to capture oxidative stress are warranted. Fluorescent oxidation products (FlOPs) can be measured in plasma and have been shown to reflect levels of oxidative stress and to predict risk of CHD in men over 6 years of follow‐up. The objective of this study is to determine whether measures of FlOPs are associated with risk of CHD in women over an extended follow‐up period.
Methods and Results
We measured FlOP by spectrofluorometer in a nested case–control study within the Nurses' Health Study, with baseline blood collection in 1990 and follow‐up of 397 incident CHD cases through 2004 matched 1:2 with controls. Level of FlOPs was independently associated with CHD. The relative risk across extreme quintiles was 1.64 (95% confidence interval [CI], 1.06 to 2.53) when adjusted for lifestyle factors, lipids and C‐reactive protein (P trend across quintiles=0.01). A slightly stronger association was observed when analyses were restricted to women fasting >8 hours at blood draw (RR, 1.91; 95% CI, 1.16 to 3.15). In exploratory time to event analyses, high levels of FlOPs measured ≥5 years before the CHD event, but not closer to the CHD event, were associated with the risk of CHD.
Conclusions
Higher levels of FlOPs were associated with the risk of CHD in women. The association appeared strongest for long‐term prediction of CHD events.
Keywords: follow‐up studies, myocardial infarction, oxidative stress, risk factors, women
Introduction
Accumulation of cholesterol and chronic low‐grade inflammation in the arterial wall are the hallmarks of atherosclerotic lesions. Reactive oxygen species released from macrophages during the inflammatory process can cause damage to cell structures and membranes, thereby affecting lipids, protein, and DNA. Elevated levels of lipid oxidation products have been associated with extensive coronary artery calcification.1 In particular, F2‐isoprostanes, which reflect the reaction of free radicals with arachidonic acid, have been directly associated with coronary heart disease (CHD) in several,2–3 although not all,4 studies. Yet other measures of oxidative damage, such as measurement of protein oxidation, have not been consistently linked with CHD.4
In previous work we reported a fluorescent assay that measures the Schiff base products from the interaction of oxidation products with proteins, phospholipids, and nucleic acids, producing chromophores with characteristic fluorescence spectra.5–6 Our fluorescent method provides a nonspecific but sensitive measure of oxidative products from lipids, protein, carbohydrate, and DNA and is considered a global measure of oxidation compared with specific oxidation measurements such as measurement of F2‐isoprostanes or malondialdehyde.6–7 We found that this global measure of fluorescent oxidation products was stable in plasma samples collected and stored for 10 years,5,8 that it was highly correlated with cigarette smoking, and that it was a strong and independent predictor of CHD in a prospective study of US men and significantly higher in patients with reduced renal function in a case–control study.9
Because women may have different underlying susceptibility to oxidative stress in response to common environmental determinants and this may affect their risk of CHD in a different manner than that observed in men,1 we aimed to address the association of plasma fluorescent oxidation products with CHD among women who participated in the prospective Nurses' Health Study. To extend our previous analyses in men in the Health Professionals Follow‐Up Study, we also explored the association of plasma fluorescent oxidation products measured across broader and more variable wavelengths.
Methods
Design and Population
The Nurses' Health Study enrolled 121 701 female nurses aged 30 to 55 at baseline in 1976. Participants have since been followed with biennial questionnaires to record newly diagnosed illnesses and to update lifestyle information, as published previously.10 Between 1989 and 1990, a blood sample was requested from all active participants in the Nurses' Health Study and collected from 32 826 women. A nested case–control study of CHD was designed among participants free of diagnosed cardiovascular disease or cancer at blood draw by identifying all incident cases of CHD between blood draw and June 2004 (n=474). Using risk‐set sampling,11 controls were selected randomly and matched in a 1:2 ratio on age, smoking, and fasting status, among participants who were free of cardiovascular disease at the time CHD was diagnosed in the case. The diagnosis of CHD included nonfatal myocardial infarction and fatal CHD. The diagnosis of myocardial infarction was confirmed on the basis of the criteria of the World Health Organization (symptoms plus either diagnostic electrocardiographic changes or elevated levels of cardiac enzymes). Deaths were identified from state vital records and the National Death Index or reported by the participant's next of kin or the postal system. Fatal CHD was confirmed by an examination of hospital or autopsy records or by the listing of CHD as the cause of death on the death certificate, if CHD was the underlying and most plausible cause and if evidence of previous CHD was available.
We performed the current analysis in all participants with complete information on 3 different fluorescent measures and all covariates. After excluding participants with any missing information, we also excluded cases and controls who had no matching control or case. Our final data sets consisted of 1161 participants (397 case–control sets).
The study protocol was approved by the institutional review board of the Brigham and Women's Hospital and the Human Subjects Committee Review Board of Harvard School of Public Health.
Measurements
Demographic, anthropometric, and lifestyle data were derived from questionnaires administered at blood draw in 1990. Body mass index was calculated as weight in kilograms divided by height in meters squared. Physical activity was expressed in terms of metabolic‐equivalent hours per week. Participants reported whether a physician had ever diagnosed them with diabetes or hypertension. The questionnaires and the validity and reproducibility of measurements have been described previously.12
Blood samples were collected in tubes treated with liquid sodium heparin. The tubes were then placed on ice packs, stored in Styrofoam containers, returned to our laboratory by overnight courier, centrifuged, and divided into aliquots for storage in liquid‐nitrogen freezers (−130°C or colder).
Measurement of fluorescent oxidation products (FlOPs) was performed with procedures previously described.7 Briefly, plasma was extracted with ethanol–ether, and measurements were assessed using a spectrofluorometer. FlOPs reflect lipid oxidation products from reactions with protein, DNA, and carbohydrate. The majority are measured in wavelengths with excitation at 360 nm and emission at 420 nm (FlOP_360), which we reported in previous studies to be stable, reproducible, and predictive of CHD in men.5,8 The fluorescence was determined as relative fluorescence units per millimeter of plasma. We also determined the relative fluorescence intensity at 2 alternative wavelengths. First, when lipid hydroperoxides and secondary products, or aldehydes, ketones, and dimeric compounds from oxidized linolenate, react with DNA in the presence of metals and reducing agents, they can generate products with excitation at 320 nm and emission at 420 nm (FlOP_320).6,13–14 Of note, malondialdehyde produced very little fluorescent products with DNA in the presence of metals and reducing agents.6,13–14 Second, products generated from malondialdehyde reacting with proteins and phospholipids usually have a higher fluorescent spectra with excitation at 400 nm and emission at 475 nm (FlOP_400).6,15
Each batch in a laboratory analysis included the matched case–control sets so that run‐to‐run variation in the analysis would not add imprecision to the differences between cases and controls. All laboratory personnel were blinded to the case–control status. The within‐run average coefficient variation (CVs) for all 3 measures were <13%.
We also measured FlOP_360, FlOP_320, and FlOP_400 in 40 Nurses' Health Study participants who donated 2 blood samples an average of 1.4 years apart (range, 0.8 to 2.2 years). After adjusting for fasting status, intraclass correlations comparing repeated measurements were 0.44 for FlOP_360, 0.55 for FlOP_320, and 0.70 for FlOP_400; within‐person CVs were 39%, 52%, and 27% for FlOP_360, FlOP_320, and FlOP_400, respectively.
Statistical Analysis
Baseline characteristics of participants who developed CHD during follow‐up and controls were assessed by means (standard deviations) or medians (interquartile ranges) for continuous variables and percentages of categorical variables. Quintiles of FlOPs were calculated using the distributions in the controls. We examined the distribution of lifestyle characteristics and cardiovascular biomarkers across quintiles of FlOPs in controls. Relative risks (RRs) and 95% confidence intervals (CIs) for CHD risk were estimated by incidence rate ratios from unconditional logistic regression, with adjustment for the matching factors (age±2 years; month of blood draw [quintiles]; fasting status [yes/no]; and smoking status [never, past <15 cigarettes/day, past 15+ cigarettes/day, current <15 cigarettes/day, current 15+ cigarettes/day]). The results were similar to conditional logistic regression models but allowed for maximum statistical power in stratified analyses without losing unmatched participants in strata.11 In multivariable analyses we adjusted for the matching factors as well as alcohol intake, body mass index, physical activity, family history of myocardial infarction, and self‐reported history of hypertension, diabetes, and hypercholesterolemia. Additional analyses further adjusted for low‐density lipoprotein and high‐density lipoprotein cholesterol, triglycerides, and C‐reactive protein. Tests for linear trend were conducted by analyzing the median value of the FlOP quintile as a continuous variable. In light of earlier results suggesting the importance of considering fasting status,5,7 we also conducted an analysis restricted to women who had fasted 8 hours or more before blood draw. We conducted analyses of FlOP1, FlOP2, and FlOP3 separately and conducted an explorative factor analysis to create a composite score of the 3 FlOP measures. We also related this common factor to risk of CHD. Finally, we examined whether the association between the FlOP measures and CHD differed by length of time between blood draw and the CHD event, by hypertension, or by smoking status. For the interaction analyses, we modeled the log‐transformed FlOPs as linear variables (standardized each FlOP by z scores). All analyses were performed using SAS 9 (SAS Institute Inc, Cary, NC).
Results
As expected, the cases had higher plasma levels of triglycerides, low‐density lipoprotein cholesterol, and C‐reactive protein compared with the controls. Median levels of the 3 FlOP measures were also higher in cases compared with controls (Table 1). FlOP_360 and FlOP_400 were highly correlated (Spearman age‐adjusted r=0.8, P<0.001) and FlOP_360 and FlOP_320 less so (r=0.4). FlOP_320 and FlOP_400 were only modestly correlated (r=0.2), and no strong correlations with plasma lipids or C‐reactive protein were observed (data not shown).
Table 1.
Baseline Characteristics of Women in Whom Coronary Heart Disease Developed During Follow‐up and Matched Controls in the NHS*
| Variable | Cases (n=397) | Controls (n=779) |
|---|---|---|
| Age, y | 60.1 (6.5) | 59.8 (6.6) |
| BMI, kg/m2 | 26.6 (5.4) | 25.1 (4.3) |
| Current smoker, % | 24 | 24 |
| Fasting >8 h, % | 75 | 73 |
| Alcohol intake (g/day), median (IQR) | 0.9 (0.0, 5.6) | 1.8 (0.0, 8.6) |
| Physical activity, METh/week | 18.2 (22.4) | 18.8 (19.7) |
| White ethnicity, % | 99 | 100 |
| Postmenopausal, % | 85 | 84 |
| Family history of MI, % | 22 | 13 |
| Diabetes,* % | 15 | 5.4 |
| Hypercholesterolemia,* % | 53 | 42 |
| Hypertension,* % | 51 | 29 |
| CVD biomarkers | ||
| Total cholesterol, mmol/L | 6.00 (1.04) | 5.87 (1.05) |
| LDL‐C, mmol/L | 3.71 (0.95) | 3.51 (0.97) |
| HDL‐C, mmol/L | 1.35 (0.39) | 1.54 (0.43) |
| Triglycerides (mmol/L), median (IQR) | 1.40 (0.99, 1.99) | 1.19 (0.86, 1.65) |
| CRP (g/L), median (IQR) | 0.26 (0.12, 0.55) | 0.19 (0.08, 0.38) |
| FlOP_360, median (IQR) | 229 (177, 307) | 216 (174, 277) |
| FlOP_320, median (IQR) | 412 (303, 712) | 380 (297, 594) |
| FlOP_400, median (IQR) | 64.6 (50.1, 89.8) | 61.0 (48.4, 83.6) |
BMI indicates body mass index; CRP, C‐reactive protein; CVD, cardiovascular disease; FlOP, fluorescent oxidation product; HDL‐C, high‐density lipoprotein cholesterol; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; METh/week, metabolic equivalent hours per week; MI, myocardial infarction; NHS, Nurses' Health Study.
Data are presented as means (SDs) unless otherwise indicated. Matching criteria were age, smoking, date of blood sampling, and fasting status. FlOPs are presented as relative fluorescent intensity/mL of plasma. To convert cholesterol concentrations from mmol/L to mg/dL, divide by 0.0259. To convert triglycerides from mmol/L to mg/dL, divide by 0.0113.
Self‐reported diagnosis before blood draw.
FlOP_360 and FlOP_400 were strongly associated with smoking status, with much higher levels observed among current smokers. Thus, we chose to standardize the characteristics presented according to quintiles of FlOPs by smoking status (Table 2). High levels of the FlOP measures were also correlated with consuming more alcohol, having hypertension, and having diagnosed hypercholesterolemia. Women with the highest FlOP concentrations were also less likely to self‐report a history of diabetes and had a slightly lower body mass index. There were no strong associations between FlOP measures and lipid markers.
Table 2.
Characteristics According to Quintiles of the 3 FlOP Measurements in Controls From the Nurses' Health Study*
| Characteristic | FlOP_360 | FlOP_320 | FlOP_400 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| Age, y | 59.0 (7.5) | 59.1 (6.7) | 60.5 (6.5) | 58.9 (6.9) | 60.2 (6.3) | 60.4 (6.2) | 58.5 (6.7) | 59.5 (6.3) | 60.7 (6.5) |
| BMI, kg/m2 | 25.8 (4.5) | 25.4 (4.6) | 24.7 (3.9) | 25.1 (3.9) | 25.3 (4.5) | 24.7 (3.9) | 26.2 (4.7) | 25.0 (4.2) | 25.0 (4.1) |
| Current smoker, % | 3 | 22 | 33 | 4 | 28 | 24 | 3 | 19 | 58 |
| Alcohol intake, g/day | 3.3 (6.8) | 5.3 (8.1) | 6.5 (10.5) | 5.6 (11.2) | 6.7 (10.6) | 5.3 (10.1) | 3.9 (7.6) | 6.0 (9.6) | 7.2 (11.4) |
| Diabetes,* % | 6 | 7 | 4 | 5 | 9 | 5 | 7 | 6 | 4 |
| High cholesterol,* % | 35 | 46 | 47 | 32 | 45 | 41 | 43 | 39 | 48 |
| Hypertension,* % | 24 | 27 | 38 | 17 | 35 | 35 | 27 | 22 | 44 |
| CVD biomarkers | |||||||||
| Total cholesterol, mmol/L | 5.3 (1.3) | 6.0 (1.1) | 5.9 (1.2) | 5.6 (1.1) | 5.9 (1.1) | 5.8 (1.0) | 5.6 (0.9) | 5.8 (1.0) | 5.9 (1.4) |
| LDL‐C, mmol/L | 3.1 (1.0) | 3.6 (1.1) | 3.6 (1.1) | 3.3 (0.9) | 3.5 (1.0) | 3.4 (0.9) | 3.3 (0.8) | 3.4 (0.9) | 3.5 (1.2) |
| HDL‐C, mmol/L | 1.4 (0.4) | 1.5 (0.5) | 1.6 (0.5) | 1.5 (0.4) | 1.5 (0.4) | 1.6 (0.4) | 1.5 (0.4) | 1.6 (0.5) | 1.6 (0.5) |
| Triglycerides, mmol/L | 1.3 (0.6) | 1.4 (0.7) | 1.3 (0.8) | 1.2 (0.5) | 1.4 (0.8) | 1.3 (0.8) | 1.4 (0.6) | 1.4 (0.9) | 1.3 (0.7) |
| CRP, g/L | 0.3 (0.4) | 0.3 (0.4) | 0.4 (0.5) | 0.3 (0.5) | 0.3 (0.3) | 0.4 (0.5) | 0.4 (0.5) | 0.4 (0.6) | 0.4 (0.4) |
Quintiles 1 (Q1), 3 (Q3), and 5 (Q5) among the controls. BMI indicates body mass index; CRP, C‐reactive protein; CVD, cardiovascular disease; FlOP, fluorescent oxidation product; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
Values are means (SDs) or percentages and are standardized to the distribution of smoking status.
Self‐reported diagnosis before blood draw.
High FlOP_360 level was associated with risk of CHD. This association was especially apparent on subsequent adjustment for lifestyle and cardiovascular disease biomarkers (Table 3). The RR in the top quintile was 1.64 (95% CI, 1.06 to 2.53) compared with the lowest quintile. The participants with the highest FlOP_320 levels had a statistically nonsignificant elevated risk of CHD. Only in the multivariable model including adjustment for biomarkers was the test for trend significant (Ptrend=0.03). FlOP_400 was associated with risk in a manner very similar to that observed for FlOP_360 (RR for the comparison of the top versus bottom quintile was 1.75; 95% CI, 1.09 to 2.80). Associations for both FlOP_360 and FlOP_400 were slightly stronger when restricted to the women who had fasted >8 hours before venipuncture. We created a composite score for the FlOP measures by factor analysis. It was determined that FlOP1 and FlOP3 contributed to most of the variation in this common factor, whereas FlOP2 did not load very high. The composite score was directly associated with risk of CHD in logistic regression models, and the fully adjusted RR for the top quintile was 1.81 (95% CI, 1.09 to 3.01; Ptrend=0.02) among the fasting women (data not shown).
Table 3.
Multivariable‐adjusted IRR and 95% Confidence Intervals of CHD According to Quintiles of the 3 FlOP Measurements in the NHS
| Q1 | Q2 | Q3 | Q4 | Q5 | P Value for Trend | |
|---|---|---|---|---|---|---|
| FlOP_360 | ||||||
| Median (range) | 149 (<166) | 182 (166 to 198) | 217 (199 to 236) | 265 (237 to 298) | 392 (>298) | |
| Unadjusted | 1 (ref) | 1.11 (0.75 to 1.64) | 0.86 (0.57 to 1.31) | 1.11 (0.73 to 1.70) | 1.52 (1.02 to 2.25) | 0.01 |
| Multivariable adjusted | — | 1.17 (0.77 to 1.78) | 0.93 (0.60 to 1.45) | 1.19 (0.76 to 1.87) | 1.53 (1.01 to 2.34) | 0.03 |
| + Biomarkers | — | 1.13 (0.74 to 1.73) | 0.94 (0.60 to 1.48) | 1.22 (0.77 to 1.94) | 1.64 (1.06 to 2.53) | 0.01 |
| + Biomarkers, fasting only | — | 1.18 (0.73 to 1.90) | 1.00 (0.59 to 1.68) | 1.31 (0.76 to 2.24) | 1.91 (1.16 to 3.15) | 0.01 |
| FlOP_320 | ||||||
| Median (range) | 246 (<284) | 312 (284 to 345) | 381 (345 to 424) | 505 (425 to 720) | 3531 (>720) | |
| Unadjusted | 1 (ref) | 0.80 (0.54 to 1.21) | 0.97 (0.65 to 1.46) | 1.06 (0.71 to 1.59) | 1.28 (0.87 to 1.80) | 0.05 |
| Multivariable adjusted | — | 0.80 (0.52 to 1.23) | 0.83 (0.54 to 1.27) | 0.99 (0.64 to 1.52) | 1.20 (0.80 to 1.81) | 0.07 |
| + Biomarkers | — | 0.75 (0.48 to 1.16) | 0.82 (0.53 to 1.27) | 0.96 (0.62 to 1.49) | 1.25 (0.82 to 1.89) | 0.03 |
| + Biomarkers, fasting only | — | 0.78 (0.48 to 1.27) | 1.01 (0.61 to 1.67) | 1.15 (0.69 to 1.92) | 1.34 (0.83 to 2.17) | 0.10 |
| FlOP_400 | ||||||
| Median (range) | 41 (<46) | 51 (46 to 56) | 61 (56 to 69) | 78 (69 to 89) | 113 (>89) | |
| Unadjusted | 1 (ref) | 1.11 (0.74 to 1.66) | 1.32 (0.88 to 1.97) | 1.10 (0.72 to 1.68) | 1.64 (1.07 to 2.53) | 0.03 |
| Multivariable adjusted | — | 1.27 (0.83 to 1.95) | 1.52 (1.00 to 2.33) | 1.16 (0.74 to 1.83) | 1.66 (1.05 to 2.62) | 0.07 |
| + Biomarkers | — | 1.31 (0.85 to 2.02) | 1.55 (1.01 to 2.40) | 1.15 (0.72 to 1.83) | 1.75 (1.09 to 2.80) | 0.05 |
| + Biomarkers, fasting only | — | 1.51 (0.92 to 2.47) | 1.65 (1.00 to 2.72) | 1.46 (0.85 to 2.50) | 1.93 (1.12 to 3.35) | 0.06 |
Medians for FlOPs are presented as relative fluorescent intensity per milliliter of plasma. Unadjusted model includes matching factors only (age, smoking, time of blood draw, and fasting status). Multivariable model includes matching factors and alcohol, physical activity, BMI, family history of MI, history of hypertension, diabetes, hypercholesterolemia. Model with biomarkers additionally adjusted for continuous measures of LDL, HDL, TG, and CRP. Fasting women only, n=889 (306 cases). Fasting >8 hours before blood draw. P for trend is test of linear trend across quintiles using medians as continuous variable. BMI indicates body mass index; CHD, coronary heart disease; CRP, C‐reactive protein; FlOP, fluorescent oxidation product; HDL, high‐density lipoprotein; IRR, incidence rate ratio; LDL, low‐density lipoprotein; MI, myocardial infarction; NHS, Nurses' Health Study; TG, triglycerides.
Although we did not detect any statistically significant effect modification by self‐reported physician‐diagnosed hypertension, hypercholesterolemia, or smoking status (all P for statistical interaction>0.3), associations with CHD were strongest among participants without hypertension and without hypercholesterolemia. Associations for FlOP_320 and FlOP_400 were strongest in past and current cigarette smokers and strongest among the current smokers (RR per each unit increase in z score for log‐transformed FlOP_320=1.44, 95% CI, 1.06 to 1.95, and for FlOP_400=1.20, 95% CI, 0.83 to 1.75).
We further explored the potential role of the FlOP measurements as acute or long‐term markers of risk for CHD by stratifying our analyses on time to event from blood draw. None of the 3 FlOP measurements were significantly associated with the short‐term risk of CHD, whereas an elevated risk of CHD was observed when the CHD event occurred ≥5 years after the blood draw (Figure). The RR of CHD per each unit increase in z score for FlOP_360 was 0.86 (0.67 to 1.11) the first 5 years of follow‐up and 1.23 (1.04 to 1.46) for events occurring ≥6 years after baseline blood collection. Similar point estimates were observed for FlOP_320 and FlOP_400. We observed that the associations in general were stronger as the duration of follow‐up increased.
Figure 1.
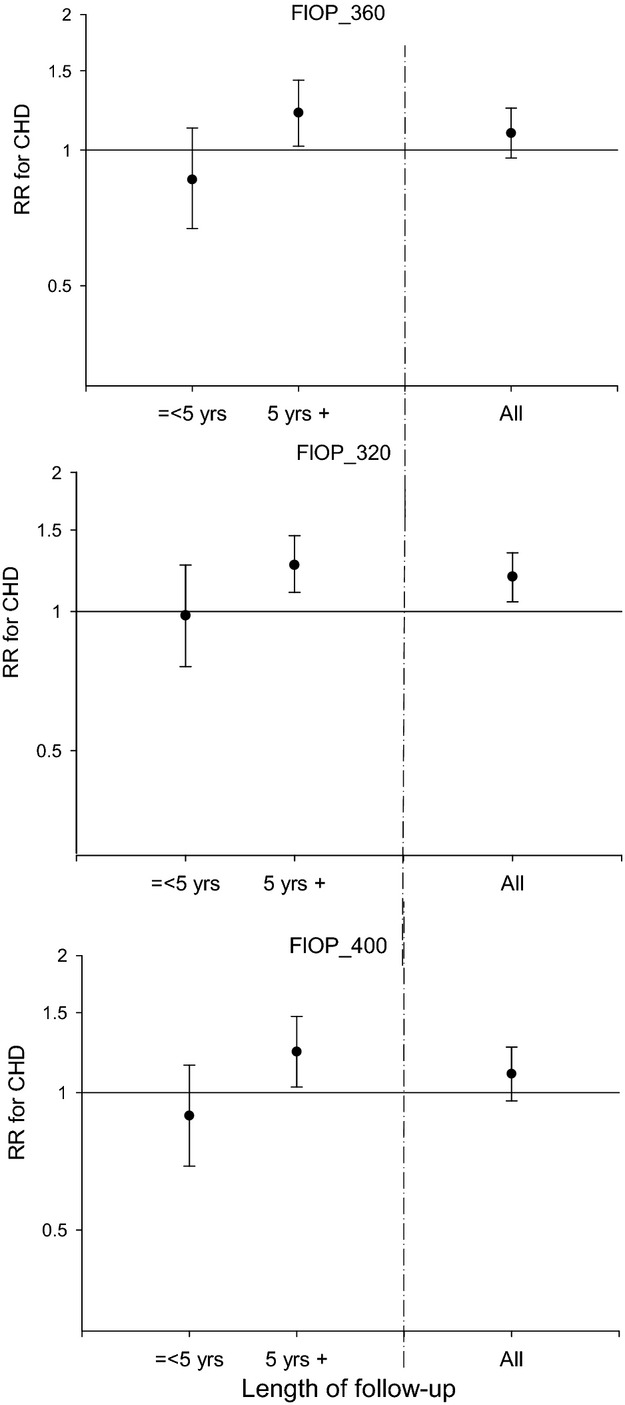
Multivariable‐adjusted RRs for CHD per 1 z‐score increase in each log‐transformed FlOP measure (relative fluorescent intensity/ml) in the Nurses' Health Study in strata of length of follow‐up (≤5 yr [112 cases occurred] and >5 yr [285 cases occurred]). RRs are incidence rate ratios (IRR) obtained from logistic regression models adjusted for matching factors (age, smoking, time of blood draw, and fasting status) and alcohol, physical activity, BMI, family history of MI, history of hypertension, diabetes, hypercholesterolemia, LDL, HDL, TG, and CRP. BMI indicates body mass index; CHD, coronary heart disease; CRP, C‐reactive protein; FlOP, fluorescent oxidation product; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; RR, relative risk; TG, triglycerides. Error bars indicate 95% confidence interval.
Discussion
In this prospective study of generally healthy middle‐aged women, a global measure of oxidative stress, as assessed by our fluorescent oxidation product assay, was associated with the risk of future CHD. Our results indicated that measures obtained at wavelengths between 360 and 475 nm were most correlated and generally showed the strongest association. In exploratory analyses we found evidence to suggest that FlOP measurements were better CHD predictors if CHD events occurred 5 years after blood draw.
Other studies have reported associations between different measures of oxidative stress and cardiovascular disease. Compared with controls, measures of malondialdehyde, a breakdown product from lipid peroxidation, have been found to be elevated in stroke patients,16 and 8‐hydroxy‐2′‐deoxyguanosine levels, a measure of DNA damage, significantly predict cardiac events among patients with chronic heart failure.17 F2‐isoprostanes, another marker specific to the oxidation of lipids, has been associated with early stages of coronary artery calcification in young healthy adults,1 and higher levels have also been observed in patients with ischemic events compared with controls.2–3 Although a link between oxidative stress and atherosclerosis has been suggested from these studies, most of them have been small and constrained to elderly populations and patients with advanced atheroslcerosis18 or have been cross‐sectional.2–3,16 Because both in vitro and animal models demonstrate that oxidative stress plays an important role in the initiation and progression of atherosclerotic diseases,19–20 it is important to evaluate the role of these oxidative markers as predictors of plaque formation in a prospective setting of generally healthy participants. In our study, we were able to perform exploratory analyses stratified by years of follow‐up. We found that FlOP levels were more strongly associated with the long‐term risk of CHD but not with CHD within the first 5 years after FlOP measurement. A study conducted in CARDIA indicated that oxidative stress measured by F2‐isoprostanes, a marker of lipid oxidation, played a role in the early stages of atherosclerosis.1 It is unlikely that participants in our older study population (age range of 43 to 70 years at the time of blood draw) are in the early stages of atherosclerosis; however, our preliminary findings may suggest that FlOP may represent a long‐term marker of elevated risk. More studies on FlOP in both early‐stage atherosclerosis and with long follow‐up are needed to examine this in greater detail. One other prospective study conducted in a generally healthy population had a median follow‐up of only 5.6 years and did not find an association between F2‐isoprotanes and risk of CHD.4 Our findings suggest that the follow‐up time may not have been sufficient or that the null association for the F2‐isoprostane marker could also be related to this marker, only capturing the lipid portion of oxidative stress. Nevertheless, our findings suggest that FlOPs might be useful as a monitoring biomarker for early prevention.
We also evaluated the association of the FlOP measures with CHD in separate subgroups. Although these analyses were limited by smaller number of cases within strata, the associations for FlOP_320 were stronger in some subgroups such as among current smokers and participants without hypertension or hypercholesterolemia. The differential patterns of FlOPs with CHD among different subgroups merit further investigation. It is likely that oxidative stress is a stronger risk factor in some compared with other subgroups, and this might reflect underlying oxidative damage.
We have previously reported on the prospective association between our global oxidative stress measure and CHD in the male Health Professionals Follow‐Up Study. In comparison with other measurements that only captured 1 aspect of oxidation (lipid peroxidation, DNA damage, or protein oxidation), a global measure may be a better marker of systematic oxidation. In the present study, we also measured FlOPs at 2 alternative wavelengths. Although FlOP_320 was not strongly associated with risk of CHD in the main analyses, the strongly correlated measures of FlOP_360 and FlOP_400 showed similar associations. A derived composite score of the measures had a slightly stronger association with risk of CHD across quintiles. It is possible that combining the 3 FlOP measures helped to reduce measurement error. The factor analysis also highlighted that FlOP_320 did not contribute much to explaining the variation in the common score. Other reasons for the lack of relationship of FlOP_320 with risk of CHD include the higher within‐person variation for this measure compared with the others. Previously, we found that the relative risk associated with high levels of FlOP_360 was greatest when restricted only to the men who had provided fasting samples. In the present study among female nurses, the analyses were matched on fasting status, and the associations were modestly stronger when restricted to fasting women. Thus, postprandial oxidation may modestly affect the overall FlOP measurement, and fasting samples might be preferable if used as a predictive marker reflecting systemic exposure to oxidation.
This study had several limitations. First, we only had a single blood sample at baseline. Although we did not observe large changes, especially in FlOP_400 (intraclass correlation=0.70), over an average of 1.4 years, we do not know if a single measurement accurately reflected average levels over a prolonged period. Second, we adjusted for a range of cardiovascular risk factors, but because of the observational study design, we cannot exclude the possibility of residual confounding.
Third, although FlOP measurements have been repeatedly shown to predict risk of CHD in both men5 and women, the detailed components of the oxidation products in FlOP measurements need to be determined in the future. Fourth, the majority of our participants were white, and the predictive ability among other ethnic groups will need to be confirmed in the future.
In summary, this prospective study extended the previously observed association between FlOP measurements obtained at wavelengths between 360 and 475 nm and risk of CHD to a population of generally healthy women. Our results suggest that the FlOP measurement may be a long‐term marker of CHD risk, a finding that warrants further investigation to advance our understanding of this measure of oxidative stress in relation to atherosclerosis progression.
Sources of Funding
The current study was partially funded by Dr Wu's American Heart Association grant 0430202N, start‐up funds, KO7‐CA138714 award from National Institute of Cancer. The Nurses' Health Study was supported by HL34594, CA87969, and HL35464 from the National Institute of Health (Bethesda, MD).
Disclosures
None.
References
- 1.Gross M, Steffes M, Jacobs DR, Jr, Yu X, Lewis L, Lewis CE, Loria CM. Plasma F2‐isoprostanes and coronary artery calcification: the CARDIA study. Clin Chem. 2005; 51:125-131 [DOI] [PubMed] [Google Scholar]
- 2.Schwedhelm E, Bartling A, Lenzen H, Tsikas D, Maas R, Brummer J, Gutzki FM, Berger J, Frolich JC, Boger RH. Urinary 8‐iso‐prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case–control study. Circulation. 2004; 109:843-848 [DOI] [PubMed] [Google Scholar]
- 3.Shishehbor MH, Zhang R, Medina H, Brennan ML, Brennan DM, Ellis SG, Topol EJ, Hazen SL. Systemic elevations of free radical oxidation products of arachidonic acid are associated with angiographic evidence of coronary artery disease. Free Radical Biol Med. 2006; 41:1678-1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodward M, Croft KD, Mori TA, Headlam H, Wang XS, Suarna C, Raftery MJ, MacMahon SW, Stocker R. Association between both lipid and protein oxidation and the risk of fatal or non‐fatal coronary heart disease in a human population. Clin Sci (Lond). 2009; 116:53-60 [DOI] [PubMed] [Google Scholar]
- 5.Wu T, Rifai N, Willett WC, Rimm EB. Plasma fluorescent oxidation products: independent predictors of coronary heart disease in men. Am J Epidemiol. 2007; 166:544-551 [DOI] [PubMed] [Google Scholar]
- 6.Frankel EN. Lipid Oxidation. 20052nd edDundee, Scotland: The Oily Press Ltd [Google Scholar]
- 7.Wu T, Willett WC, Rifai N, Rimm EB. Plasma fluorescent oxidation products as potential markers of oxidative stress for epidemiologic studies. Am J Epidemiol. 2007; 166:552-560 [DOI] [PubMed] [Google Scholar]
- 8.Wu T, Rifai N, Roberts LJ, II, Willett WC, Rimm EB. Stability of measurements of biomarkers of oxidative stress in blood over 36 hours. Cancer Epidemiol Biomarkers Prev. 2004; 13:1399-1402 [PubMed] [Google Scholar]
- 9.Rebholz CM, Wu T, Hamm LL, Arora R, Khan IE, Liu Y, Chen CS, Mills KT, Rogers S, Kleinpeter MA, Simon EE, Chen J. The association of plasma fluorescent oxidation products and chronic kidney disease: a case–control study. Am J Nephrol. 2012; 36:297-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20‐year contribution to the understanding of health among women. J Womens Health. 1997; 6:49-62 [DOI] [PubMed] [Google Scholar]
- 11.Prentice RL, Breslow NE. Retrospective studies and failure time models. Biometrika. 1978; 65:153-158 [Google Scholar]
- 12.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self‐administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992; 135:1114-1126 [DOI] [PubMed] [Google Scholar]
- 13.Frankel EN, Neff WE, Brooks DD, Fujimoto K. Fluorescence formation from the interaction of DNA with lipid oxidation degradation products. Biochim Biophys Acta. 1987; 919:239-244 [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto K, Neff WE, Frankel EN. The reaction of DNA with lipid oxidation products, metals and reducing agents. Biochim Biophys Acta. 1984; 795:100-107 [DOI] [PubMed] [Google Scholar]
- 15.Flynn TP, Allen DW, Johnson GJ, White JG. Oxidant damage of the lipids and proteins of the erythrocyte membranes in unstable hemoglobin disease. Evidence for the role of lipid peroxidation. J Clin Investig. 1983; 71:1215-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polidori MC, Cherubini A, Stahl W, Senin U, Sies H, Mecocci P. Plasma carotenoid and malondialdehyde levels in ischemic stroke patients: relationship to early outcome. Free Radical Res. 2002; 36:265-268 [DOI] [PubMed] [Google Scholar]
- 17.Susa T, Kobayashi S, Tanaka T, Murakami W, Akashi S, Kunitsugu I, Okuda S, Doi M, Wada Y, Nao T, Yamada J, Ueyama T, Okamura T, Yano M, Matsuzaki M. Urinary 8‐hydroxy‐2′‐deoxyguanosine as a novel biomarker for predicting cardiac events and evaluating the effectiveness of carvedilol treatment in patients with chronic systolic heart failure. Circ J. 2012; 76:117-126 [DOI] [PubMed] [Google Scholar]
- 18.Mezzetti A, Zuliani G, Romano F, Costantini F, Pierdomenico SD, Cuccurullo F, Fellin R. Vitamin E and lipid peroxide plasma levels predict the risk of cardiovascular events in a group of healthy very old people. J Am Geriatr Soc. 2001; 49:533-537 [DOI] [PubMed] [Google Scholar]
- 19.Ross R, Glomset JA. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976; 295:420-425 [DOI] [PubMed] [Google Scholar]
- 20.Burgoyne JR, Mongue‐Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res. 2012; 111:1091-1106 [DOI] [PubMed] [Google Scholar]


