Abstract
Background
The intersection of heart failure (HF) and atrial fibrillation (AF) is common, but the burden of AF among black patients with HF is poorly characterized. We sought to determine the prevalence of AF, characteristics, in‐hospital outcomes, and warfarin use associated with AF in patients hospitalized with HF as a function of race.
Methods and Results
We analyzed data on 135 494 hospitalizations from January 2006 through January 2012 at 276 hospitals participating in the American Heart Association's Get With The Guidelines HF Program. Multivariable logistic regression models using generalized estimating equations approach for risk‐adjusted comparison of AF prevalence, in‐hospital outcomes, and warfarin use. In this HF population, 53 389 (39.4%) had AF. Black patients had markedly less AF than white patients (20.8% versus 44.8%, P<0.001). Adjusting for risk factors and hospital characteristics, black race was associated with significantly lower odds of AF (adjusted odds ratio 0.52, 95% CI 0.48 to 0.55, P<0.0001). There were no racial differences in in‐hospital mortality; however, black patients had a longer length of stay relative to white patients. Black patients compared with white patients with AF were less likely to be discharged on warfarin (adjusted odds ratio 0.76, 95% CI 0.69 to 0.85, P<0.001).
Conclusions
Despite having many risk factors for AF, black patients, relative to white patients hospitalized for HF, had a lower prevalence of AF and lower prescription of guideline‐recommended warfarin therapy.
Keywords: anticoagulation, atrial fibrillation, heart failure, racial disparity, risk factors
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in the United States with a prevalence of 2.7 to 6.1 million in 2010 and is expected to rise to between 5.6 and 12 million in 2050.1 Similarly, heart failure (HF) is a burgeoning epidemic that currently affects ≈6.6 million US adults with an expected 25% increase in prevalence by 2030.1 The intersection of AF and HF is an intricate pathophysiologic imbalance that results in worse morbidity and mortality than either separately. The black population has the highest risk of developing HF and, when present, has an earlier diagnosis and more often leads to hospitalization.2 Despite a higher or similar occurrence of established risk factors associated with the development of AF, studies have suggested a lower prevalence of AF in black patients relative to white patients.3–5 Less is known regarding the racial prevalence and outcomes of AF in patients with HF.
The goal of this study was 3‐fold: in patients hospitalized with HF, (1) determine whether the prevalence of AF was lower in black patients compared with white patients and to evaluate racial differences in characteristics among those with AF, (2) evaluate the effect of race on in‐hospital mortality and length of stay (LOS), and (3) assess the prescription of guideline‐recommended warfarin therapy for eligible patients hospitalized with AF and HF as a function of race.
Methods
Get With The Guidelines‐Heart Failure (GWTG‐HF) is a voluntary registry‐ and hospital‐based performance‐improvement program for patients hospitalized with HF, and it has been previously described.6–8 Patients hospitalized with new or worsening HF as primary diagnosis or patients who developed significant HF symptoms such that HF was the primary discharge diagnosis were included. Patients were enrolled into the program regardless of their left ventricular function. Hospitals participating in this registry use a Web‐based, point‐of‐care, patient management tool (PMT; Outcome Sciences Inc) to collect and submit clinical information on patients' in‐hospital care and outcomes. Outcome Sciences, Inc serves as the data collection (through PMT) and coordination center for GWTG. The Duke Clinical Research Institute (DCRI) serves as the data analysis center and has an agreement to analyze the aggregate deidentified data for research purposes.
Trained personnel at participating sites abstract data on consecutive eligible patients using standardized definitions for all data fields and submit these data to the GWTG database. Using methodology similar to other national cardiovascular registries and randomized trials, race and ethnicity were collected for the purpose of evaluating subgroup differences. Admission and/or medical staff recorded race/ethnicity, usually as the patient was registered. Patients were assigned to race and ethnicity categories using options defined by the case report forms as follows: race—American Indian or Alaska Native, Asian, black or African American, Native Hawaiian or Pacific Islander, white, or unable to be determined; and ethnicity—Hispanic, yes, no, or unable to be determined.
All participating institutions were required to comply with local regulatory and privacy guidelines and to obtain approval from their institutional review board. Because data were used primarily at the local site for quality improvement, sites were granted a waiver of informed consent under the common rule. Computerized edit checks are performed and data quality monitored to ensure completeness and accuracy of reported data. Data included in GWTG‐HF were demographic and clinical characteristics, comorbidities, previous therapies and procedures, contraindications to evidence‐based therapies, LOS, and in‐hospital procedures and outcomes.
Data collection on rhythm status for this analysis included the following definitions: preexisting AF (had a prior history of AF) and new diagnosis (diagnosis of AF at hospital presentation or during hospitalization and did not have a prior history of AF). Either of the above was characterized as “AF” for the purposes of this analysis.
Study Population
From January 1, 2006, through January 13, 2012, we identified 164 044 patients from 286 hospitals in the GWTG‐HF database. We excluded 5815 (3.5%) with missing information on AF history and diagnosis, 11 731 (7.2%) of Hispanic ethnicity, 4480 (2.7%) with no data on race, and 6524 (4.0%) with race other than black or white. For the purpose of this analysis, we included only patients who were recorded as non‐Hispanic black (black) and non‐Hispanic white (white). The final overall population included 135 494 patients from 276 hospitals. Among the 135 494 patients, we identified 53 389 patients with AF who were ultimately used for comparing in‐hospital mortality, LOS, and the prescription of warfarin at discharge by race.
Outcome Measures
The principal objective of the study was to assess AF prevalence and patient characteristics among black and white patients with AF hospitalized for HF. The outcomes of interest in the primary analyses included in‐hospital mortality, LOS, and warfarin use at discharge in eligible patients with AF without documented contraindications or intolerance as a function of race. To determine the association between magnitude of stroke risk and warfarin use, patients were stratified by CHADS2 (congestive heart failure, hypertension, age >75, diabetes mellitus, and prior stroke or transient ischemic attack) score.
Statistical Analysis
Sociodemographic factors, clinical variables, and hospital characteristics were compared between blacks and whites using Pearson χ2 test for categorical variables and Wilcoxon rank sum test for continuous variables. Categorical and continuous variables were reported in percentages and median (25th and 75th percentiles) as appropriate. A multivariable logistic regression analysis was performed to examine independent predictors of AF in patients hospitalized for HF. Generalized estimating equations approach was used to account for clustering of patients within hospitals.9 Additionally, multivariable regression analyses using generalized estimating equations were performed in patients with AF to assess the relationships between race and the following outcomes: in‐hospital mortality, LOS, and warfarin use at discharge. In the analyses, the type of AF (preexisting versus new diagnosis) and its interaction with race were tested to evaluate whether the relationship between race and outcomes differs across patients with preexisting AF and new diagnosis AF. Since there was no significant interaction between race and AF type (new diagnosis or preexisting), the relationship between race and outcomes was reported among all patients with AF inclusive of new diagnosis and preexisting patients. For anticoagulation, the analysis also adjusted for CHADS2 risk stratification score to eliminate the influence of CHADS2 score on the relationship between race and warfarin use. The analysis of warfarin at discharge included those individuals with appropriate eligibility. The following contraindications to warfarin use led to exclusion: allergy to warfarin, pregnancy, excess bleeding risk, excess fall risk, other medical contraindications, or patient reasons. We tested the interaction of race and gender on the prescription of warfarin at discharge based on prior analyses suggesting women with AF were less likely to receive oral anticoagulation for stroke prophylaxis. For LOS, log transformation was performed first because LOS had a heavily rightward‐skewed distribution, and then regression analysis was conducted for the log‐transformed time variable that had an approximate normal distribution. Therefore, an exponentiation was performed to the estimates of race effect from the model and ratio of LOS between black and white race was reported. These multivariable analyses were adjusted for patient and hospital characteristics that may have affected the association of race with outcomes. Patient‐level variables included demographics (age, gender), insurance status (Medicare, Medicaid, other including Veterans Administration, health maintenance organization, etc, and no insurance), medical history (chronic obstructive pulmonary disease [COPD] or asthma, diabetes, hyperlipidemia, hypertension, peripheral vascular disease [PVD], coronary artery disease [CAD], prior myocardial infarction [MI], presence of valvular heart disease [VHD], prior percutaneous coronary intervention [PCI], prior coronary artery bypass graft surgery, stroke or transient ischemic attack [TIA], anemia, implantable cardioverter‐defibrillator [ICD] or pacemaker placement, end‐stage renal disease, chronic renal insufficiency, depression, cigarette smoking in the past 12 months), HF etiology (ischemic or nonischemic), and admission vital signs (heart rate and systolic blood pressure [BP]). Hospital characteristics included in the models were number of beds, academic center and/or staffing by residents, and region of the country (midwest, northeast, west, and south). The confounder variables had less than 5% missing, and thus a simple imputation was done to replace the missing with the median value for a continuous variable and the dominant category for a categorical variable.
All P values were based on 2‐sided tests and α=.05 was used to establish statistical significance of tests. We used SAS software version 9.2 (SAS Institute Inc, Cary, NC). All authors had access to the data and assume responsibility for the accuracy of the analysis.
Results
Baseline Characteristics of Patients Hospitalized for HF
Of the 135 494 patients hospitalized with HF in this analysis, 53 389 (39.4%) had AF. As shown in Table 1, compared with white patients with AF, black patients with AF were younger, had higher body mass index (BMI), and had similar gender distribution. Black patients with AF had a higher prevalence of hypertension, diabetes mellitus, COPD or asthma, HF, smoking, anemia, renal insufficiency, or dialysis dependence, relative to white patients. Black patients with AF were more likely to have a history of stroke or TIA. Conversely, proportionate to white patients, black patients with AF had less CAD or prior MI, PVD, and VHD. At admission, black patients had higher heart rates, and BP but similar brain natriuretic peptide (BNP) levels relative to white patients. Black patients had a significantly lower median left ventricular ejection fraction (LVEF) (35% versus 45%) and a greater proportion with LVEF <40% (54% versus 38%, P<0.0001). Black patients with AF were slightly more likely than white patients to undergo cardioversion during their hospitalization (2.9% versus 2.4%, P=0.05). Black patients without AF (23 989) differed from black patients with AF (6301). Notably, black patients without AF were younger, had a higher prevalence of diabetes, but had less CAD or prior MI, HF, VHD, COPD, or asthma and had fewer strokes or TIAs. At admission, black patients without AF had higher median heart rates (87 versus 83 bpm), systolic (148 versus 135 mm Hg) and diastolic (84 versus 78 mm Hg) BP, and higher BNP levels (Table 1). Similar to the comparison of black patients with and without AF, white patients with AF differed from those without AF most pronounced in the younger age of those without AF.
Table 1.
Patient and Hospital Characteristics
| Variable | White Patients With AF (47 088) | Black Patients With AF (6301) | P Value* | Black Patients Without AF (23 989) | P Value* | White Patients Without AF (58 116) | P Value* |
|---|---|---|---|---|---|---|---|
| Age, y | 80.0 (72.0, 86.0) | 69.0 (58.0, 79.0) | <0.001 | 60.0 (50.0, 72.0) | <0.001 | 75 (64, 84) | <0.001 |
| Male | 49.9 | 51.2 | 0.136 | 50.9 | 0.816 | 48.6 | <0.001 |
| Insurance | <0.001 | <0.001 | <0.001 | ||||
| No insurance/not documented/unknown | 1.2 | 4.6 | 10.5 | 3.3 | |||
| Medicare | 68.2 | 49.4 | 36.8 | 59.6 | |||
| Medicaid | 4.2 | 20.8 | 21.9 | 6.9 | |||
| Other | 25.2 | 21.7 | 25.5 | 27.9 | |||
| BMI,§, kg/m2 | 27 (23, 32) | 29 (24, 36) | <0.001 | 29 (24, 36) | 0.418 | 28 (23, 34) | <0.001 |
| EF, % | 45 (30, 55) | 35 (20, 53) | <0.001 | 31 (20, 52) | 0.025 | 40 (25, 55) | <0.001 |
| EF <40% | 38.4 | 54.5 | <0.001 | 57.1 | <0.001 | 44.4 | <0.001 |
| Medical history of | |||||||
| Anemia | 20.3 | 21.5 | 0.024 | 16.9 | <0.001 | 18.4 | <0.001 |
| Atrial flutter | 3.5 | 6.4 | <0.001 | 1.3 | <0.001 | 1.2 | <0.001 |
| CAD | 52.0 | 37.8 | <0.001 | 33.8 | <0.001 | 52.5 | 0.111 |
| COPD or asthma | 31.6 | 33.1 | 0.020 | 27.7 | <0.001 | 30.6 | <0.001 |
| CVA/TIA | 16.9 | 19.1 | <0.001 | 12.6 | <0.001 | 13.6 | <0.001 |
| Diabetes | 36.6 | 42.6 | <0.001 | 45.8 | <0.001 | 43.3 | <0.001 |
| Heart failure | 69.5 | 75.9 | <0.001 | 64.4 | <0.001 | 59.7 | <0.001 |
| Hyperlipidemia | 46.7 | 43.6 | <0.001 | 37.4 | <0.001 | 47.0 | 0.363 |
| Hypertension | 72.7 | 83.2 | <0.001 | 83.5 | 0.608 | 72.9 | 0.583 |
| ICD | 10.8 | 19.1 | <0.001 | 13.2 | <0.001 | 9.3 | <0.001 |
| IHD | 60.7 | 46.2 | <0.001 | 40.3 | <0.001 | 61.8 | <0.001 |
| Pacemaker | 20.5 | 15.2 | <0.001 | 6.8 | <0.001 | 11.3 | <0.001 |
| PVD | 13.6 | 9.3 | <0.001 | 7.5 | <0.001 | 13.8 | 0.530 |
| Prior CABG | 19.0 | 9.3 | <0.001 | 6.6 | <0.001 | 16.3 | <0.001 |
| Prior MI | 20.1 | 16.5 | <0.001 | 14.2 | <0.001 | 23.1 | <0.001 |
| Prior PCI | 11.5 | 7.5 | <0.001 | 6.8 | 0.062 | 12.5 | <0.001 |
| Renal insufficiency | 21.1 | 24.5 | <0.001 | 23.8 | 0.228 | 20.8 | 0.250 |
| Smoking | 9.6 | 19.6 | <0.001 | 30.2 | <0.001 | 18.0 | <0.001 |
| Valvular heart disease | 20.2 | 16.9 | <0.001 | 8.2 | <0.001 | 12.0 | <0.001 |
| Vital signs and laboratory data at admission | |||||||
| Heart rate, bpm | 81 (70, 97) | 83 (71, 100) | <0.001 | 87 (75, 100) | <0.001 | 81 (70, 95) | <0.001 |
| Systolic blood pressure, mm Hg | 132 (115, 151) | 135 (116, 156) | <0.001 | 148 (125 to 172) | <0.001 | 139 (119, 160) | <0.001 |
| Diastolic blood pressure, mm Hg | 72 (62, 84) | 78 (67, 91) | <0.001 | 84 (72 to 99) | <0.001 | 73 (63, 85) | <0.001 |
| BNP,* pg/mL | 726 (374, 1390) | 758 (287, 1699) | 0.582 | 956 (374 to 2100) | <0.001 | 862 (404, 1759) | <0.001 |
| Hemoglobin,* g/dL | 11.8 (10.5, 13.2) | 11.7 (10.3, 13.1) | <0.001 | 11.9 (10.4 to 13.4) | <0.001 | 11.9 (10.5, 13) | <0.001 |
| Sodium,* mEq/L | 138 (135, 140) | 139 (136, 141) | <0.001 | 139 (136 to 141) | 0.334 | 138 (135, 140) | <0.001 |
| Creatinine,* mg/dL | 1.3 (1.0, 1.8) | 1.4 (1.1, 2.0) | <0.001 | 1.4 (1.1 to 2.1) | 0.631 | 1.3 (1.0, 1.8) | 0.611 |
| Blood urea nitrogen,* mg/dL | 27 (19, 40) | 24 (17, 38) | <0.001 | 22 (15, 35) | <0.001 | 25 (17, 38) | <0.001 |
| In‐hospital procedures | |||||||
| Cardioversion | 2.4 | 2.9 | 0.053 | 0.3 | <0.001 | 0.3 | <0.001 |
| Pacemaker | 1.1 | 0.6 | <0.001 | 0.4 | 0.049 | 0.6 | <0.001 |
| ICD implantation | 2.4 | 2.7 | 0.180 | 3.6 | 0.001 | 4.0 | <0.001 |
| PCI or PCI with stent | 1.0 | 0.5 | <0.001 | 1.1 | <0.001 | 1.6 | <0.001 |
| Hospital characteristics | |||||||
| Hospital bed size | 375 (243 to 581) | 472 (342 to 617) | <0.001 | 470 (330 to 617) | <0.001 | 372 (240, 575) | <0.001 |
| Trainees* | 53.8 | 61.8 | <0.001 | 63.4 | <0.001 | 51.0 | <0.001 |
| Academic hospital* | 55.5 | 80.8 | <0.001 | 76.7 | <0.001 | 52.3 | <0.001 |
| Region | <0.001 | <0.001 | <0.001 | ||||
| West | 11.8 | 4.9 | 5.5 | 12.7 | |||
| South | 23.9 | 29.0 | 34.9 | 29.0 | |||
| Midwest | 26.7 | 22.5 | 21.8 | 26.2 | |||
| Northeast | 37.7 | 43.5 | 37.8 | 32.2 | |||
Values are presented as % or median (25th, 75th percentiles). P values are based on Pearson χ2 tests for all categorical row variables. AF indicates atrial fibrillation; BMI, body mass index; BNP, brain natriuretic peptide; CABG, coronary artery bypass graft surgery; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; EF, ejection fraction; ICD, implantable cardioverter‐defibrillator; IHD, ischemic heart disease; PCI, percutaneous intervention; PVD, peripheral vascular disease; TIA, transient ischemic attack.
P value comparing black and white patients with AF.
P value comparing black patients with and without AF.
P value comparing white patients with and without AF.
23% missing data.
Laboratory values had missing data between 21% and 36%.
14.8% missing data.
11.8% missing data.
In the study population, 20.8% of black patients and 44.8% of white patients had AF. In a multivariable analysis of all patients hospitalized for HF, adjusting for patient factors (age, gender, type of insurance, past medical history of COPD or asthma, diabetes mellitus, hyperlipidemia, hypertension, PVD, prior stroke/TIA, ICD, prior HF, anemia, pacemaker, dialysis, renal insufficiency, depression ischemic heart disease, smoking history, systolic BP, and heart rate on admission) and hospital characteristics (region, bed size, and teaching/resident status), black patients had a lower odds of AF compared to white patients (adjusted odds ratio [aOR] 0.52, 95% CI 0.48 to 0.55) (Table 2). Age was a strong predictor of AF in the multivariable model, and black patients were considerably younger than white patients. Thus, to further examine the effect of age on the lower prevalence of AF in black patients, we performed an age‐stratified analysis of AF prevalence in black patients compared with white patients. At every age group, black patients had a significantly lower prevalence of AF relative to white patients (Appendix A).
Table 2.
Characteristics Associated With Atrial Fibrillation
| Characteristic | OR (95% CI) | P value |
|---|---|---|
| Age (per 10‐y increase) | 1.40 (1.38 to 1.42) | <0.001 |
| Sex (female vs male) | 0.82 (0.79 to 0.85) | <0.001 |
| Race (black vs white) | 0.52 (0.48 to 0.55) | <0.001 |
| Medical history | ||
| Anemia | 1.00 (0.97 to 1.03 | 0.895 |
| CVA/TIA | 1.26 (1.20 to 1.32) | <0.001 |
| Depression | 0.96 (0.92 to 1.00) | 0.078 |
| Diabetes | 0.87 (0.84 to 0.89) | <0.001 |
| Dialysis | 0.77 (0.71 to 0.84) | <0.001 |
| Heart failure | 1.43 (1.38 to 1.48) | <0.001 |
| Hyperlipidemia | 1.00 (0.97 to 1.04) | 0.767 |
| Hypertension | 1.02 (0.98 to 1.06) | 0.337 |
| ICD | 1.11 (1.05 to 1.18) | <0.001 |
| Ischemic heart disease | 0.85 (0.82 to 0.88) | <0.001 |
| Pacemaker | 1.70 (1.63 to 1.78) | <0.001 |
| Peripheral vascular disease | 0.96 (0.93 to 1.00) | 0.046 |
| Pulmonary | 1.15 (1.10 to 1.19) | <0.001 |
| Renal insufficiency | 0.96 (0.92 to 1.00) | 0.080 |
| Smoker | 0.69 (0.67 to 0.72) | <0.001 |
| Vital signs | ||
| Heart rate at admission (per 10‐unit increase) | 1.10 (1.09 to 1.11) | <0.001 |
| Systolic BP at admission (per 10‐unit increase) | 0.92 (0.92 to 0.93) | <0.001 |
| Hospital variables | ||
| Hospital bed size (per 500 units) | 1.16 (1.04 to 1.29) | 0.006 |
| Hospital teaching/residents | 1.07 (0.97 to 1.18) | 0.157 |
| Hospital region: west vs northeast | 0.88 (0.76 to 1.01) | 0.072 |
| Hospital region: south vs northeast | 0.75 (0.66 to 0.84) | <0.001 |
| Hospital region: midwest vs northeast | 0.86 (0.75 to 0.97) | 0.018 |
BP indicates blood pressure; CVA, cerebrovascular accident; ICD, implantable cardioverter‐defibrillator; OR, odds ratio; TIA, transient ischemic attack.
Hospital Outcomes and Warfarin Use Among Patients Hospitalized for HF
After adjusting for patient and hospital factors, black race was not significantly associated with lower in‐hospital mortality (aOR 0.79, 95% CI 0.61 to 1.02, P=0.08). However, black race was associated with a longer LOS (aOR 1.04, 95% CI 1.01 to 1.07, P=0.007) (Table 3). Among eligible patients with AF hospitalized for HF and no contraindication to warfarin therapy (pregnancy, excess bleeding or fall risk, allergy to warfarin, or other documented contraindications), 68.6% were discharged on warfarin. Adjusting for patient and hospital factors, black patients were less likely to be prescribed warfarin at discharge (aOR 0.76, 95% CI 0.69 to 0.85, P<0.0001) (Table 3). We assessed the interaction between race and gender and its effect on warfarin use at discharge. The interaction between race and gender was significant (aOR 1.17, 95% CI 1.02 to 1.35, P=0.02), driven by white men having a greater odds of warfarin prescription at discharge relative to white women. Both black men and women hospitalized for HF with AF were less likely to be discharged on warfarin than were their white counterparts (women: aOR 0.83, 95% CI 0.74 to 0.93; men: aOR 0.71, 95% CI 0.62 to 0.80).
Table 3.
Hospital Outcomes by Race
| Clinical Outcomes | Black Patients | White Patients | Adjusted OR, 95% CI | P value |
|---|---|---|---|---|
| Black vs white | ||||
| In‐hospital mortality, % | 2.6 | 4.1 | 0.79 (0.61 to 1.02) | 0.076 |
| LOS,* d | 5 (3 to 8) | 4 (3 to 7) | 1.04 (1.01 to 1.07)* | 0.007 |
| Warfarin use at discharge, % | 65.0 | 69.1 | 0.76 (0.69 to 0.85) | <0.001 |
Adjusted for patient and hospital characteristics. LOS indicates length of stay; OR, odds ratio reported for outcomes in‐hospital mortality and warfarin use at discharge.
Median (25th, 75th percentile).
Multivariable regression model analyzed logarithm transformed LOS, and adjusted ratio of LOS was reported.
Warfarin Use and Risk Stratification in Patients Hospitalized for HF
The American College of Cardiology (ACC)/American Heart Association (AHA)/Physician Consortium AF and Atrial Flutter Performance Measures10 call for assessment and documentation of thromboembolic risk factors. Thus, we examined the relationship between risk factors for stroke and prescription of warfarin at discharge using the CHADS2 scheme.11 The mean CHADS2 score for the overall AF population was 3.1. Black patients (2.96) had a statistically significant but not clinically relevant lower mean CHADS2 score than white patients (3.07, P<0.0001) hospitalized with HF. Ninety‐four percent of white patients and 93% of black patients had a CHADS2 score ≥2. Adjusting for CHADS2 score and patient and hospital factors, black patients were less likely than white patients to be prescribed warfarin at discharge (aOR 0.76, 95% CI 0.69 to 0.85, P<0.0001). The higher prescription of warfarin at discharge was present and significant in patients with CHADS2 scores 1 to 5 (Table 4). As seen in Figure 1, among white patients, there was a risk treatment mismatch in that higher CHADS2 score was associated with lower prescription of warfarin at discharge. This mismatch was not as evident in black patients.
Table 4.
Anticoagulation Use by CHADS2 Score Stratified by Race
| Variable | Adjusted OR, 95% CI | P value |
|---|---|---|
| Black vs white | ||
| CHADS2=1 | 0.61 (0.44 to 0.86) | 0.005 |
| CHADS2=2 | 0.70 (0.60 to 0.82) | <0.001 |
| CHADS2=3 | 0.77 (0.67 to 0.89) | <0.001 |
| CHADS2=4 | 0.77 (0.62 to 0.96) | 0.022 |
| CHADS2=5 | 0.69 (0.54 to 0.89) | 0.003 |
| CHADS2=6 | 1.07 (0.67 to 1.68) | 0.786 |
Adjusted for patient and hospital characteristics. CHADS2 indicates congestive heart failure, hypertension, age >75, diabetes mellitus, prior stroke or transient ischemic attack; OR, odds ratio.
Figure 1.
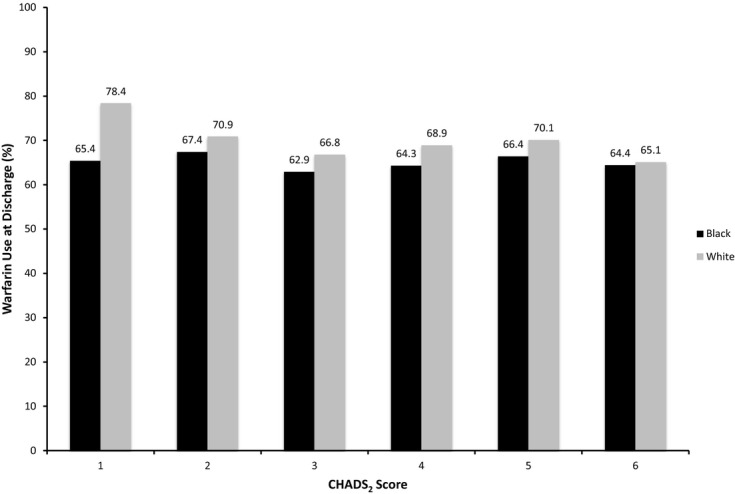
Warfarin use at discharge by race and CHADS2 score. Warfarin use at discharge among patients hospitalized for HF with AF as a function of race. AF indicates atrial fibrillation; CHADS2, congestive heart failure, hypertension, age >75, diabetes mellitus, and prior stroke or transient ischemic attack; HF, heart failure.
Combination Warfarin/Antiplatelet Therapy
In patients hospitalized for HF with AF, 16% of black and 14% of white individuals were discharged on aspirin. Triple therapy with aspirin, clopidogrel, and warfarin was infrequent and did not vary according to race. Overall, 10% of patients hospitalized for HF with AF were discharged without aspirin, clopidogrel, or warfarin (Figure 2).
Figure 2.
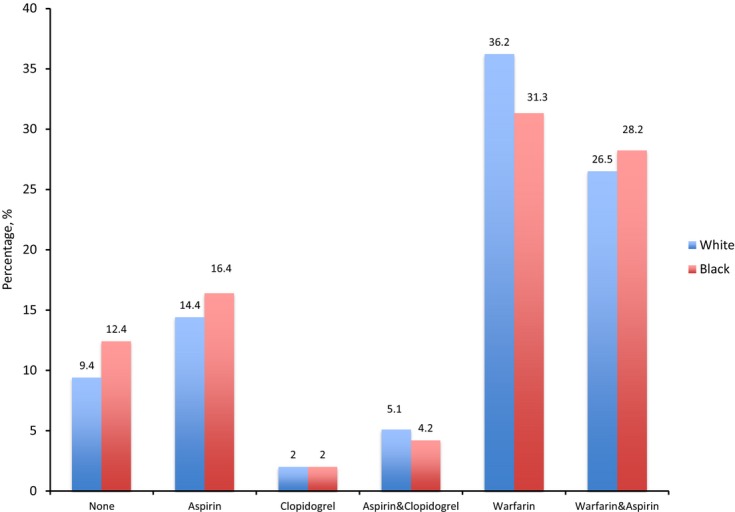
Antithrombotic therapy at discharge by race.
Discussion
Using GWTG‐HF, a large, national, multicenter clinical practice registry of patients hospitalized with HF, we examined racial differences in the prevalence, characteristics, hospital outcomes, and prescription of warfarin at discharge among patients with AF. Our study has the following major findings. First, black patients hospitalized for HF had a significantly lower prevalence of AF than white patients. Black patients with AF have different clinical characteristics compared with white patients. Despite having a different clinical profile and longer LOS, black patients hospitalized for HF with AF have similar in‐hospital mortality relative to white patients. Finally, despite national guideline recommendations and an ACC/AHA performance measure for anticoagulation at discharge, warfarin use remains suboptimal and there are significant racial disparities in the prescription of warfarin at discharge among at‐risk eligible patients hospitalized for HF.
A large HF population offers a unique opportunity to assess the prevalence of AF by race. Our finding of 52% lower odds of AF in black patients with HF compared with white patients represents one of the largest reported racial differences in the prevalence of AF. Except for age, black patients in our analysis had higher rates of risk factors for AF such as hypertension, diabetes mellitus, COPD or asthma, HF, and higher body mass indices. Traditional AF risk factors have largely been derived from racially/ethnically homogeneous populations and may not be generalizable. Moreover, while risk factors for AF are well known, they do not explain all the attributable risk.12–13 The emerging field of AF genetics may provide insight into AF risk. A recent study found that European genetic ancestry increased AF risk among blacks in 2 different cohorts.14 Based on the hypothesis that racial differences in AF prevalence may involve genetic factors, Schnabel et al15 conducted a widespread gene analysis in white individuals and African Americans to identify common variants (single nucleotide polymorphisms [SNPs]) associated with AF. SNP rs4845625 in the IL6R gene was associated with AF in white patients but did not reach statistical significance in African American patients.15 The known chromosome 4 locus near PITX2 in whites also was associated with AF in African Americans. This study should be considered hypothesis generating and provide the rational for including African American patients in additional genetic analyses associated with AF. Whether genetic, environmental, or a combination, there are likely protective mechanisms in blacks or factors that increase AF susceptibility in white patients.
To our knowledge, this is the first analysis to specifically assess racial differences in hospital outcomes for patients hospitalized for HF with AF. AF is associated with higher mortality and longer hospitalizations among patients hospitalized for HF.16 We found that among individuals hospitalized for HF, black patients with AF had a trend toward lower in‐hospital mortality compared with white patients and had a longer LOS than whites independent of hospital characteristics or comorbid conditions. The rising costs associated with hospitalizations for AF and HF make decreasing LOS while reducing rehospitalization rates an important hospital performance indicator.
Patients with HF and AF, as evidenced by the recommendation for anticoagulation from the Heart Failure Society of America and ACC/AHA, are at increased risk for stroke. Despite the absence of contraindications to warfarin, all patients with HF and a disproportionately lower percentage of black patients may be less often treated with this guideline recommended therapy.17–18 Since 2005, the ACC/AHA guidelines for the management of chronic HF have advocated the use of warfarin for stroke prophylaxis in patients with paroxysmal or persistent AF (Class I recommendation; Level of evidence A).19–20 After adjusting for patient and hospital factors, black patients in our study remained 24% less likely to receive warfarin at discharge compared with white patients. The Reasons for Geographical Differences in Stroke Study (REGARDS) investigators assessed AF awareness and warfarin use in 432 individuals (88 black individuals). Compared with white patients, black patients were less aware of their AF diagnosis and treated less often with Warfarin (OR 0.28, 95% CI 0.13 to 0.60).18
Similar to other studies, we observed a risk paradox with low rates of warfarin use among patients at highest risk for stroke based on the CHADS2 index.17,21 In the most recent update to the American College of Chest Physician Evidence‐Based Clinical Practice Guidelines for managing AF, intermediate (CHADS2 1) and high risk (CHADS2 ≥2) individuals should be treated with oral anticoagulation (Grade 1B and 1A, respectively).22 Despite a median CHADS2 score of 3.0 and 93% of black patients with CHADS2 score ≥2, only 65% were prescribed warfarin for AF. Given the significant risk of thromboembolic stroke associated with AF, the lower prescription of warfarin among eligible black patients is concerning and warrants further exploration. A potential explanation for this disparity is the concern for intracranial hemorrhage (ICH) among blacks. Black patients have a 2‐fold higher risk of stoke relative to white patients23 partially attributable to ischemic strokes. ICH accounts for 20% to 30% of strokes in black patients and only 10% to 15% in white patients.24 Moreover, black patients hospitalized for HF with AF treated with warfarin are at higher risk of ICH than similarly treated white patients.23 Warfarin decreases the risk of thromboembolic stroke by 50% to 60% at the expense of doubling the risk of ICH. Additionally, black patients in GWTG‐HF had higher BP on admission and were more often discharged with suboptimal BP control (Appendix *). BP remains one of the strongest independent predictors for ICH. This ideology may have contributed to the disparity yet is unlikely to explain it fully. In most instances, the risk of thromboembolic stroke associated with AF outweighs that of ICH.25–26 The role of patient preference as an explanation for racial differences in anticoagulation is unknown and should be explored in future analyses. These issues notwithstanding, this disparity in the context of a quality improvement initiative suggests the need for more intensive investigation to understand the reasons for lower anticoagulation in blacks and solutions to provide higher‐quality equitable care.
Limitations
Although the GWTG‐HF registry provides detailed data to assess the characteristics and treatment patterns of patients hospitalized with HF, our analysis has several limitations. Data were obtained from patients enrolled in a voluntary quality improvement initiative, and the characteristics and treatment patterns may not reflect those of the general HF population with AF not cared for in these hospitals. However, findings from other HF registries and analyses suggest that participants in quality improvement registries have similar characteristics and in‐hospital outcomes to that of nonparticipating individuals and that data from this registry may be representative of data on a national level.27 Although we controlled for many variables in our analyses, measured and unmeasured residual confounding may remain, such as those related to bleeding risk. We had no longitudinal data to ascertain how the overall low prescription of warfarin at discharge and the racial disparity affected patient outcomes. Finally, there were no direct measures of socioeconomic status or bias in GWTG‐HF; thus, the contribution of certain elements affecting similar or disparate health care can only be inferred but not proved with these data. This study was not a prospective randomized trial, and residual measured and unmeasured confounders may have influenced outcomes.
Conclusions
Black patients hospitalized for HF have many risk factors for AF but have a lower prevalence of AF compared with white patients. Black and white patients with HF and AF had similar in‐hospital outcomes. Overall, guideline recommended warfarin for patients with AF remains inadequate, and black patients at significant thromboembolic risk were less often prescribed warfarin at discharge than whites. Future studies should continue to explore explanations for the lower prevalence of AF in black patients and examine policies that can both increase oral anticoagulation for all indicated patients and eliminate racial disparities in anticoagulation use.
Sources of Funding
The Get With The Guidelines‐Heart Failure (GWTG‐HF) program is provided by the American Heart Association. GWTG‐HF has been funded in the past through support from Medtronic, GlaxoSmithKline, Ortho‐McNeil, and the American Heart Association Pharmaceutical Roundtable.
Disclosures
The following relationships exist related to this presentation: Dr Thomas reports research support from American Heart Association (modest), consulting for Boston Scientific (modest), Janssen Pharmaceuticals (modest) Sanofi Aventis (modest). For Drs Piccini, Peterson, and Hernandez, see those reported at http://www.dcri.org/about-us/conflict-of-interest. Dr Fonarow reports research support from the NHLBI (significant) AHRQ (significant), consulting for Novartis (significant), Medtronic (modest), and Gambro (modest). Dr Yancy is on the Patient Centered Outcomes Research Institute selection committee.
Appendix A.
Racial difference in atrial fibrilation prevalence, age‐adjusted
| Variable | AF Prevalence | Adjusted OR (95% CI) | P Value | |
|---|---|---|---|---|
| Black | White | |||
| Black vs white | ||||
| Age <60 y | 13.2 | 22.1 | 0.57 (0.51 to 0.64) | <0.001 |
| 60 to 69 y | 22.1 | 35.6 | 0.54 (0.49 to 0.60) | <0.001 |
| 70 to 79 y | 27.9 | 46.5 | 0.49 (0.45 to 0.52) | <0.001 |
| 80 to 89 y | 33.1 | 53.5 | 0.47 (0.42 to 0.53) | <0.001 |
| ≤90 y | 29.1 | 53.7 | 0.37 (0.31 to 0.44) | <0.000 |
Appendix B.
Quality of heart failure Care as a function of race
| Heart Failure Quality Metrics | White Patients (n=105 204) | Black Patients (n=30 290) | P Value |
|---|---|---|---|
| Performance measures | |||
| Discharge instructions | 90.5 | 91.6 | 0.017 |
| Smoking history and cessation counseling | 96.6 | 97.0 | 0.512 |
| Anticoagulation for atrial fibrillation | 69.1 | 65.0 | <0.001 |
| Blood pressure <140/90 at discharge | 81.3 | 78.3 | <0.001 |
| Aldosterone antagonist for LVSD at discharge | 29.4 | 31.4 | 0.036 |
| Hydralazine/isordil for blacks at discharge | — | 25.1 | — |
| ICD counseling or ICD placed/prescribed at discharge for ejection fraction ≤35% | 50.4 | 53.2 | 0.019 |
| Achievement measures | |||
| ACEI/ARB for LVSD | 90.2 | 91.9 | 0.009 |
| Evidence‐based β‐blocker for LVSD | 74.2 | 77.6 | <0.001 |
| Ejection fraction assessment | 98.0 | 99.0 | <0.001 |
Acknowledgments
The authors Adrian Hernandez and Gregg Fonarow had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
References
- 1.Roger VL, Go AS, Lloyd‐Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012; 125:e2-e220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yancy CW. Heart failure in African Americans. Am J Cardiol. 2005; 96:3i-12i [DOI] [PubMed] [Google Scholar]
- 3.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African‐Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009; 158:111-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borzecki AM, Bridgers DK, Liebschutz JM, Kader B, Kazis LE, Berlowitz DR. Racial differences in the prevalence of atrial fibrillation among males. J Natl Med Assoc. 2008; 100:237-245 [DOI] [PubMed] [Google Scholar]
- 5.Bush D, Martin LW, Leman R, Chandler M, Haywood LJ. Atrial fibrillation among African Americans, hispanics and caucasians: clinical features and outcomes from the AFFIRM trial. J Natl Med Assoc. 2006; 98:330-339 [PMC free article] [PubMed] [Google Scholar]
- 6.LaBresh KA, Ellrodt AG, Gliklich R, Liljestrand J, Peto R. Get with the guidelines for cardiovascular secondary prevention: pilot results. Arch Intern Med. 2004; 164:203-209 [DOI] [PubMed] [Google Scholar]
- 7.Hernandez AF, Fonarow GC, Liang L, Al‐Khatib SM, Curtis LH, LaBresh KA, Yancy CW, Albert NM, Peterson ED. Sex and racial differences in the use of implantable cardioverter‐defibrillators among patients hospitalized with heart failure. JAMA. 2007; 298:1525-1532 [DOI] [PubMed] [Google Scholar]
- 8.Piccini JP, Hernandez AF, Dai D, Thomas KL, Lewis WR, Yancy CW, Peterson ED, Fonarow GC. Use of cardiac resynchronization therapy in patients hospitalized with heart failure. Circulation. 2008; 118:926-933 [DOI] [PubMed] [Google Scholar]
- 9.Zeger SL, Liyang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988; 44:1049-1060 [PubMed] [Google Scholar]
- 10.Estes NA, III, Halperin JL, Calkins H, Ezekowitz MD, Gitman P, Go AS, McNamara RL, Messer JV, Ritchie JL, Romeo SJ, Waldo AL, Wyse DG, Bonow RO, DeLong E, Goff DC, Jr, Grady K, Green LA, Hiniker A, Linderbaum JA, Masoudi FA, Pina IL, Pressler S, Radford MJ, Rumsfeld JS. ACC/AHA/Physician Consortium 2008 clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and the Physician Consortium for Performance Improvement (Writing Committee to Develop Clinical Performance Measures for Atrial Fibrillation) developed in collaboration with the Heart Rhythm Society. Circulation. 2008; 117:1101-1120 [DOI] [PubMed] [Google Scholar]
- 11.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA. 2001; 285:2864-2870 [DOI] [PubMed] [Google Scholar]
- 12.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994; 271:840-844 [PubMed] [Google Scholar]
- 13.Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001; 37:371-378 [DOI] [PubMed] [Google Scholar]
- 14.Marcus GM, Alonso A, Peralta CA, Lettre G, Vittinghoff E, Lubitz SA, Fox ER, Levitzky YS, Mehra R, Kerr KF, Deo R, Sotoodehnia N, Akylbekova M, Ellinor PT, Paltoo DN, Soliman EZ, Benjamin EJ, Heckbert SR. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010; 122:2009-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnabel RB, Kerr KF, Lubitz SA, Alkylbekova EL, Marcus GM, Sinner MF, Magnani JW, Wolf PA, Deo R, Lloyd‐Jones DM, Lunetta KL, Mehra R, Levy D, Fox ER, Arking DE, Mosley TH, Muller‐Nurasyid M, Young TR, Wichmann HE, Seshadri S, Farlow DN, Rotter JI, Soliman EZ, Glazer NL, Wilson JG, Breteler MM, Sotoodehnia N, Newton‐Cheh C, Kaab S, Ellinor PT, Alonso A, Benjamin EJ, Heckbert SR. Large‐scale candidate gene analysis in whites and African Americans identifies IL6R polymorphism in relation to atrial fibrillation: the National Heart, Lung, and Blood Institute's Candidate Gene Association Resource (CARE) project. Circ Cardiovasc Genet. 2011; 4:557-564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mountantonakis SE, Grau‐Sepulveda MV, Bhatt DL, Hernandez AF, Peterson ED, Fonarow GC. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: an analysis of Get With the Guidelines–Heart Failure. Circ Heart Fail. 2012; 5:191-201 [DOI] [PubMed] [Google Scholar]
- 17.Piccini JP, Hernandez AF, Zhao X, Patel MR, Lewis WR, Peterson ED, Fonarow GC. Quality of care for atrial fibrillation among patients hospitalized for heart failure. J Am Coll Cardiol. 2009; 54:1280-1289 [DOI] [PubMed] [Google Scholar]
- 18.Meschia JF, Merrill P, Soliman EZ, Howard VJ, Barrett KM, Zakai NA, Kleindorfer D, Safford M, Howard G. Racial disparities in awareness and treatment of atrial fibrillation: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010; 41:581-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). Circulation. 2005; 112:e154-e235 [DOI] [PubMed] [Google Scholar]
- 20.Bonow RO, Bennett S, Casey DE, Jr, Ganiats TG, Hlatky MA, Konstam MA, Lambrew CT, Normand SL, Pina IL, Radford MJ, Smith AL, Stevenson LW, Bennett SJ, Burke G, Eagle KA, Krumholz HM, Linderbaum J, Masoudi FA, Ritchie JL, Rumsfeld JS, Spertus JA. ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures) endorsed by the Heart Failure Society of America. Circulation. 2005; 112:1853-1887 [DOI] [PubMed] [Google Scholar]
- 21.Sandhu RK, Bakal JA, Ezekowitz JA, McAlister FA. Risk stratification schemes, anticoagulation use and outcomes: the risk‐treatment paradox in patients with newly diagnosed non‐valvular atrial fibrillation. Heart. 2011; 97:2046-2050 [DOI] [PubMed] [Google Scholar]
- 22.You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, Hylek EM, Schulman S, Go AS, Hughes M, Spencer FA, Manning WJ, Halperin JL, Lip GY. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012; 141:e531S-e575S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007; 50:309-315 [DOI] [PubMed] [Google Scholar]
- 24.Falk RH. Ethnic disparity in intracranial hemorrhage among anticoagulated patients with atrial fibrillation: an answer in search of a question? J Am Coll Cardiol. 2007; 50:316-318 [DOI] [PubMed] [Google Scholar]
- 25.Fang MC, Go AS, Hylek EM, Chang Y, Henault LE, Jensvold NG, Singer DE. Age and the risk of warfarin‐associated hemorrhage: the anticoagulation and risk factors in atrial fibrillation study. J Am Geriatr Soc. 2006; 54:1231-1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogacka R, Chieffo A, Michev I, Airoldi F, Latib A, Cosgrave J, Montorfano M, Carlino M, Sangiorgi GM, Castelli A, Godino C, Magni V, Aranzulla TC, Romagnoli E, Colombo A. Dual antiplatelet therapy after percutaneous coronary intervention with stent implantation in patients taking chronic oral anticoagulation. JACC Cardiovasc Interv. 2008; 1:56-61 [DOI] [PubMed] [Google Scholar]
- 27.Curtis LH, Greiner MA, Hammill BG, DiMartino LD, Shea AM, Hernandez AF, Fonarow GC. Representativeness of a national heart failure quality‐of‐care registry: comparison of OPTIMIZE‐HF and non‐OPTIMIZE‐HF medicare patients. Circ Cardiovasc Qual Outcomes. 2009; 2:377-384 [DOI] [PMC free article] [PubMed] [Google Scholar]


