Abstract
Background
With adoption of telemedicine, physicians are increasingly asked to diagnose ST‐segment elevation myocardial infarctions (STEMIs) based on electrocardiograms (ECGs) with minimal associated clinical information. We sought to determine physicians' diagnostic agreement and accuracy when interpreting potential STEMI ECGs.
Methods and Results
A cross‐sectional survey was performed consisting of 36 deidentified ECGs that had previously resulted in putative STEMI diagnoses. Emergency physicians, cardiologists, and interventional cardiologists participated in the survey. For each ECG, physicians were asked, “based on the ECG above, is there a blocked coronary artery present causing a STEMI?” The reference standard for ascertaining the STEMI diagnosis was subsequent emergent coronary arteriography. Responses were analyzed with generalized estimating equations to account for nested and repeated measures. One hundred twenty‐four physicians interpreted a total of 4392 ECGs. Among all physicians, interreader agreement (kappa) for ECG interpretation was 0.33, reflecting poor agreement. The sensitivity to identify “true” STEMIs was 65% (95% CI: 63 to 67) and the specificity was 79% (95% CI: 77 to 81). There was a 6% increase in the odds of accurate ECG interpretation for every 5 years of experience since medical school graduation (OR 1.06, 95% CI: 1.02 to 1.10, P=0.01). After adjusting for experience, there was no significant difference in the odds of accurate interpretation by specialty—Emergency Medicine (reference), General Cardiology (AOR 0.97, 95% CI: 0.79 to 1.2, P=0.80), or Interventional Cardiology physicians (AOR 1.24, 95% CI: 0.93 to 1.7, P=0.15).
Conclusions
There is significant physician disagreement in interpreting ECGs with features concerning for STEMI. Such ECGs lack the necessary sensitivity and specificity to act as a suitable “stand‐alone” diagnostic test.
Keywords: electrocardiogram, myocardial infarction, quality, telemedicine
Introduction
Timely reperfusion therapy for ST‐segment elevation myocardial infarction (STEMI) is critically important for myocardial salvage and improved survival.1–2 A number of strategic recommendations have been proposed to reduce delays in STEMI treatment with percutaneous coronary intervention (PCI).3–4 Notably, an emphasis on rapid diagnosis and triage of STEMI patients may lead increasingly to ECG interpretations without the benefit of robust or first‐hand clinical information, a process whose diagnostic accuracy is uncertain. Such scenarios include the use of telemedicine as a means to triage patients to designated heart centers or to activate cardiac catheterization teams without evaluation in the emergency department (ED).
Reported rates of clinical STEMI misdiagnoses (ie, “false positive” STEMI diagnoses) range from 15% to 36%.5–7 A modest rate of false positive misdiagnoses is expected in order to maximize diagnostic sensitivity, but frequent false positive activations of cardiac catheterization teams may erode trust between responsible physicians and over‐extend human and financial resources. Conversely, false negative interpretations of STEMI ECGs may harm patients. The extent to which STEMI misdiagnoses may be attributable to difficulty with interpretation of the inciting ECG is unclear, and physician accuracy in interpreting potential STEMI ECGs is unknown. Data from a statewide registry suggest that 11% of all STEMI activations are cancelled prior to angiography following secondary evaluation of the putative STEMI ECG8 and this does not account for the patients who went on to angiography but ultimately lacked a culprit artery occlusion.
In this study, we sought to determine the interreader agreement and accuracy of physicians' interpretations of potential STEMI ECGs when scant clinical data are available. Secondarily, in order to test the hypothesis that physician proficiency may be a key factor in accurate ECG interpretation, we also assessed the impact of physician specialty and experience in this process. These data may help in devising systems of care for potential STEMI patients and effective use of novel telemedicine technologies.
Methods
Data Source
The Activate‐SF registry is a registry of clinical STEMI diagnoses made by emergency physicians at a tertiary care and a county trauma center with prospective ascertainment of outcomes. Details of this registry have been described elsewhere.5
Thirty‐six cases from the registry were selected from the database via a random number generator to serve as the basis for our study (Data S1). Patients whose inciting STEMI diagnosis ECGs demonstrated isolated left bundle branch block or predominant ventricular rhythms were excluded as were patients who did not undergo emergent angiography, which served as the reference standard for accurate versus inaccurate STEMI diagnosis. The total number of ECGs to be included in this study was established through preliminary polling and subsequent pilot tests of 15 cardiologists and emergency physicians regarding the number of ECGs each would be willing to read at one time without compensation. Emergency coronary arteriography revealed 12 (33%) of the 36 cases that formed the basis of this study had no culprit lesion present and had Thrombolysis In Myocardial Infarction (TIMI) grade III flow in all coronary arteries, consistent with a lack of STEMI. The corresponding overall prevalence of “false positive” STEMI diagnoses in the Activate‐SF registry at that time was similarly 36%.5
All ECGs were standard 12 lead tracings obtained using Philips Pagewriter machines (Philips) at 25 mm paper speed. The survey ECG images were directly downloaded in electronic format and batch processed to ensure uniform quality. All ECGs were previously deidentified and over‐read for 12 key characteristics in blinded fashion by 2 cardiologists (E.J.A. and K.S.H.) with adjudication by a third cardiologist (J.M.M.) for disagreement beyond prespecified boundaries per registry protocols.
Physician Participants
Physicians were recruited to participate in this study from a cross‐section of specialties that regularly manage STEMI patients. Accordingly, study participants were recruited from Interventional Cardiology, Noninvasive/General Cardiology, Emergency Medicine, and Internal Medicine (provided they worked in an Urgent Care or ED setting). Participation was encouraged by “team leaders” identified within each specialty. No remuneration was offered for participation. All responses were anonymously submitted and reviewed in the aggregate. Access to the study survey was provided through a generic internet link and rates of participation were not tracked. Responses were collected electronically using an internet‐based survey (Survey Monkey).
Each participating physician was provided with the same 36 ECGs. The survey instructions included the introductory statement: “We recognize that activation of the cardiac catheterization team is a clinical decision; please focus on whether or not the ECG in question meets your diagnostic criteria for an acutely blocked coronary artery (a STEMI).” Each ECG was then followed by a single question: “Based on the ECG above, is there a blocked coronary artery present causing a STEMI? (please provide your best guess).” No clinical details were provided for individual cases and all were described as a “patient with moderate risk of acute coronary syndrome” to isolate ECG interpretation characteristics from the heterogeneous process of risk stratification and assignment of pretest probability.9–10 Respondents were not informed that each ECG was obtained from a patient referred for emergent angiography.
Definitions
We defined a “false positive” ECG as one from a patient who lacked a thrombotic coronary artery occlusion and had TIMI grade III blood flow in all coronary arteries. A “true‐positive” STEMI ECG was one from a patient who had either a thrombotic coronary occlusion or less than TIMI III blood flow. Of note, the median ECG‐to‐angiography time for all cases that form the basis of this study was 49 minutes (interquartile range [IQR] 33 to 68 minutes). Left ventricular hypertrophy criteria were defined previously.11 ST‐segment elevation (STE) was defined as J‐point elevation in 2 or more contiguous leads of 2 mm or more in leads V1, V2, or V3 or 1 mm or more in other leads.12 A computer‐based STEMI diagnosis was recorded if the ECG header listed a diagnosis of “Acute Myocardial Infarction” based on Phillips' proprietary algorithm.
Statistical Analysis
Using the binary outcome of accurate versus inaccurate ECG interpretation, we first calculated the sensitivity, specificity, positive predictive values (PPV), negative predictive values (NPV), and their 95% CIs for our respondents' ECG interpretations of the 24 “true” and 12 “false” STEMI ECGs. These analyses were repeated following stratification of the cohort based on respondent level of training and training discipline. Kappa statistics were also calculated for the degree of interobserver agreement in ECG interpretation and compared using an inverted modified Wald test approach.13 Receiver operating characteristic (ROC) curves were based on the binary outcome of accurate versus inaccurate ECG interpretation and applied to the same respondent classification schemes. To compare the effects of reader characteristics (length and discipline of experience) and ECG characteristics (average height of STE, number of leads with diagnostic STE, territory with maximal STE) on accuracy of interpretation, we created serial univariate models using log‐binomial generalized estimating equations (GEEs) to account for nested and repeated measures per respondent. Multivariate models were also used to adjust for baseline differences in length of experience between cardiologists and noncardiologists. We then performed a sensitivity analysis repeating our calculations following exclusion of ECGs originally obtained from patients not having a STEMI (ie, the 12 “false” STEMI ECGs).
For tabular data, simple comparisons were performed with t Tests or Wilcoxon ranksum tests for normally and nonnormally distributed data respectively. Continuous variables are presented as means±standard deviations (SD) or median values and IQRs for nonnormally distributed variables. A 2‐tailed P<0.05 was considered statistically significant. All statistical analyses were performed with Stata version 11.
Results
One hundred twenty‐four physicians interpreted an average 35.4 of 36 ECGs (total ECG interpretations: 4392). Fifty‐two (42%) of participants were fully licensed physicians board‐certified in their area of specialty (“attendings”), 33 (27%) were cardiology or emergency medicine fellows, and 37 (30%) were internal medicine or emergency medicine residents (2 respondents did not provide level of experience). Participant distribution by specialty is listed in Table 1. The median interval since medical school graduation (“experience”) among all participants was 7 years (IQR 3 to 13, range 1 to 57 years). Noncardiology participants tended to have less experience than participants within cardiology (median 3 versus 8 years since medical school graduation, P<0.01), although this was not true when limited to attending physicians (median 14 versus 17 years, P=0.09). There was no significant difference in the number of cardiologists and noncardiologists who spent less than 50% of their time on clinical work (P=0.50) (Table 1). Ninety‐two percent of participants were based at a university or teaching hospital, 5% were in private practice, and 3% worked in governmental or community centers.
Table 1.
Participants Demographics
| N | ECGs Read | Median Years Since Medical School (IQR) | >50% Time Clinical (%) | |
|---|---|---|---|---|
| All participants* | 124 | 4392 | 7 (3 to 13) | 97 (80) |
| All residents | 37 | 1332 | 2 (1 to 3) | 35 (98) |
| Medicine residents | 26 | 936 | 2 (1 to 3) | 25 (96) |
| Emergency residents | 11 | 396 | 2 (1 to 3) | 11 (100) |
| All fellows | 33 | 1188 | 6 (5 to 7) | 27 (82) |
| Emergency fellows | 3 | 108 | 4 (4 to 6) | 1 (33) |
| General cardiology fellows | 25 | 900 | 6 (5 to 8) | 21 (84) |
| Interventional cardiology fellows | 5 | 180 | 7 (6 to 7) | 5 (100) |
| All attendings | 52 | 1872 | 16 (10 to 24) | 34 (65) |
| Emergency attendings | 26 | 936 | 14 (10 to 18) | 17 (65) |
| General cardiology attendings | 17 | 612 | 20 (11 to 40) | 9 (52) |
| Interventional cardiology attendings | 9 | 324 | 17 (12 to 19) | 8 (89) |
ECG indicates electrocardiogram; IQR, 25th to 75th interquartile range.
Two participants did not provide their current level of training.
Table 2 outlines the survey results by training level and specialty. Among all participants, interreader agreement (kappa) for ECG interpretation was 0.33 (where 1 is perfect agreement and −1 is perfect disagreement). Only 6 of the study ECGs (17%) had near universal (>90%) agreement in interpretation among all participants while 7 (19%) of the ECGs resulted in nearly split (40% to 60%) levels of agreement (Figure 1). The overall sensitivity to identify “true” STEMI ECGs was 65% (95% CI: 64 to 67). Participants' specificity in determining which ECGs did not represent a STEMI among those without a culprit artery occlusion was 79% (95% CI: 77 to 81). The PPV of a STEMI interpretation among all readers was 86% (95% CI: 85 to 88) and the NPV of a high‐risk ECG—the probability that a patient did not have a culprit artery occlusion when the ECG was interpreted as such—was 53% (95% CI: 51 to 55). The resultant area under the ROC curve (c), which quantifies the discrimination of readers' ECG interpretation to distinguish a “true” STEMI pattern from “false” STEMI ECG pattern (where 1 is perfect discrimination and 0.5 is no better than chance), was 0.72 (95% CI: 0.71 to 0.74).
Table 2.
Physicians' ECG Interpretation Accuracy by Specialty and Experience
| # of ECG Interpretations | Sens | Spec | PPV | NPV | Kappa | C | |
|---|---|---|---|---|---|---|---|
| Computer algorithm | 36 | 46 | 83 | 85 | 44 | n/a | 0.65 |
| All participants | 4365 | 65 | 79 | 86 | 53 | 0.33 | 0.72 |
| By training level | |||||||
| All residents | 1332 | 61 | 73 | 82 | 48 | 0.27 | 0.67 |
| All fellows | 1188 | 63 | 86 | 90 | 54 | 0.33 | 0.74 |
| All attendings | 1872 | 70 | 79 | 87 | 57 | 0.36 | 0.75 |
| By specialty | |||||||
| Interventional cardiologists | 502 | 70 | 89 | 92 | 59 | 0.42 | 0.79 |
| Non‐invasive cardiologists | 1505 | 63 | 85 | 90 | 54 | 0.41 | 0.74 |
| All cardiologists | 2007 | 65 | 86 | 90 | 55 | 0.41 | 0.75 |
| Cardiology trainees | 1080 | 63 | 87 | 90 | 54 | 0.4 | 0.75 |
| Cardiology attendings | 936 | 67 | 86 | 90 | 57 | 0.43 | 0.76 |
| Emergency physicians | 1259 | 70 | 72 | 83 | 55 | 0.3 | 0.71 |
| All non‐cardiologists | 2358 | 66 | 73 | 83 | 52 | 0.28 | 0.7 |
| Non‐cardiology trainees* | 1457 | 61 | 74 | 82 | 49 | 0.26 | 0.67 |
| Emergency med attendings | 936 | 74 | 72 | 84 | 58 | 0.35 | 0.73 |
Highest values are bolded. Attendings are physicians board‐certified in their area of specialty. C is the area under the ROC curve. ECG indicates electrocardiogram; NPV, negative predictive values; PPV, positive predictive values; ROC, receiver operating characteristic; Sens, sensitivity; Spec, specificity.
Noncardiology trainees were internal medicine and emergency medicine residents and fellows.
Figure 1.
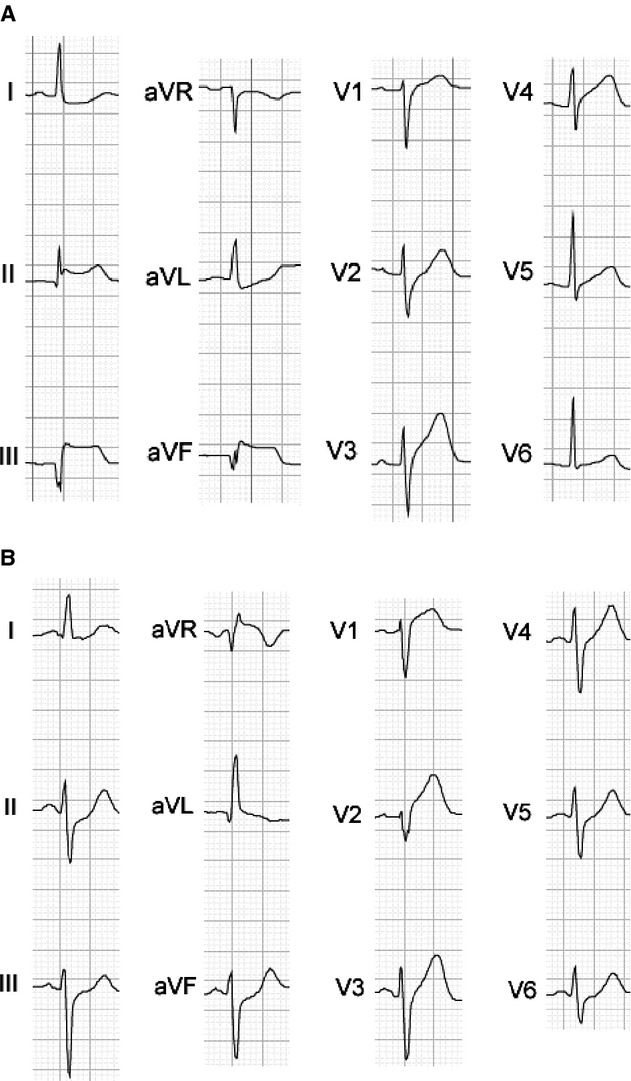
Twelve lead electrocardiographic morphologies demonstrating electrocardiograms (ECGs) with high (A) and low (B) interreader agreement. Patient A had a thrombotic occlusion of the right coronary artery. Patient B had a thrombotic occlusion of the left anterior descending coronary artery. aVF indicates augmented vector foot lead; aVL, lead augmented vector left; aVR, lead augmented vector right
Irrespective of specialty, when compared to resident physicians, fellows had a 26% greater odds of accurate ECG interpretation (OR 1.26, 95% CI: 1.02 to 1.57, P=0.03) and attending physicians had a 45% greater odds of accurate ECG diagnosis (OR 1.45, 95% CI: 1.18 to 1.77, P<0.01) (Table 3). Furthermore, among all participants there was a 6% increase in the odds of accurate ECG interpretation for every 5 years since medical school graduation (ie, “experience”) (OR 1.06, 95% CI: 1.02 to 1.10, P=0.01). After adjusting for years of physician experience, there was no significant difference in the odds of accurate interpretation between emergency medicine, general cardiology, or interventional cardiology physicians. This remained true when the analysis was restricted only to attending‐level physicians (Table 3).
Table 3.
Direct Comparisons of Participants' ECG Interpretation Accuracy for All ECGs (36) and Limited to ECGs Just From Those With Culprit Lesions on Angiography (24)
| All ECGs | True STEMI ECGs Only | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| By experience | ||||||
| Per 5 years experience* | 1.06 | 1.02 to 1.10 | 0.01 | 1.05 | 0.61 to 1.78 | 0.87 |
| Resident | Ref | — | — | Ref | — | — |
| Fellow | 1.26 | 1.02 to 1.57 | 0.03 | 1.07 | 0.84 to 1.38 | 0.56 |
| Attending | 1.45 | 1.19 to 1.77 | <0.01 | 1.42 | 1.06 to 1.89 | 0.02 |
| By specialty | ||||||
| Non‐cardiologists | Ref | — | — | Ref | — | — |
| General cardiologists | 0.97 | 0.79 to 1.2 | 0.8 | 0.91 | 0.72 to 1.14 | 0.42 |
| Interventional cardiologists | 1.24 | 0.93 to 1.67 | 0.15 | 1.2 | 0.88 to 1.62 | 0.25 |
| Attending emergency physicians | Ref | — | — | Ref | — | — |
| Attending general cardiologists | 0.91 | 0.67 to 1.23 | 0.53 | 0.77 | 0.50 to 1.20 | 0.25 |
| Attending interventional cardiologists | 1.06 | 0.73 to 1.53 | 0.77 | 0.91 | 0.57 to 1.45 | 0.69 |
CI indicates confidence interval; ECG, electrocardiogram; OR, odds ratio; STEMI, ST‐segment elevation myocardial infarction.
Experience since medical school graduation.
Sensitivity Analysis for True STEMIs Only
Since diagnosing a true‐STEMI ECG is arguably more important than correctly recognizing a concerning ECG that does not represent a STEMI, a sensitivity analysis was performed utilizing only those ECGs from patients with true STEMIs (24 cases). The overall accuracy of interpretation among true STEMIs was 66% (1899 of 2899 ECG reads were correctly diagnosed). In this setting, attending physicians continued to be more accurate than resident physicians and training discipline did not significantly affect the odds of accurate “true” STEMI interpretation after adjusting for years since medical school graduation (Table 3).
ECG Characteristics
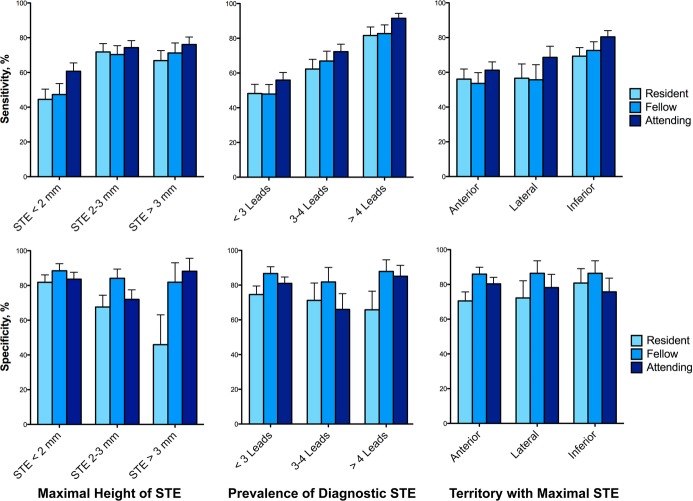
Among the 36 ECG's used for this analysis, there were no statistically significant differences in key measured components between true‐positive STEMI and false‐positive STEMI ECGs, though the height of the ST elevations, median number of leads with ST elevations, and percent without voltage criteria for left ventricular hypertrophy were numerically greater among true‐positive STEMI ECGs (Table 4). ECG reader agreement was significantly greater for inferior ST elevations than for all other territories (kappa 0.44 versus kappa 0.26, respectively; P<0.01). The sensitivity and specificity of all participants' ECG interpretations relative to specific ECG criteria are displayed in Figure 2. Among the true STEMI ECGs, the odds of accurate diagnosis were 42% greater per lead with diagnostic ST elevations (OR 1.42, 95% CI: 1.36 to 1.48, P<0.01) and 14% greater per millimeter of maximal ST elevation (OR 1.14, 95% CI: 1.09 to 1.19, P<0.01). Conversely, the presence of voltage criteria for left ventricular hypertrophy on the ECG led to a 64% reduction in odds of an accurate interpretation (OR 0.36, 95% CI: 0.30 to 0.42, P<0.01).
Table 4.
Adjudicated ECG Characteristics Stratified by Angiographic Results
| Culprit Lesion (n=24) | No Culprit Lesion (n=12) | P Value | |
|---|---|---|---|
| STE territory, % | 0.41 | ||
| Anterior | 9 (38) | 8 (66) | |
| Lateral | 4 (17) | 2 (17) | |
| Inferior | 8 (32) | 2 (17) | |
| Posterior | 3 (13) | 0 (0) | |
| Median height STE, mm (IQR) | 2.1 (1 to 4) | 1.8 (1.3 to 2.3) | 0.21 |
| Median # of leads with STE, (IQR) | 3 (2 to 4.3) | 1.8 (0.5 to 3.5) | 0.19 |
| LVH present, % | 5 (21) | 5 (42) | 0.19 |
| Sinus, no conduction block (%) | 20 (83) | 11 (92) | 0.49 |
ECG indicates electrocardiogram; IQR, 25th to 75th interquartile range; LVH, left ventricular hypertrophy; STE, ST‐segment elevation; mm, millimeters.
Figure 2.
Sensitivity and specificity of physicians' STEMI diagnosis stratified by experience and ECG characteristics. Residents, light blue bars; fellows, royal blue bars; attendings, dark blue bars. Whiskers represent one‐sided 95% CIs. ECG indicates electrocardiogram; STE, electrocardiographic ST‐segment elevations; STEMI, ST‐segment elevation myocardial infarction.
Discussion
This study demonstrates that ECGs concerning for STEMI lack the necessary sensitivity and specificity to be considered a reliable “stand‐alone” diagnostic test. The modest diagnostic accuracy and high interobserver disagreement in interpreting concerning ECGs for the presence of an acute coronary thrombotic lesion may help explain the high rates of “false positive” STEMI diagnoses recently reported by us and others.5,7,14 These data lend credibility to the notion that an increasing reliance on electrocardiograms as the sole tool for STEMI diagnoses, as may be done increasingly with telemedicine technology implementation, is associated with high levels of inaccurate diagnoses, particularly false positive STEMI diagnoses. Such findings reinforce the notion that the ECG is one of multiple modalities necessary to establish the STEMI diagnosis.
An emphasis on diagnostic sensitivity in ECG interpretation and resultant high rates of false positive errors, as suggested by this study, particularly among noncardiologists and younger providers are arguably less dangerous than false negative errors. Nevertheless, high rates of false positive diagnoses have the potential to significantly tax the human and capital resources invested in 24‐hour emergency STEMI programs and put patients at risk for unnecessary procedures. However, these data do suggest that diagnostic accuracy in interpreting potential STEMI ECGs is related to the experience of the interpreting physician; thus, targeted educational efforts may accelerate learning and help reduce unnecessary complications and health care expenditures. Of course, perfect diagnostic accuracy and a PPV of 100% is both unobtainable on a broad scale and undesirable because a certain number of “false positive” diagnoses are necessary to ensure appropriate diagnostic sensitivity.
While accuracy of physicians' interpretation does improve with physician experience, this was not true among the subgroup of “true positive” STEMI ECGs, suggesting that younger physicians are more likely to emphasize sensitivity. As compared to cardiologists, noncardiologists were also more likely to emphasize sensitivity with a corresponding decrement in specificity. However, in multivariable analyses, the odds of an accurate diagnosis of coronary artery occlusion were not significantly different based on specialty training. Among all physicians, the maximal height of the ST elevations, the number of leads with diagnostic ST elevations, and the lack of left ventricular hypertrophy all increased the odds of an accurate “true positive” STEMI diagnosis.
Improvements in telecommunication technologies and an increasing acceptance of telemedicine15 have led to a growing interest in remote prehospital STEMI diagnoses as a mechanism for expediting time to reperfusion.16–17 Such prehospital STEMI diagnoses allow for appropriate triage to designated heart centers and/or direct transport to the cardiac catheterization laboratory without evaluation in the ED.18–21 Implementation of telemedicine technologies is thus an extremely powerful and valuable process. However, remote STEMI diagnoses confirmed by ED physicians or cardiologists through telecommunication systems still rely largely on accurate interpretation of concerning ECGs from at‐risk patients with scant additional diagnostic information available. While prior small analyses have suggested that electronic transmission of prehospital ECGs to emergency physicians or cardiologists may improve the specificity of out‐of‐hospital STEMI diagnoses,22–23 two small observation studies of 7 and 15 interventional cardiologists respectively both demonstrated significant heterogeneity in interpretation of potential‐STEMI ECGs.24–25 Our data shed further light on the potential limitations of telemedicine strategies that rely predominantly on ECG interpretation for establishing the STEMI diagnosis. As such, these data may be useful in designing STEMI care systems that continue to leverage the advantages of telemedicine to improve patient care while recognizing the inherent limitations in diagnostic accuracy that may be associated with such technologies.
Given the implications of our data on improving regional primary PCI systems, we chose to evaluate the accuracy of physicians' ECG interpretations using the subsequent angiogram as the reference standard. One could choose, instead, to evaluate the appropriateness of the physicians' determinations relative to published criteria for electrocardiographic evidence of a myocardial infarction.12,26 However, the impetus for this study sprung largely from the notion that categorization of ECGs into dichotomous STEMI and not‐STEMI groups is often over‐simplified. This notion has importance particularly in respect to appropriateness criteria for STEMI team activation protocols. Many analyses of STEMI team activations categorize electrocardiographic ST segment elevations as a binary variable—present or not present. Such dichotomies fail to capture the graded nature of ST‐segment elevations and may grossly oversimplify the challenging task of diagnosing true STEMI patients from the much larger cohort of at‐risk patients presenting with chest pain or equivalent symptoms.27 Varying degrees of electrocardiographic ST segment elevation in the absence of culprit coronary artery lesions have been previously described5,12 and, notably, in this study the median height of the ST‐segment elevations among ECGs from patients without culprit lesions on angiography was 1.8 mm above the isoelectric T‐P segment. While these data speak to the difficult nature of discerning accurate from inaccurate STEMI diagnoses on the basis of ECGs alone, they also suggest that considering electrocardiographic ST elevations as a dichotomous variable for the purposes of catheterization activation protocols or appropriateness analyses may be insufficiently discerning.
This study has a number of strengths. Each ECG is from a real STEMI team activation and each corresponding patient underwent diagnostic angiography, which provides a reference standard. Furthermore, we successfully recruited 124 physicians into this study and had very high rates of study completion. This study also has some inherent limitations. Pilot testing for this study suggested that enrolling such a large number of physicians and having them complete the task would require limiting the number of ECGs. In addition, it is recognized that culprit coronary occlusions may on occasion resolve spontaneously leading to a spurious disparity between the inciting ECG and the subsequent coronary arteriogram. While this is statistically unlikely to meaningfully affect our analysis, we did account for this possibility by accepting nonocclusive thrombotic coronary lesions or reduced TIMI blood flow without apparent culprit lesion as consistent with a STEMI diagnosis.
Additionally, our study was meant to assess practitioners' discernment among high‐risk ECGs and may not reflect lower‐risk conditions. Since each ECG was drawn from a patient who was sent for emergent coronary arteriography due to concern for a possible STEMI, our study ECGs were enriched for concerning characteristics relative to the population of ECGs evaluated in the ED as a whole. This enrichment will falsely decrease negative predictive values by artificially raising pretest probability and will decrease specificity since patients with low‐risk and “normal” ECGs not originally diagnosed with a STEMI clinically were not incorporated into the registry. Nevertheless, the NPV for all ECGs interpreted in a standard ED setting is of limited comparative use since it will always be high given the relatively low incidence of STEMIs in an unenriched population. As noted, increasing attention has also been afforded to “appropriateness” of STEMI team activations.8,12,28 Such criteria cannot be assessed in this study since appropriate activations are generally defined by considering ECG characteristics and an associated clinical scenario. Specific scenarios were not provided in this study in an effort to focus specifically on physicians' ECG interpretations.
In summary, physicians' accuracy in evaluating high‐risk ECGs for the presence of culprit coronary artery occlusions requiring activation of the STEMI team demonstrates only modest sensitivity and specificity and relatively high levels of interobserver disagreement. Such difficulties may explain higher than expected levels of inaccurate STEMI diagnoses. These findings should be considered when devising systems of care for potential STEMI patients. Directed educational efforts may aid in reducing inaccurate assessments of potentially concerning ECGs.
Disclosures
The authors are solely responsible for the design and conduct of the study as well as the analyses, the drafting and editing of the article, and its final contents. There are no disclosures to report. No extramural funding was used to support this work.
References
- 1.Cannon CP, Gibson CM, Lambrew CT, Shoultz DA, Levy D, French WJ, Gore JM, Weaver WD, Rogers WJ, Tiefenbrunn AJ. Relationship of symptom‐onset‐to‐balloon time and door‐to‐balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA. 2000; 283:2941-2947 [DOI] [PubMed] [Google Scholar]
- 2.McNamara RL, Wang Y, Herrin J, Curtis JP, Bradley EH, Magid DJ, Peterson ED, Blaney M, Frederick PD, Krumholz HMNRMI Investigators Effect of door‐to‐balloon time on mortality in patients with ST‐segment elevation myocardial infarction. J Am Coll Cardiol. 2006; 47:2180-2186 [DOI] [PubMed] [Google Scholar]
- 3.Bradley EH, Herrin J, Wang Y, Barton BA, Webster TR, Mattera JA, Roumanis SA, Curtis JP, Nallamothu BK, Magid DJ, McNamara RL, Parkosewich J, Loeb JM, Krumholz HM. Strategies for reducing the door‐to‐balloon time in acute myocardial infarction. N Engl J Med. 2006; 355:2308-2320 [DOI] [PubMed] [Google Scholar]
- 4.Krumholz HM, Bradley EH, Nallamothu BK, Ting HH, Batchelor WB, Kline‐Rogers E, Stern AF, Byrd JR, Brush JE. A campaign to improve the timeliness of primary percutaneous coronary intervention: door‐to‐balloon: an alliance for quality. JACC Cardiovasc Interv. 2008; 1:97-104 [DOI] [PubMed] [Google Scholar]
- 5.McCabe JM, Armstrong EJ, Kulkarni A, Hoffmayer KS, Bhave PD, Garg S, Patel A, MacGregor JS, Hsue P, Stein JC, Kinlay S, Ganz P. Prevalence and factors associated with false‐positive ST‐segment elevation myocardial infarction diagnoses at primary percutaneous coronary intervention‐capable centers: a report from the Activate‐SF registry. Arch Intern Med. 2012; 172:864-871 [DOI] [PubMed] [Google Scholar]
- 6.Larson DM, Menssen KM, Sharkey SW, Duval S, Schwartz RS, Harris J, Meland JT, Unger BT, Henry TD. “False‐positive” cardiac catheterization laboratory activation among patients with suspected ST‐segment elevation myocardial infarction. JAMA. 2007; 298:2754-2760 [DOI] [PubMed] [Google Scholar]
- 7.Kontos MC, Kurz MC, Roberts CS, Joyner SE, Kreisa L, Ornato JP, Vetrovec GW. An evaluation of the accuracy of emergency physician activation of the cardiac catheterization laboratory for patients with suspected ST‐segment elevation myocardial infarction. Ann Emerg Med. 2010; 55:423-430 [DOI] [PubMed] [Google Scholar]
- 8.Garvey JL, Monk L, Granger CB, Studnek JR, Roettig ML, Corbett CC, Jollis JG. Rates of cardiac catheterization cancelation for ST‐segment elevation myocardial infarction after activation by emergency medical services or emergency physicians: results from the North Carolina Catheterization Laboratory Activation Registry. Circulation. 2012; 125:308-313 [DOI] [PubMed] [Google Scholar]
- 9.Schulman KA, Berlin JA, Harless W, Kerner JF, Sistrunk S, Gersh BJ, Dubé R, Taleghani CK, Burke JE, Williams S, Eisenberg JM, Escarce JJ. The effect of race and sex on physicians' recommendations for cardiac catheterization. N Engl J Med. 1999; 340:618-626 [DOI] [PubMed] [Google Scholar]
- 10.Cruz MF, Edwards J, Dinh MM, Barnes EH. The effect of clinical history on accuracy of electrocardiograph interpretation among doctors working in emergency departments. Med J Aust. 2012; 197:161-165 [DOI] [PubMed] [Google Scholar]
- 11.Armstrong EJ, Kulkarni AR, Bhave PD, Hoffmayer KS, MacGregor JS, Stein JC, Kinlay S, Ganz P, McCabe JM. Electrocardiographic criteria for ST‐elevation myocardial infarction in patients with left ventricular hypertrophy. Am J Cardiol. 2012; 110:977-983 [DOI] [PubMed] [Google Scholar]
- 12.Wagner GS, Macfarlane P, Wellens H, Josephson M, Gorgels A, Mirvis DM, Pahlm O, Surawicz B, Kligfield P, Childers R, Gettes LS. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. J Am Coll Cardiol. 2009; 53:1003-1011 [DOI] [PubMed] [Google Scholar]
- 13.Zou G, Donner A. Confidence interval estimation of the intraclass correlation coefficient for binary outcome data. Biometrics. 2004; 60:807-811 [DOI] [PubMed] [Google Scholar]
- 14.Mixon TA, Suhr E, Caldwell G, Greenberg RD, Colato F, Blackwell J, Jo C‐H, Dehmer GJ. Retrospective description and analysis of consecutive catheterization laboratory ST‐segment elevation myocardial infarction activations with proposal, rationale, and use of a new classification scheme. Circ Cardiovasc Qual Outcomes. 2012; 5:62-69 [DOI] [PubMed] [Google Scholar]
- 15.Clemmensen P, Loumann‐Nielsen S, Sejersten M. Telemedicine fighting acute coronary syndromes. J Electrocardiol. 2010; 43:615-618 [DOI] [PubMed] [Google Scholar]
- 16.Müller D, Schnitzer L, Brandt J, Arntz H‐R. The accuracy of an out‐of‐hospital 12‐lead ECG for the detection of ST‐elevation myocardial infarction immediately after resuscitation. Ann Emerg Med. 2008; 52:658-664 [DOI] [PubMed] [Google Scholar]
- 17.Carmody BJ. A novel approach to transmission of the out‐of‐hospital EKG in patients with ST segment elevation myocardial infarction. Ann Emerg Med. 2008; 52:183-184 [DOI] [PubMed] [Google Scholar]
- 18.Dhruva VN, Abdelhadi SI, Anis A, Gluckman W, Hom D, Dougan W, Kaluski E, Haider B, Klapholz M. ST‐segment analysis using wireless technology in acute myocardial infarction (STAT‐MI) trial. J Am Coll Cardiol. 2007; 50:509-513 [DOI] [PubMed] [Google Scholar]
- 19.Sanchez‐Ross M, Oghlakian G, Maher J, Patel B, Mazza V, Hom D, Dhruva V, Langley D, Palmaro J, Ahmed S, Kaluski E, Klapholz M. The STAT‐MI (ST‐segment analysis using wireless technology in acute myocardial infarction) trial improves outcomes. JACC Cardiovasc Interv. 2011; 4:222-227 [DOI] [PubMed] [Google Scholar]
- 20.Chen K‐C, Yen DH‐T, Chen C‐D, Young MS, Yin W‐H. Effect of emergency department in‐hospital tele‐electrocardiographic triage and interventional cardiologist activation of the infarct team on door‐to‐balloon times in ST‐segment‐elevation acute myocardial infarction. Am J Cardiol. 2011; 107:1430-1435 [DOI] [PubMed] [Google Scholar]
- 21.Sejersten M, Sillesen M, Hansen PR, Nielsen SL, Nielsen H, Trautner S, Hampton D, Wagner GS, Clemmensen P. Effect on treatment delay of prehospital teletransmission of 12‐lead electrocardiogram to a cardiologist for immediate triage and direct referral of patients with ST‐segment elevation acute myocardial infarction to primary percutaneous coronary intervention. Am J Cardiol. 2008; 101:941-946 [DOI] [PubMed] [Google Scholar]
- 22.Youngquist ST, Shah AP, Niemann JT, Kaji AH, French WJ. A comparison of door‐to‐balloon times and false‐positive activations between emergency department and out‐of‐hospital activation of the coronary catheterization team. Acad Emerg Med. 2008; 15:784-787 [DOI] [PubMed] [Google Scholar]
- 23.Davis DP, Graydon C, Stein R, Wilson S, Buesch B, Berthiaume S, Lee DM, Rivas J, Vilke GM, Leahy DR. The positive predictive value of paramedic versus emergency physician interpretation of the prehospital 12‐lead electrocardiogram. Prehosp Emerg Care. 2007; 11:399-402 [DOI] [PubMed] [Google Scholar]
- 24.Tran V, Huang HD, Diez JG, Kalife G, Goswami R, Paniagua D, Jneid H, Wilson JM, Sherron SR, Birnbaum Y. Differentiating ST‐elevation myocardial infarction from nonischemic ST‐elevation in patients with chest pain. Am J Cardiol. 2011; 108:1096-1101 [DOI] [PubMed] [Google Scholar]
- 25.Jayroe JB, Spodick DH, Nikus K, Madias J, Fiol M, De Luna AB, Goldwasser D, Clemmensen P, Fu Y, Gorgels AP, Sclarovsky S, Kligfield PD, Wagner GS, Maynard C, Birnbaum Y. Differentiating ST elevation myocardial infarction and nonischemic causes of ST elevation by analyzing the presenting electrocardiogram. Am J Cardiol. 2009; 103:301-306 [DOI] [PubMed] [Google Scholar]
- 26.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HDJoint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Third universal definition of myocardial infarction. Eur Heart J. 2012; 33:2551-2567 [DOI] [PubMed] [Google Scholar]
- 27.Smith SW, Khalil A, Henry TD, Rosas M, Chang RJ, Heller K, Scharrer E, Ghorashi M, Pearce LA. Electrocardiographic differentiation of early repolarization from subtle anterior ST‐segment elevation myocardial infarction. Ann Emerg Med. 2012; 60:45.e2-56.e2 [DOI] [PubMed] [Google Scholar]
- 28.Rokos IC, French WJ, Mattu A, Nichol G, Farkouh ME, Reiffel J, Stone GW. Appropriate cardiac cath lab activation: optimizing electrocardiogram interpretation and clinical decision‐making for acute ST‐elevation myocardial infarction. Am Heart J. 2010; 160:995-1003 [DOI] [PubMed] [Google Scholar]