Abstract
Background
The adipocyte‐derived hormone leptin is elevated in obesity and may contribute to vascular risk associated with obesity. The mechanism(s) by which leptin affects vascular disease is unclear, although leptin has been shown to increase sympathetic activity. The aim of this study was to investigate the effect of leptin treatment on endothelial function and the role of the local sympathetic nervous system in mediating these effects.
Methods and Results
Recombinant leptin was administered to C57BL6/J mice every other day for 1 week. Mesenteric arteriole myography revealed that leptin treatment caused significant impairment of endothelium‐dependent vasorelaxation. Although leptin alone did not raise aortic blood pressure, leptin treatment augmented the blood pressure response to angiotensin II. The effects of leptin on mesenteric arteriolar function and aortic blood pressure response to angiotensin II were neutralized following sympathetic denervation to the mesenteric vasculature. The superoxide scavenger TEMPOL was also effective in preventing the effects of leptin on endothelial dysfunction.
Conclusions
Leptin causes endothelial dysfunction and enhances the effects of angiotensin II on blood pressure. These effects of leptin are mediated by sympathetic nervous system activation and superoxide and may contribute to vascular stiffness and hypertension in obesity.
Keywords: ganglionectomy, hypertension, nervous system, obesity, superoxide
Introduction
Obesity is a risk factor for cardiovascular diseases; however, mechanisms by which obesity increases vascular risk remain ill‐defined.1 Leptin is produced by the adipocyte and provides feedback to the hypothalamus regarding energy stores.2 Leptin may also affect many other physiological processes via central or peripheral leptin receptor signaling pathways.3–6 Elevated leptin levels in humans have been associated with cardiovascular complications in some studies7 and with atherosclerosis,4–5 thrombosis,3,5 neointimal hyperplasia,8–10 and hypertension11–12 in preclinical studies. Because endothelial dysfunction may represent an early form of vascular disease that precedes atherosclerosis, we investigated the effects of leptin on endothelial function and the role of the sympathetic nervous system in mediating these effects.
Methods
Animals
Wild‐type C57BL6/J male mice were purchased from the Jackson Laboratory. Eight‐week‐old male mice were fed a standard laboratory rodent diet (#5001; TestDiet) in specific pathogen‐free facilities. All procedures complied with the Principles of Laboratory and Animal Care established by the National Society for Medical Research and were approved by the University of Michigan Committee on Use and Care of Animals.
Leptin Treatment
Ten micrograms of recombinant murine leptin (R&D Systems, Inc) was injected subcutaneously every other day for a total of 4 doses. Functional properties of mesenteric arterioles or aortic blood pressure measurement were performed the day following the last subcutaneous leptin injection.
Leptin Concentration Measurement
Blood was collected from the periorbital sinus of mice before and 3 and 10 hours after 1 dose of subcutaneous injection of 10 μg of leptin. Leptin concentration in the serum was measured with a murine leptin ELISA kit (R&D Systems, Inc) according to the manufacturer's instructions.
TEMPOL Treatment
4‐Hydroxy‐2,2,6,6‐tetramethylpiperidinyloxy (TEMPOL; Sigma) was used to deplete superoxide anions in vivo.13 TEMPOL was dissolved in water to make a 2 mmol/L solution. Mice were supplied with TEMPOL in the drinking water for 3 days before and during leptin treatment.
Celiac Sympathetic Ganglionectomy
Celiac ganglionectomy was performed as previously described.14 Briefly, mice were anesthetized with an IP injection of sodium pentobarbital (67 mg/kg). The abdomen was opened through a ventral midline laparotomy. The small intestine was gently moved aside and covered by sterile saline‐soaked gauze. Celiac sympathetic ganglionectomy was performed by locating and stripping the celiac plexus between the aorta, celiac artery, and cranial mesenteric artery. The abdominal cavity was closed in 2 layers with interrupted 6‐0 nylon sutures. For sham‐operated animals, only fat was rubbed off the surface of the ganglia, and nerves were kept intact. Mice received leptin or PBS treatment beginning 2 weeks after surgery.
Functional Properties of Mesenteric Arterioles
Mesenteric arteriole myography was performed as previously described.15 Briefly, mice were euthanized with IP pentobarbital (80 mg/kg), and a segment of small intestine with attached mesentery was removed and placed into a silastic‐elastomer–lined petri dish filled with cold physiologic salt solution (PSS; containing [in mmol/L]: NaCl, 120; KCl, 4.7; MgSO4, 1.18; CaCl2, 2.5; KH2PO4, 1.18; NaHCO3, 25; glucose, 5.5; and EDTA, 0.026 [at pH 7.4]) equilibrated with 5% CO2 and 95% O2. The second‐order branches of mesenteric arteries were dissected, and surrounding fat and connective tissue were cleared. Vessel segments 2 to 3 mm in length were mounted onto glass cannulae of a pressure myograph (Living Systems Instrumentation). Cannulae were adjusted to the axial direction of the vessel until the vessel walls were parallel without any stretch. Vessels were equilibrated in PSS at 37°C for 60 minutes at 45 mm Hg intraluminal pressure. The real‐time dimension of the vessel wall was detected and analyzed by a video dimension analyzer (Living Systems Instrumentation). Vascular reactivity was tested under no‐flow conditions. After equilibration, vascular viability was tested using extraluminal applied norepinephrine (NE; Sigma) 10−5 mol/L plus KCl 125 mmol/L. The vessels were considered viable when the constriction of the luminal area exceeded 60%. After washing, vascular contraction was assessed by measuring constriction in response to cumulatively applied NE (10−8 to 10−4 mol/L). After washing and equilibration, endothelium‐dependent relaxation was assessed by measuring the dilatory response to acetylcholine (10−9 to 10−4 mol/L; Sigma) in NE (10−5 mol/L) precontracted vessels. Endothelium‐independent relaxation was assessed by extraluminally applied sodium nitroprusside (10−9 to 10−3 mol/L; Sigma) on the same vessel precontracted with NE (10−5 mol/L).
Aortic Blood Pressure Measurement
Blood pressure monitoring was performed via carotid arterial catheterization as previously described.16 Briefly, animals were anesthetized with IP injection of urethane (1.0 g/kg). Body temperature was maintained at 37°C on a controlled heating pad. After clearing surrounding tissue from the right common carotid artery, an arteriotomy was performed using fine scissors. A 1.4F microtip catheter sensor (model SPR‐671; Millar Instruments Inc) was inserted into the carotid artery toward the heart into the ascending aorta. Blood pressure was equilibrated for 10 minutes to reach a steady state. Then cumulative dosages of angiotensin II (0.2 to 200 μg/kg) were administrated via jugular vein by a GENIE Plus Infusion Syringe Pump (Kent Scientific). Blood pressure was recorded using a data acquisition system (Powerlab 8/30) and Chart software (AdInstruments).
Osmotic Pump Implantation and Peripheral Blood Pressure Measurement
To determine more chronic effects of angiotensin II and leptin on blood pressure, 10 μg of leptin or PBS was injected subcutaneously every other day for 8 days. Mice were then implanted with osmotic minipumps (model 2004; Alzet) infusing angiotensin II (500 ng/kg per minute; Sigma). Leptin or PBS administration was continued using the same protocol for an additional 12 days. During this time blood pressure was measured daily during the afternoon (2 to 4 pm) to control circadian variation, using the tail‐cuff method as previously described.17 For blood pressure measurements, mice were gently restrained on a platform at 37°C (Visitech BP‐2000) for 10 minutes before measurement. For additional controls, mice were administered leptin or PBS using the same protocol and implanted with osmotic minipumps infusing only PBS.
Statistical Analysis
All data are presented as mean±SE. Statistical analysis was done using GraphPad Prism software (GraphPad). Results were analyzed using the unpaired Mann–Whitney test for comparison between 2 groups, and median and P values are presented. For multiple comparisons, results were analyzed using 2‐way ANOVA, followed by Bonferroni posttest analysis. Probability values of P<0.05 were considered statistically significant.
Results
Effect of Leptin Treatment on Mesenteric Endothelial Function and Blood Pressure
To investigate the chronic effect of leptin on endothelial function in mice, 10 μg of leptin (n=6) or PBS control (n=5) was injected subcutaneously every other day for 8 days. There was no difference in body weight between mice treated with PBS or leptin (25.86±1.11 versus 26.35±0.95 g; median, 26.00 versus 26.05 g; P=0.70). The leptin levels in the serum increased to 21.11±2.4 ng/mL 3 hours after subcutaneous leptin injection from a baseline level of 2.49±0.3 ng/mL. Leptin levels had returned to basal levels when measured 10 hours following the injection (2.16±0.4 ng/mL). Mesenteric pressure myography revealed that NE‐induced concentration‐dependent contractile responses were similar in mice treated with PBS and mice treated with leptin (Figure 1A). Endothelium‐independent vasorelaxation responses to sodium nitroprusside were also similar in the groups (Figure 1B). However, endothelial‐dependent vasorelaxation responses to acetylcholine were significantly reduced in mice treated with leptin compared with PBS‐treated mice (Figure 1C). Though there was no significant difference in aortic blood pressure between mice treated with PBS and mice treated with leptin (96.2±11.8 versus 107.6±5.0 mm Hg; median, 100.1 versus 106.9 mm Hg; P=0.052), the systolic blood pressure increased to a greater degree following angiotensin II challenge in the mice treated with leptin compared with mice treated with PBS (Figure 1D). Heart rate was also increased in mice receiving leptin compared with mice receiving PBS (629.8±10.53 versus 597.0±10.20 bpm; median, 633.0 versus 587.0 bpm; P=0.046). To investigate the effect of leptin on sustained blood pressure elevations, mice were implanted with osmotic minipumps infusing angiotensin II. Ten microgram of leptin (n=6) or PBS control (n=6) was injected subcutaneously every other day for 8 days before pump implantation and for 12 days following pump implantation. Mice infused with angiotensin II developed elevations in blood pressure compared with mice infused with PBS (n=6). Leptin alone did not induce hypertension (n=6). However, leptin administration with angiotensin II increased blood pressure compared with mice infused with angiotensin II alone (Figure 1E).
Figure 1.
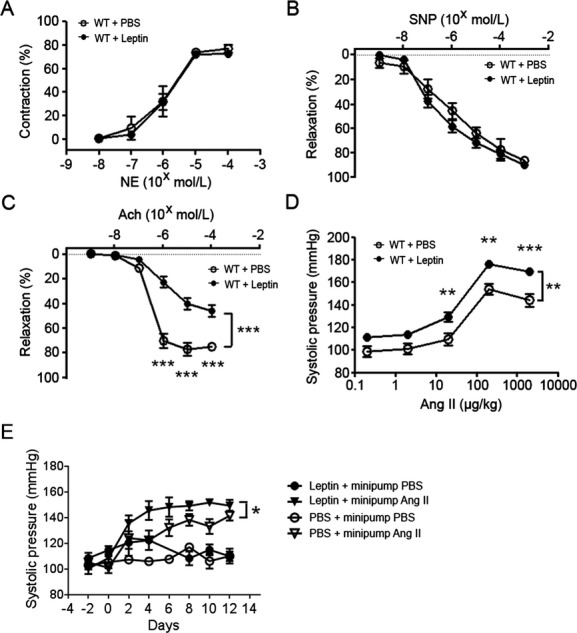
Leptin‐induced endothelial dysfunction and enhanced pressor response to angiotensin II. A, Vasoconstriction responses of mesenteric arterioles to NE. B, Vasorelaxation responses of mesentery arterioles to SNP. C, Vasorelaxation responses of mesentery arterioles to Ach. D, Response of systolic blood pressure to angiotensin II. **P<0.01, ***P<0.001. E, Effect of leptin on chronic blood pressure elevation in response to angiotensin II. *P<0.05. Ach indicates acetylcholine; Ang II, angiotensin II; NE, norepinephrine; PBS, phosphate‐buffered saline; SNP, sodium nitroprusside; WT, wild type.
Celiac Ganglionectomy Blocks Vascular Effects of Exogenous Leptin
The effects of 1 week of leptin treatment were also determined following celiac ganglionectomy. Again, there was no significant difference in body weight between mice treated with PBS and mice treated with leptin (24.94±0.68 versus 23.30±0.67 g; median, 24.80 versus 23.20 g; P=0.22). Celiac ganglionectomy did not impair the NE‐induced contraction or sodium nitroprusside–induced endothelium‐independent vasorelaxation of mesenteric arterioles (n=5 for each group; Figure 2A and 2B). However, leptin‐induced impairment of endothelial‐dependent relaxation in response to acetylcholine was completely recovered in mice receiving ganglionectomy compared with mice receiving sham operation and leptin treatment (n=5 for each group; Figure 2C). Similarly, the leptin‐induced augmentation of systolic blood pressure to angiotensin II was blocked by celiac ganglionectomy compared with sham‐operated mice (n=7 for PBS treatment+ganglionectomy, n=9 for leptin treatment+ganglionectomy, and n=5 for sham‐operated mice combined with leptin treatment; Figure 2D).
Figure 2.
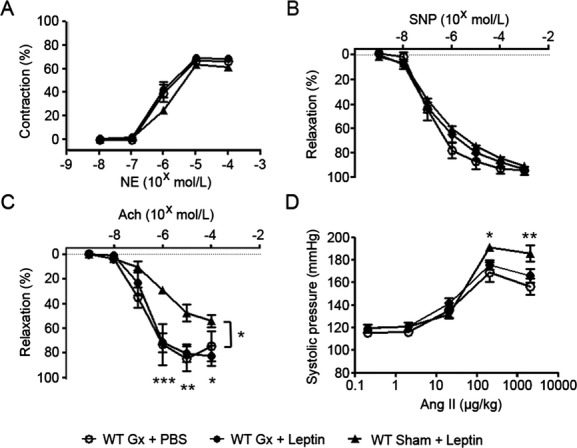
Celiac ganglionectomy blocked the effect of leptin on endothelial function and blood pressure response. A, Vasoconstriction responses of mesenteric arterioles to NE. B, Vasorelaxation responses of mesentery arterioles to SNP. C, Vasorelaxation responses of mesentery arterioles to Ach. D, Response of systolic blood pressure to angiotensin II. *P<0.05, **P<0.01, ***P<0.001 for comparisons between mice receiving celiac ganglionectomy or sham operation with subsequent leptin treatment. Ach indicates acetylcholine; Ang II, angiotensin II; Gx, ganglionectomy; NE, norepinephrine; PBS, phosphate‐buffered saline; SNP, sodium nitroprusside; WT, wild type.
Superoxide Anion Depletion Blocked the Effect of Chronic Leptin Treatment
To determine whether generation of superoxide anion played a role in mediating the effect of leptin, mice were treated with TEMPOL, a superoxide scavenger, before and during leptin treatment. Compared with PBS, TEMPOL alone did not affect endothelial function (n=5; Figure 3C). However, TEMPOL completely blocked the effect of leptin on endothelial dysfunction (n=6 for leptin treatment alone and n=5 for leptin plus TEMPOL treatment; Figure 3C). There was no significant difference between the group administered leptin and TEMPOL and the control group given PBS alone (Figure 3C).
Figure 3.
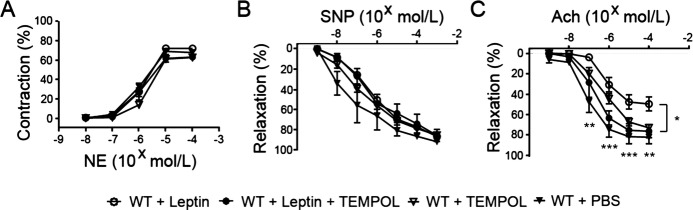
TEMPOL blocked the effect of leptin on endothelial function. A, Vasoconstriction responses of mesenteric arterioles to NE. B, Vasorelaxation responses of mesentery arterioles to SNP. C, Vasorelaxation responses of mesentery arterioles to Ach. *P<0.05, **P<0.01, ***P<0.001 for comparisons between mice treated with leptin and those treated with both leptin and TEMPOL. Ach indicates acetylcholine; NE, norepinephrine; PBS, phosphate‐buffered saline; SNP, sodium nitroprusside; TEMPOL, 4‐hydroxy‐2,2,6,6‐tetramethylpiperidinyloxy; WT, wild type.
Discussion
One of the earliest detectable vascular abnormalities associated with obesity is impaired vascular relaxation.18–19 Impaired vascular function has been shown to be predictive of later cardiovascular complications.20 Mouse models of diet‐induced obesity have also been shown to exhibit endothelial dysfunction,15 and this coincides with increased leukocyte‐endothelial interactions and precedes overt atherosclerosis. Because so many factors are altered in states of obesity, it is difficult to determine the causal contribution of individual obesity‐related factors in obese models.
Leptin is a hormone secreted by adipocytes that is critically important in the regulation of energy balance.21 Mice and humans deficient in leptin or leptin receptor signaling are severely obese, and leptin deficiency states can be successfully treated with leptin replacement therapy.22–24 However, leptin deficiency in humans is rare, and most obese humans have elevated leptin levels. These elevated levels do not prevent obesity, indicating the presence of central resistance to the effects of leptin and/or other overriding stimuli that promote obesity. Resistance to chronically elevated levels of leptin may be tissue specific, as previous reports have demonstrated the presence of central leptin resistance to changes in food intake while vascular responses to leptin are preserved.12
Because the mechanism(s) by which leptin affects properties of endothelial cells remains controversial, we studied the effect of chronic leptin treatment on endothelial dysfunction and the role of local sympathetic innervation in chronic leptin‐mediated endothelial dysfunction. Recombinant leptin has been previously shown to affect atherosclerosis,5 thrombosis,3–4 and blood pressure12 in mice. The specific effects of leptin on vascular tone and endothelial function are controversial.25 This may be because of divergent mechanisms with both positive and negative effects in different models. For example, leptin may simultaneously induce balanced activation of the sympathetic nervous system, leading to a pressor effect, and also increased nitric oxide (NO), leading to a depressor effect.25–26 Obese mice deficient in leptin have been shown to have impaired endothelial relaxation responses to acetylcholine that are corrected with exogenous leptin treatment.27 However, the severe obesity associated with leptin deficiency may exert detrimental effects on the endothelium via multiple mechanisms, and leptin replacement may provide indirect beneficial effects in the setting of leptin deficiency. Direct effects of leptin have been shown on rat aortic and mesenteric vascular rings preconstricted with phenylephrine.28 Leptin receptors were also demonstrated on endothelial cells, and the effects of leptin were blocked following endothelial denudation.28 Several investigators have reported a role for leptin in increasing NO production.29–31 However, the in vivo relevance of leptin‐induced endothelial NO upregulation remains unclear. For example, to address the possibility that leptin mediates a balanced NO depressor effect with a sympathetic nervous system pressor effect, the effect of leptin was studied in various vascular beds of rats, and no effect was observed with or without the α1‐adrenoceptor blocker prazosin.32 The reason for the discrepancy between studies is unclear, although the effects of leptin may vary depending on the state of leptin sensitivity, the duration of leptin therapy, the dosing of leptin, the vascular bed being studied, and whether the intact animal is being studied versus isolated tissue.
In our study leptin was given to lean mice at a dose that did not affect body weight in order to study the direct vascular effects of leptin. Levels of leptin resulted that were in the physiologic range of obesity33 but in a pulsatile pattern, returning to baseline within 10 hours of administration. This pattern of leptin adiminstration may limit the effects of leptin resistance on the vasculature because the vessels are not exposed to sustained elevated levels of leptin, which induced leptin resistance.34–35 Levels of endogenous leptin have also been shown to fluctuate because of circadian rhythms and acute stressors,36–38 although the effect of these fluctuations on leptin sensitivity in humans is unclear. Our study showed that 1 week of leptin treatment contributes to impairment of endothelial relaxation in resistance vessels. Although the dose of leptin used in this study did not raise aortic blood pressure, it did enhance the pressor response to angiotensin II following both acute and chronic treatment with angiotensin II. Some studies have shown that the blood pressure response to angiotensin II is enhanced in obese rodents,39 although the role for leptin in this enhanced sensitivity has not been previously shown, to our knowledge.
To determine the role of mesenteric sympathetic nervous system innervation in the effects of leptin toward mesenteric endothelial dysfunction and the aortic pressure response to angiotensin II, we performed celiac ganglionectomy. The sympathetic nervous system has been implicated in oxidative stress,40–41 which may contribute to vascular dysfunction related to sympathetic nervous system activation. Mice tolerated this procedure well with no change in weight compared with control mice. Mesenteric sympathetic denervation blocked the adverse effects of leptin on mesenteric endothelial function and also blocked the enhanced pressor response to angiotensin II. To further explore the potential mechanisms for the vascular effects of leptin in this model, we determined the role of the superoxide anion because leptin has previously been shown to induce endothelial oxidative stress.42 To determine the functional importance of the superoxide anion, mice were treated with the superoxide scavenger TEMPOL. This treatment completely blocked the effect of leptin on endothelial dysfunction.
This study has established the important role of sympathetic innervation in the vascular effects of leptin and the potential of leptin to contribute to endothelial dysfunction and hypertension. These findings could be relevant to the early vascular changes observed in obesity. These results also suggest therapeutic targets such as targeting reactive oxygen species or sympathetic outflow may prevent obesity‐related vascular functional abnormalities. Of particular interest to these findings, catheter‐based renal sympathectomy has been shown to reduce blood pressure in patients with resistant hypertension.43–44
Sources of Funding
This work was supported by National Institutes of Health grant HL‐073150 (to Dr Eitzman) and a Veterans Health Administration (VA) Merit Award (BX000353; to Dr Eitzman).
Disclosures
None.
References
- 1.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world—a growing challenge. N Engl J Med. 2007; 356:213-215 [DOI] [PubMed] [Google Scholar]
- 2.Woods AJ, Stock MJ. Leptin activation in hypothalamus. Nature. 1996; 27:745. [DOI] [PubMed] [Google Scholar]
- 3.Bodary PF, Westrick RJ, Wickenheiser KJ, Shen Y, Eitzman DT. Effect of leptin on arterial thrombosis following vascular injury in mice. JAMA. 2002; 287:1706-1709 [DOI] [PubMed] [Google Scholar]
- 4.Konstantinides S, Schäfer K, Koschnick S, Loskutoff DJ. Leptin‐dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. J Clin Invest. 2001; 108:1533-1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodary PF, Gu S, Shen Y, Hasty AH, Buckler JM, Eitzman DT. Recombinant leptin promotes atherosclerosis and thrombosis in apolipoprotein E‐deficient mice. Arterioscler Thromb Vasc Biol. 2005; 25:e119-e122 [DOI] [PubMed] [Google Scholar]
- 6.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor‐mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997; 100:270-278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, Sattar N. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation. 2001; 104:3052-3056 [DOI] [PubMed] [Google Scholar]
- 8.Schäfer K, Halle M, Goeschen C, Dellas C, Pynn M, Loskutoff DJ, Konstantinides S. Leptin promotes vascular remodeling and neointimal growth in mice. Arterioscler Thromb Vasc Biol. 2004; 24:112-117 [DOI] [PubMed] [Google Scholar]
- 9.Stephenson K, Tunstead J, Tsai A, Gordon R, Henderson S, Dansky HM. Neointimal formation after endovascular arterial injury is markedly attenuated in db/db mice. Arterioscler Thromb Vasc Biol. 2003; 23:2027-2033 [DOI] [PubMed] [Google Scholar]
- 10.Bodary PF, Shen Y, Ohman M, Bahrou KL, Vargas FB, Cudney SS, Wickenheiser KJ, Myers MG, Jr, Eitzman DT. Leptin regulates neointima formation after arterial injury through mechanisms independent of blood pressure and the leptin receptor/STAT3 signaling pathways involved in energy balance. Arterioscler Thromb Vasc Biol. 2007; 27:70-76 [DOI] [PubMed] [Google Scholar]
- 11.De Haro Moraes C, Figueiredo VN, de Faria AP, Barbaro NR, Sabbatini AR, Quinaglia T, Ferreira‐Melo SE, Martins LC, Demacq C, Júnior HM. High‐circulating leptin levels are associated with increased blood pressure in uncontrolled resistant hypertension. J Hum Hypertens. 2012; 27:225-230 [DOI] [PubMed] [Google Scholar]
- 12.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet‐induced obesity hypertension. Diabetes. 2005; 54:2012-2018 [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood‐brain barrier permeability via oxidative stress. Neuroscience. 2010; 171:852-858 [DOI] [PubMed] [Google Scholar]
- 14.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007; 50:547-556 [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Luo W, Wang J, Guo C, Wang X, Wolffe SL, Bodary PF, Eitzman DT. Obesity‐induced endothelial dysfunction is prevented by deficiency of P‐selectin glycoprotein ligand‐1. Diabetes. 2012; 61:3219-3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Villacorta L, Chang L, Fan Z, Hamblin M, Zhu T, Chen CS, Cole MP, Schopfer FJ, Deng CX, Garcia‐Barrio MT, Feng YH, Freeman BA, Chen YE. Nitro‐oleic acid inhibits angiotensin II‐induced hypertension. Circ Res. 2010; 107:540-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang L, Villacorta L, Zhang J, Garcia‐Barrio MT, Yang K, Hamblin M, Whitesall SE, D'Alecy LG, Chen YE. Vascular smooth muscle cell‐selective peroxisome proliferator‐activated receptor‐gamma deletion leads to hypotension. Circulation. 2009; 119:2161-2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharjee R, Alotaibi WH, Kheirandish‐Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in obese non‐hypertensive children without evidence of sleep disordered breathing. BMC Pediatr. 2010; 10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valle Jiménez M, Estepa RM, Camacho RM, Estrada RC, Luna FG, Guitarte FB. Endothelial dysfunction is related to insulin resistance and inflammatory biomarker levels in obese prepubertal children. Eur J Endocrinol. 2007; 156:497-502 [DOI] [PubMed] [Google Scholar]
- 20.Aggoun Y, Farpour‐Lambert NJ, Marchand LM, Golay E, Maggio AB, Beghetti M. Impaired endothelial and smooth muscle functions and arterial stiffness appear before puberty in obese children and are associated with elevated ambulatory blood pressure. Eur Heart J. 2008; 29:792-799 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994; 372:425-432 [DOI] [PubMed] [Google Scholar]
- 22.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999; 341:879-884 [DOI] [PubMed] [Google Scholar]
- 23.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995; 269:540-543 [DOI] [PubMed] [Google Scholar]
- 24.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight‐reducing effects of the plasma protein encoded by the obese gene. Science. 1995; 269:543-546 [DOI] [PubMed] [Google Scholar]
- 25.Bełtowski J. Leptin and the regulation of endothelial function in physiological and pathological conditions. Clin Exp Pharmacol Physiol. 2012; 39:168-178 [DOI] [PubMed] [Google Scholar]
- 26.Frühbeck G. Pivotal role of nitric oxide in the control of blood pressure after leptin administration. Diabetes. 1999; 48:903-908 [DOI] [PubMed] [Google Scholar]
- 27.Winters B, Mo Z, Brooks‐Asplund E, Kim S, Shoukas A, Li D, Nyhan D, Berkowitz DE. Reduction of obesity, as induced by leptin, reverses endothelial dysfunction in obese (Lep(ob)) mice. J Appl Physiol. 2000; 89:2382-2390 [DOI] [PubMed] [Google Scholar]
- 28.Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, d'Amati G, Trimarco B. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes. 2000; 49:293-297 [DOI] [PubMed] [Google Scholar]
- 29.Beltowski J, Wójcicka G, Borkowska E. Human leptin stimulates systemic nitric oxide production in the rat. Obes Res. 2002; 10:939-946 [DOI] [PubMed] [Google Scholar]
- 30.Bełtowski J, Jochem J, Wójcicka G, Zwirska‐Korczala K. Influence of intravenously administered leptin on nitric oxide production, renal hemodynamics and renal function in the rat. Regul Pept. 2004; 120:59-67 [DOI] [PubMed] [Google Scholar]
- 31.Kimura K, Tsuda K, Baba A, Kawabe T, Boh‐oka S, Ibata M, Moriwaki C, Hano T, Nishio I. Involvement of nitric oxide in endothelium‐dependent arterial relaxation by leptin. Biochem Biophys Res Commun. 2000; 273:745-749 [DOI] [PubMed] [Google Scholar]
- 32.Mitchell JL, Morgan DA, Correia ML, Mark AL, Sivitz WI, Haynes WG. Does leptin stimulate nitric oxide to oppose the effects of sympathetic activation? Hypertension. 2001; 38:1081-1086 [DOI] [PubMed] [Google Scholar]
- 33.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive‐leptin concentrations in normal‐weight and obese humans. N Engl J Med. 1996; 334:292-295 [DOI] [PubMed] [Google Scholar]
- 34.Montez JM, Soukas A, Asilmaz E, Fayzikhodjaeva G, Fantuzzi G, Friedman JM. Acute leptin deficiency, leptin resistance, and the physiologic response to leptin withdrawal. Proc Natl Acad Sci USA. 2005; 102:2537-2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tajmir P, Kwan JJ, Kessas M, Mozammel S, Sweeney G. Acute and chronic leptin treatment mediate contrasting effects on signaling, glucose uptake, and GLUT4 translocation in L6‐GLUT4myc myotubes. J Cell Physiol. 2003; 197:122-130 [DOI] [PubMed] [Google Scholar]
- 36.Anubhuti Arora S. Leptin and its metabolic interactions: an update. Diabetes Obes Metab. 2008; 10:973-993 [DOI] [PubMed] [Google Scholar]
- 37.Brydon L, Wright CE, O'Donnell K, Zachary I, Wardle J, Steptoe A. Stress‐induced cytokine responses and central adiposity in young women. Int J Obes (Lond). 2008; 32:443-450 [DOI] [PubMed] [Google Scholar]
- 38.Konishi N, Otaka M, Odashima M, Jin M, Wada I, Komatsu K, Sato T, Kato S, Matsuhashi T, Watanabe S. Systemic stress increases serum leptin level. J Gastroenterol Hepatol. 2006; 21:1099-1102 [DOI] [PubMed] [Google Scholar]
- 39.Müller‐Fielitz H, Lau M, Jöhren O, Stellmacher F, Schwaninger M, Raasch W. Blood pressure response to angiotensin II is enhanced in obese Zucker rats and is attributed to an aldosterone‐dependent mechanism. Br J Pharmacol. 2012; 166:2417-2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golling M, Jahnke C, Fonouni H, Ahmadi R, Urbaschek R, Breitkreutz R, Schemmer P, Kraus TW, Gebhard MM, Büchler MW, Mehrabi A. Distinct effects of surgical denervation on hepatic perfusion, bowel ischemia, and oxidative stress in brain dead and living donor porcine models. Liver Transpl. 2007; 13:607-617 [DOI] [PubMed] [Google Scholar]
- 41.Chaswal M, Das S, Prasad J, Katyal A, Fahim M. Cardiac autonomic function in acutely nitric oxide deficient hypertensive rats: role of the sympathetic nervous system and oxidative stress. Can J Physiol Pharmacol. 2011; 89:865-874 [DOI] [PubMed] [Google Scholar]
- 42.Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999; 13:1231-1238 [PubMed] [Google Scholar]
- 43.Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Böhm M, Hoppe UC. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012; 59:901-909 [DOI] [PubMed] [Google Scholar]
- 44.Symplicity HTN‐2 Investigators Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment‐resistant hypertension (The Symplicity HTN‐2 Trial): a randomised controlled trial. Lancet. 2010; 376:1903-1909 [DOI] [PubMed] [Google Scholar]


