Abstract
Background
We investigated whether disease location influences survival in patients with peripheral arterial disease.
Methods and Results
Patients (n=12 731; mean age, 67.5±12.7 years; 57.4% male) who underwent outpatient noninvasive lower extremity arterial evaluation were followed up for 5.9±3.1 years for all‐cause mortality. Peripheral arterial disease (n=8930) was defined as a resting or postexercise ankle‐brachial index (ABI) ≤0.90, and normal ABI (n=3 801) was defined as a resting and postexercise ABI of 1.00 to 1.30. Presence or absence of disease at the proximal location or distal location was determined on the basis of Doppler signals in leg arteries; 42% had no PD or DD, 45% had proximal (14% postexercise PD only), 30% had distal disease, 17% had both proximal and distal disease, 28% had proximal only and 14% had distal only. We performed multivariable logistic regression to identify factors associated with disease location, and Cox proportional hazard regression to assess the respective effects of proximal or distal disease on survival. Older age, male sex, diabetes, heart failure, and critical limb ischemia were associated with distal disease, whereas female sex, smoking, hypertension, dyslipidemia, coronary heart disease, cerebrovascular disease, chronic obstructive pulmonary disease, and critical limb ischemia were associated with proximal disease. Over a mean follow‐up of 5.9±3.1 years, 3039 patients (23.9%) died. After adjustment for potential confounders, the hazard ratios (HRs) of death associated with PD only and DD only were 1.3 (1.3 to 1.4) and 1.5 (1.4 to 1.6), respectively. After additional adjustment for resting ABI, there was no significant association between proximal disease and death, whereas the association of distal disease with death remained significant (HR, 1.2; 95% CI, 1.1 to 1.3).
Conclusions
In patients with peripheral arterial disease, proximal and distal disease locations were associated with distinctive risk factor and comorbidity profiles. Distal disease was associated with worse survival even after adjustment for risk factors, comorbidities, and resting ABI.
Keywords: atherosclerosis, Doppler, peripheral arterial disease, prognosis, survival
Introduction
Peripheral artery disease (PAD) is a common manifestation of atherosclerosis and affects ≈8 million people in the United States.1 PAD is associated with increased risk of death and adverse cardiovascular events,2–3 causes leg pain with exertion, impairs functional capacity and quality of life, and is frequently associated with coronary, cerebral, and renal artery disease.3–4 PAD involves the arteries distal to the aortic bifurcation in a nonuniform manner, and factors such as arterial geometry and anatomic, cellular, or biochemical properties of the arterial wall may influence disease location.5–6 For example, iliac arteries are relatively elastic, whereas infragenicular arteries contain progressively more muscular elements.6
Several modalities have been used to define affected arteries in PAD, including Doppler, computed tomography angiography, contrast‐enhanced magnetic resonance angiography, and digital subtraction angiography.7–8 Digital subtraction angiography is considered the “gold standard” for assessing location and severity of PAD but is not generalizable to the entire PAD population because patients with severe and symptomatic disease are more likely to be referred for angiography. On the other hand, Doppler evaluation is relatively inexpensive, easily performed, and widely used in noninvasive vascular laboratories.
Several studies have shown that the distribution, extent, and progression of PAD are influenced by cardiovascular risk factors, but the findings are not consistent.5–6,5–11 A few studies suggest that the prognosis of patients with PAD varies according to disease localization.6,9,12–14 The goal of the present study was to describe the patterns of disease location in patients with known or suspected PAD who underwent evaluation in a noninvasive vascular laboratory that included noninvasive Doppler examination and to examine the association of such patterns with survival.
Methods
Study Population
The study population was drawn from 22 859 consecutive outpatients who underwent lower extremity arterial evaluation in the noninvasive vascular laboratory of the Gonda Vascular Center, Mayo Clinic, Rochester, Minnesota, between January 1, 1998, and December 31, 2007. We identified 8930 PAD patients who (1) had appropriate research authorization, (2) were ≥18 years at the time of arterial evaluation, (3) resided within a 500‐mile radius of Rochester, Minnesota (to ensure appropriate information would be available in the electronic medical record), (4) were not hospitalized at the time of arterial evaluation, (5) had results available from both arterial and Doppler ultrasound evaluation, and (6) had an ankle brachial index (ABI) ≤0.90. An additional reference group of 3801 patients with normal ABI (1.00 to 1.30) and no evidence of disease from Doppler ultrasound evaluation was identified in the same manner, resulting in a total of 12 731 unique patients available for analysis.
The study was approved by the Mayo Clinic Institutional Review Board and informed consent was obtained from participants.
Noninvasive Arterial Evaluation and Definition of Disease Location
Noninvasive arterial evaluation was performed in the vascular laboratory of the Mayo Clinic as described in detail in the Supplement. The Mayo Vascular Laboratory is certified by the Intersocietal Commission for the Accreditation of Vascular Laboratories. Quality control and maintenance procedures are in place as required by the Intersocietal Commission for the Accreditation of Vascular Laboratories, including for interpretation of Doppler waveforms (see Supplement for details). Patients were determined to have PAD if they had a resting or postexercise ABI ≤0.90. Normal ABI was defined as a resting and postexercise ABI of 1.00 to 1.30. Disease location was inferred from the results of continuous‐wave Doppler interrogation of lower extremity arteries. A Doppler probe is placed directly over the artery and held at a 45‐ to 60‐degree angle to obtain a Doppler waveform from each major lower extremity artery, including common femoral (CF), superficial femoral, popliteal, posterior tibial (PT), and dorsalis pedis (DP) arteries. A standardized algorithm was used by highly trained vascular laboratory technicians to categorize the Doppler signals (see Supplement). Triphasic, normal, and biphasic Doppler signals were considered normal, whereas reduced biphasic, slightly abnormal, abnormal, monophasic, and absent signals were considered abnormal. The rating of severity of abnormal Doppler signals was defined as follows: reduced biphasic<slightly abnormal<abnormal<monophasic<absent.
The CF artery was assessed at rest and again after exercise if the patient was able to walk on a treadmill; all other vessels were assessed only at rest. The right and left legs were evaluated separately at each arterial location. Disease status was assigned for each leg at the proximal and distal regions on the basis of involvement of the CF and PT/DP arteries, respectively. The most severe Doppler signal was used if multiple results were available at the same arterial location for the same leg. Therefore, each person had up to 4 disease status assignments, whereas missing assignments reflected evaluations not performed. CF Doppler results were used to characterize proximal disease status as normal, abnormal only after exercise, or abnormal at rest. PT/DP Doppler results were used to characterize distal disease status as normal or abnormal at rest. To address the possibility that Doppler abnormality in distal arteries might reflect reduced flow in the proximal vessels, we considered distal disease to be present only when the Doppler signals worsened at the PT/DP arteries compared with the readings at the CF and/or superficial femoral/popliteal arteries, using the severity rating shown above. Although each person had at least one Doppler signal available, 905 (7.1%) patients were only assessed at the distal arteries and 54 (0.4%) patients were only assessed at the proximal vessels.
Clinical Characteristics
Demographic characteristics were obtained from the Mayo electronic medical record, as detailed in the Supplement.15 Cardiovascular risk factors were ascertained from the electronic medical record using previously validated algorithms that have good sensitivity and specificity compared with manual chart abstraction.16 The follow‐up period for each patient was the time between first arterial evaluation and date of death or final censoring date (September 30, 2009).
Statistical Analysis
Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, NC). Continuous variables were expressed as mean±standard deviation, whereas categorical variables were expressed as number (percentage). P<0.05 was considered statistically significant.
Univariable and multivariable generalized estimating equations logistic regression models were fit to identify variables associated with abnormal resting Doppler results within each region separately, adjusting for age and sex. To assess whether the risk factor and comorbidity associations differed between proximal and distal locations, both regions and both legs were analyzed as separate observations in the same generalized estimating equation model with terms for location and for the interactions between location (proximal or distal) and risk factors. For this analysis, patients with any abnormal Doppler results in the proximal region only after exercise for the left and/or right leg were excluded (n=2324) to allow comparison of characteristics of patients with abnormalities in the proximal and distal regions at rest. Each patient had between 1 and 4 Doppler results available, and generalized estimating equation models were used to account for repeated measures. An exchangeable correlation structure was used for analysis within each region (up to 2 observations per patient). Those patients missing results in both legs within a particular region were excluded from the corresponding regional analysis (n=905 missing proximal results, n=54 missing distal results).
Survival curves for the study groups were depicted using the Kaplan–Meier method. Univariable and multivariable Cox proportional hazards regression was used to estimate hazard ratios for death according to the presence or absence of disease in each region (proximal or distal) using the most severe disease status across both legs within each region. For this analysis, proximal Doppler results were classified as an ordinal variable, with normal as the reference level, postexercise abnormality as the intermediate level, and resting abnormality as the highest level. To analyze the joint effect of proximal and distal disease without excluding patients in whom either proximal or distal information was missing, we used logistic regression models to calculate the predicted probabilities of proximal and distal disease and used them as imputations for the missing disease status variables. Both proximal and distal disease status variables were then included in the Cox models, both as main effects and including their interaction. Adjustment was performed sequentially for (1) age and sex; (2) age, sex, obesity, smoking, diabetes, hypertension, dyslipidemia, coronary heart disease (CHD), heart failure, chronic kidney disease (CKD), cerebrovascular disease (CVD), chronic obstructive pulmonary disease (COPD), malignancy, and medication (lipid‐lowering and aspirin) use; and finally (3) also for resting ABI.
Results
After applying the exclusion criteria mentioned above, 12 731 patients were identified for analyses. The mean age of patients was 67.5±12.7 years, and 57.4% were men. Postexercise proximal disease was present in 10.2%, resting proximal disease in 18.0%, distal disease in 13.6%, postexercise proximal and distal disease in 4.0%, resting proximal and distal disease in 12.5%, and no proximal or distal disease in 41.7% (Table 1).
Table 1.
Patient Characteristics by Doppler Results
| No Proximal or Distal Disease (n=5313) | Postexercise Proximal Disease and No Distal Disease (n=1298) | Resting Proximal Disease and No Distal Disease (n=2287) | Distal Disease and No Proximal Disease (n=1729) | Postexercise Proximal and Distal Disease (n=515) | Resting Proximal and Distal Disease (n=1589) | |
|---|---|---|---|---|---|---|
| Age, y | 64.5±13.7 | 65.0±11.8 | 68.4±11.3 | 73.0±11.0 | 70.6±9.9 | 71.1±10.9 |
| Men | 2876 (54.1%) | 786 (60.6%) | 1309 (57.2%) | 1085 (62.8%) | 333 (64.7%) | 915 (57.6%) |
| Decedents | 714 (13.4%) | 213 (16.4%) | 714 (31.2%) | 579 (33.5%) | 118 (22.9%) | 701 (44.1%) |
| Years of follow‐up | 5.8±2.8 | 6.4±3.0 | 5.9±3.2 | 5.6±3.1 | 6.5±3.0 | 5.8±3.4 |
| Obesity | 2214 (41.7%) | 519 (40.0%) | 760 (33.2%) | 626 (36.2%) | 157 (30.5%) | 522 (32.9%) |
| Systolic BP, mm Hg | 134.9±20.3 | 138.6±20.5 | 144.7±24.0 | 141.8±22.3 | 140.3±20.8 | 146.4±24.4 |
| Missing | 175 | 37 | 99 | 110 | 18 | 77 |
| Diastolic BP, mm Hg | 76.0±10.5 | 74.6±10.6 | 74.0±11.4 | 73.6±10.6 | 73.7±10.6 | 74.3±11.4 |
| Missing | 341 | 56 | 182 | 201 | 21 | 144 |
| Ever smoked | 4032 (75.9%) | 1147 (88.4%) | 2128 (93.0%) | 1340 (77.5%) | 462 (89.7%) | 1400 (88.1%) |
| Diabetes | 1033 (19.4%) | 328 (25.3%) | 575 (25.1%) | 633 (36.6%) | 134 (26.0%) | 493 (31.0%) |
| Hypertension | 2994 (56.4%) | 928 (71.5%) | 1752 (76.6%) | 1257 (72.7%) | 385 (74.8%) | 1255 (79.0%) |
| Dyslipidemia | 3565 (67.1%) | 1074 (82.7%) | 1874 (81.9%) | 1246 (72.1%) | 409 (79.4%) | 1218 (76.7%) |
| CHD | 1996 (37.6%) | 655 (50.5%) | 1366 (59.7%) | 913 (52.8%) | 283 (55.0%) | 964 (60.7%) |
| Heart failure | 424 (8.0%) | 98 (7.6%) | 299 (13.1%) | 317 (18.3%) | 32 (6.2%) | 317 (19.9%) |
| CKD | 244 (4.6%) | 63 (4.9%) | 158 (6.9%) | 137 (7.9%) | 35 (6.8%) | 140 (8.8%) |
| CVD | 921 (17.3%) | 422 (32.5%) | 883 (38.6%) | 522 (30.2%) | 155 (30.1%) | 595 (37.4%) |
| COPD | 553 (10.4%) | 192 (14.8%) | 492 (21.5%) | 250 (14.5%) | 88 (17.1%) | 369 (23.2%) |
| Lipid‐lowering medication use | 1641 (30.9%) | 588 (45.3%) | 969 (42.4%) | 552 (31.9%) | 216 (41.9%) | 540 (34.0%) |
| Aspirin use | 1903 (35.8%) | 598 (46.1%) | 1031 (45.1%) | 717 (41.5%) | 238 (46.2%) | 640 (40.3%) |
| Resting ABI | 1.04±0.18 | 0.87±0.18 | 0.52±0.19 | 0.67±0.20 | 0.69±0.15 | 0.49±0.19 |
| Missing | 5 | 10 | 10 | 11 | 1 | 7 |
| CLI | 273 (5.1%) | 13 (1.0%) | 390 (17.1%) | 435 (25.2%) | 8 (1.6%) | 536 (33.7%) |
Shown are mean±standard deviation for continuous variables and frequency (%) for categorical variables and missing values. Proximal and distal disease for each subject is classified separately based on the most severe disease status (no disease < postexercise disease < resting disease) across both legs. Those with no proximal Doppler assessments were classified based on imputed disease status (similarly for those with no distal Doppler assessment). ABI indicates ankle brachial index; BP, blood pressure; CHD, coronary heart disease; CKD, chronic kidney disease; CLI, critical limb ischemia; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease.
Risk Factors and Comorbidities
There were significant associations between older age and male sex with resting distal disease (both P<0.001) and between older age and resting proximal disease (P<0.001), as shown in Table 2. These associations were stronger with distal disease than with proximal disease (interaction P<0.001 and P=0.004, respectively). The presence of cardiovascular risk factors and comorbidities were compared after adjustment for age and sex. Among cardiovascular risk factors, body mass index <30, smoking, diabetes, hypertension, and dyslipidemia were significantly associated with proximal disease, whereas diabetes and hypertension were significantly associated with distal disease. In models that considered interactions between disease location and risk factors, diabetes was more strongly associated with distal disease (interaction P<0.001), whereas smoking and hypertension were more strongly associated with proximal disease (interaction P<0.001 and P=0.005, respectively) (Table 2).
Table 2.
Risk Factors and Comorbid Conditions Associated With Resting Disease Status: Univariable Regression Analyses
| Variable | Proximal (n=9502 Patients) | Distal (n=10 353 Patients) | Interaction between location and variable(n=10 407 Patients) | ||
|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | P Value | |
| Age* | 1.18 (1.15 to 1.22) | <0.001 | 1.60 (1.54 to 1.66) | <0.001 | <0.001 |
| Male sex | 1.02 (0.93 to 1.11) | 0.692 | 1.21 (1.11 to 1.31) | <0.001 | 0.004 |
| Obesity | 0.79 (0.72 to 0.87) | <0.001 | 0.94 (0.86 to 1.02) | 0.145 | 0.189 |
| Smoking | 3.47 (3.01 to 4.01) | <0.001 | 1.07 (0.96 to 1.19) | 0.211 | <0.001 |
| Diabetes | 1.18 (1.07 to 1.30) | 0.001 | 2.03 (1.85 to 2.22) | <0.001 | <0.001 |
| Hypertension | 2.06 (1.85 to 2.28) | <0.001 | 1.42 (1.29 to 1.57) | <0.001 | 0.005 |
| Dyslipidemia | 1.82 (1.63 to 2.02) | <0.001 | 1.01 (0.92 to 1.11) | 0.757 | <0.001 |
| CHD | 2.11 (1.92 to 2.31) | <0.001 | 1.33 (1.22 to 1.45) | <0.001 | <0.001 |
| Heart failure | 1.43 (1.25 to 1.62) | <0.001 | 1.89 (1.69 to 2.12) | <0.001 | <0.001 |
| CKD | 1.32 (1.11 to 1.56) | 0.002 | 1.47 (1.26 to 1.73) | <0.001 | 0.123 |
| CVD | 2.28 (2.07 to 2.50) | <0.001 | 1.31 (1.20 to 1.44) | <0.001 | <0.001 |
| COPD | 1.98 (1.77 to 2.21) | <0.001 | 1.21 (1.09 to 1.35) | <0.001 | <0.001 |
| Lipid‐lowering medication use | 1.42 (1.30 to 1.56) | <0.001 | 0.82 (0.75 to 0.90) | <0.001 | <0.001 |
| Aspirin use | 1.23 (1.13 to 1.35) | <0.001 | 0.94 (0.87 to 1.03) | 0.189 | 0.014 |
| Resting ABI* | 0.54 (0.53 to 0.55) | <0.001 | 0.75 (0.74 to 0.76) | <0.001 | <0.001 |
| Critical limb ischemia | 2.38 (2.12 to 2.67) | <0.001 | 4.06 (3.66 to 4.49) | <0.001 | <0.001 |
Proximal and distal results are reported from models within each region separately and reflect number of persons with specific regional results. Interaction results are reported from the model with all data together and required data from either or both regions. (ie, those with at least one Doppler assessment). People missing proximal results (n=905) were excluded only from the proximal region analysis, people missing distal results (n=54) were excluded only from the distal region analysis. Patients with postexercise proximal disease in either leg (n=2324) were excluded from this analysis. No disease was the reference group. ABI indicates ankle brachial index; CHD, coronary heart disease; 95% CI, 95% confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; OR, odds ratio.
Per 10‐year increase.
Per 0.1‐unit increase.
Among comorbid conditions, CHD, heart failure, CKD, CVD, COPD, and critical limb ischemia were associated with both proximal and distal disease. CHD, CVD, and COPD were more strongly associated with proximal disease (all interaction P<0.001), whereas heart failure and critical limb ischemia were more strongly associated with distal disease (both interactions P<0.001); see Table 2. Of the remaining variables, lower ABI was more strongly associated with proximal disease than with distal disease (both interactions P<0.001; Table 2). Use of lipid‐lowering medication was positively associated with proximal disease and negatively associated with distal disease, whereas aspirin use was only associated with proximal disease.
Multivariable models were fit to identify joint associations of risk factors and comorbid conditions with proximal and distal disease (Table 3). Results were similar to those from univariable analysis except that age, diabetes, heart failure, CKD, and use of aspirin or lipid‐lowering medication were no longer significantly independently associated with proximal disease, whereas CHD, CKD, and COPD were no longer associated with distal disease. In the multivariable models, male sex was now inversely associated with proximal disease, and body mass index <30 was now associated with distal disease.
Table 3.
Risk Factors and Comorbid Conditions Associated With Resting Disease Status: Multivariable Regression Analyses
| Variable | Proximal (n=9502 Patients) | Distal (n=10 353 Patients) | Interaction between location and variable (n=10 407 Patients) | ||
|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | P Value | |
| Age* | 1.03 (0.99 to 1.07) | 0.179 | 1.46 (1.40 to 1.52) | <0.001 | <0.001 |
| Male sex | 0.81 (0.74 to 0.89) | <0.001 | 1.21 (1.10 to 1.32) | <0.001 | <0.001 |
| Obesity | 0.79 (0.72 to 0.87) | <0.001 | 0.86 (0.79 to 0.94) | 0.001 | 0.280 |
| Smoking | 2.97 (2.56 to 3.44) | <0.001 | 1.01 (0.91 to 1.13) | 0.811 | <0.001 |
| Diabetes | 0.95 (0.85 to 1.06) | 0.334 | 1.75 (1.58 to 1.93) | <0.001 | <0.001 |
| Hypertension | 1.55 (1.39 to 1.74) | <0.001 | 1.16 (1.05 to 1.29) | 0.005 | <0.001 |
| Dyslipidemia | 1.33 (1.18 to 1.51) | <0.001 | 0.96 (0.86 to 1.07) | 0.420 | <0.001 |
| CHD | 1.54 (1.38 to 1.71) | <0.001 | 1.10 (1.00 to 1.22) | 0.052 | <0.001 |
| Heart failure | 0.97 (0.85 to 1.12) | 0.720 | 1.41 (1.25 to 1.60) | <0.001 | <0.001 |
| CKD | 0.90 (0.75 to 1.08) | 0.250 | 1.05 (0.88 to 1.25) | 0.606 | 0.274 |
| CVD | 1.80 (1.63 to 1.98) | <0.001 | 1.23 (1.11 to 1.35) | <0.001 | <0.001 |
| COPD | 1.48 (1.31 to 1.66) | <0.001 | 1.03 (0.91 to 1.16) | 0.632 | <0.001 |
| Lipid‐lowering medication use | 1.06 (0.95 to 1.18) | 0.297 | 0.78 (0.70 to 0.87) | <0.001 | <0.001 |
| Aspirin use | 1.00 (0.91 to 1.11) | 0.979 | 0.99 (0.90 to 1.08) | 0.781 | 0.996 |
| Critical limb ischemia | 2.25 (1.99 to 2.54) | <0.001 | 3.47 (3.13 to 3.85) | <0.001 | <0.001 |
Proximal and distal results are reported from models within each region separately and reflect number of persons with specific regional results. Interaction results are reported from the model with all data together and required data from either or both regions.(ie, those with at least one Doppler assessment). People missing proximal results (n=905) were excluded only from the proximal region analysis, people missing distal results (n=54) were excluded only from the distal region analysis. Patients with postexercise proximal disease in either leg (n=2324) were excluded from this analysis. No disease was the reference group. CHD indicates coronary heart disease; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; OR, odds ratio.
Per 10‐year increase.
Survival
Of the 12 731 patients followed for a mean 5.9±3.1 years, 3039 (23.9%) died. Unadjusted Kaplan–Meier survival curves according to proximal and distal disease status are shown in Figure. We estimated the relative hazard of death for proximal and distal disease together, using multivariable Cox proportional hazards regression both with and without the interaction between proximal and distal disease (Table 4). In the main effects model, after adjustment for age and sex, the relative hazard of death was 1.3 for proximal disease only and 1.5 for distal disease only. However, there was a highly significant interaction (P<0.001) such that the presence of both proximal and distal disease was not associated with higher risk than the presence of either disease separately (HR, 1.4 proximal only; HR, 1.9 distal only; HR, 2.2 proximal and distal). In the model that further adjusted for risk factors and comorbidities, the same pattern was seen. In the final model that adjusted for resting ABI, there was no significant association between proximal disease and death, whereas the hazard ratio for death was 1.2 for those with distal disease. The same models were fitted on a subset of patients, excluding those with critical limb ischemia, with similar findings (see Table S1). Survival curves adjusted for age and adjusted for all covariates in the final survival model are provided in the Supplement.
Figure 1.
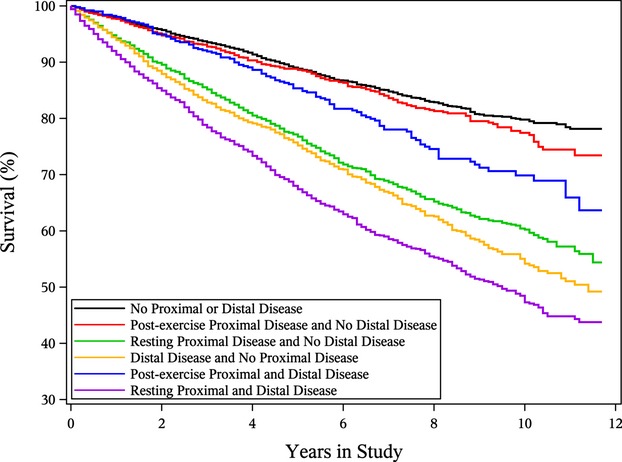
Kaplan–Meier survival curves for survival free of death over 12 years of follow‐up by proximal and distal disease status.
Table 4.
Hazard of Death Associated With Disease Status
| Model (n=12 731) | Main‐Effects Model | Models With Interaction between proximal and distal disease | |||
|---|---|---|---|---|---|
| Proximal Only | Distal Only | Proximal Only | Distal Only | Proximal and Distal | |
| A | |||||
| HR (95% CI) | 1.31 (1.25 to 1.36) | 1.52 (1.41 to 1.63) | 1.43 (1.36 to 1.51) | 1.90 (1.70 to 2.13) | 2.16 (1.69 to 2.78) |
| P Value | <0.001 | <0.001 | <0.001 | <0.001 | * |
| B | |||||
| HR (95% CI) | 1.22 (1.17 to 1.28) | 1.39 (1.29 to 1.49) | 1.33 (1.26 to 1.41) | 1.71 (1.53 to 1.92) | 1.84 (1.43 to 2.37) |
| P Value | <0.001 | <0.001 | <0.001 | <0.001 | * |
| C | |||||
| HR (95% CI) | 1.04 (0.99 to 1.10) | 1.17 (1.08 to 1.27) | 1.06 (0.99 to 1.14) | 1.23 (1.08 to 1.40) | 1.25 (0.93 to 1.67) |
| P Value | 0.127 | <0.001 | 0.083 | 0.002 | — |
Proximal disease was defined as an ordinal variable: no disease (reference level), postexercise disease (intermediate level), resting disease (highest level). The hazard ratios represent a change of one level. Distal disease was defined as a binary variable: no disease (reference level), resting disease (highest level). The disease status at each region for each person was defined as the most severe disease status across both legs. Those with no proximal Doppler assessment were classified based on imputed disease status (similarly for those with no distal Doppler assessment). Adjustments were as follows—model A, age and sex; model B, age, sex, obesity, CHD, cerebrovascular disease, heart failure, diabetes, hypertension, chronic kidney disease, malignancy, chronic obstructive pulmonary disease, smoking, lipid‐lowering medication use, and aspirin use; model C, model B variables+resting ABI. ABI indicates ankle brachial index; CHD, coronary heart disease; 95% CI, confidence interval; HR, hazard ratio.
Interaction of proximal and distal disease status significant at P<0.001.
Discussion
There are 2 main findings of our study. First, there appear to be 2 subtypes of PAD, proximal and distal. Each disease location is associated with a distinct risk factor and comorbidity profile. Female sex, smoking, hypertension, dyslipidemia, CHD, CVD, and COPD were more significantly associated with proximal disease, whereas older age, male sex, diabetes, heart failure, and critical limb ischemia were more significantly associated with distal disease. Second, patients with distal disease had poorer prognosis compared with patients without distal disease, independent of age, sex, comorbid conditions, medication (lipid‐lowering and aspirin) use and resting ABI, whereas patients with proximal disease showed no difference in prognosis after similar adjustment compared with patients without proximal disease. The inferences remained unchanged even after excluding patients with critical limb ischemia.
Like most atherosclerotic vascular disease, PAD affects the arterial bed in a nonuniform fashion.6,17–18 Disease location in patients with PAD is often classified as “proximal” or “distal,” but the definition has varied in different studies.5 Some investigators define aortoiliac arteries as proximal arteries, and femoral/popliteal and infragenicular arteries as “distal” arteries.11,17,19 Others define arteries above the knee20 and even above the ankle9,12 as “proximal.” In the present study, we classified the disease location as proximal and/or distal on the basis of involvement at the CF and PT/DP anatomical levels, respectively, as ascertained by noninvasive Doppler evaluation.
Diehm et al,6 in 2659 patients undergoing endovascular intervention for PAD, classified disease location of the 4205 atherosclerotic lesions as iliac, femoral‐popliteal, and infragenicular. The femoral‐popliteal location was the most common (51.2%). Similar results were reported by Vogt et al.14 Among 575 PAD patients with only single‐segment disease ascertained by noninvasive segmental blood pressure gradients, femoral‐popliteal disease was present in 47.3%, aortoiliac disease in 33.6%, and tibioperoneal disease in 19.1% of patients.14 Ozkan et al18 studied 626 patients with symptomatic PAD who underwent angiography and found that the crural segment (from the anterior tibial arteries to the ankle) was the site most commonly affected (70%). Of 8930 patients with PAD in the present study, CF was more commonly affected than the PT/DP location (57.6% and 42.9%, respectively). Because distal Doppler abnormality can be secondary to reduced flow in a proximal vessel, to avoid misclassification, a worsening of Doppler signals was required to label disease as distal.
Predilection for certain anatomical sites may be a result of differences in wall composition, arterial diameter, or hemodynamic factors.14,18 In addition, certain conventional risk factors may predispose differentially to proximal versus distal disease in PAD patients. Several clinical and epidemiologic studies have investigated the association of risk factors with disease location in PAD.6,9,11,14,18–19 In the present study, we found that those with only distal disease were older (73.0±11.0 years), consistent with the results of previous studies.6,9,14,17,19 Disease location also significantly differed between men and women,6 with men more often having distal disease than proximal disease. Some studies reported that men more often had iliac disease,11,19,21 whereas a study including younger (<50 years) patients with PAD showed that female sex was associated with the aortoiliac disease.22 Another study showed that sex was not related to disease distribution.18
Of the conventional risk factors for PAD, smoking status and diabetes mellitus were strongly associated with disease distribution. In the present study, history of smoking was most frequent in patients with only resting proximal disease (93.0%) and was more significantly associated with proximal disease than with distal disease (interaction P<0.001), consistent with other reports.6,9,18–19 Our univariable results are concordant with prior studies showing that diabetes was more strongly associated with distal disease than with proximal disease (interaction P<0.001).5–6,9,14,18 In multivariable regression analyses, smoking history was again more associated with proximal disease, whereas diabetes was more strongly associated with distal disease. We also found that dyslipidemia was also more strongly associated with proximal disease than with distal disease. In Ozkan et al's study, serum lipid levels were not related to the segmental distribution of PAD.18 A study of younger patients (mean age, 42.7± 4.2 years) with PAD showed that dyslipidemia (based on either total cholesterol or triglyceride level) was more prevalent in aortoiliac disease than in distal disease.17 The data on the association between hypertension and PAD localization are not consistent, either. In the present study, history of hypertension was more strongly associated with proximal disease. However, a trend toward more distal lesions in hypertensive patients was noted in other studies.17,19 Diehm et al6 found no relationship between hypertension and lesion sites.
The prognosis of PAD patients has been reported to differ by disease location.6,12,14,19,23 In the present study, patients with distal disease had an increased risk of death compared with those with normal distal signals even after adjustment for risk factors for atherosclerosis, comorbid conditions, and resting ABI levels, whereas the risk of death was not significantly increased among those with a proximal disease location after adjustment for resting ABI. This finding differs from previous studies.12,14,19,23 Two studies that assessed PAD patients with flow velocity by Doppler ultrasound and segmental blood pressure ratios showed higher mortality in proximal PAD.12,14 In a study of 400 patients with PAD, ascertained by digital subtraction angiography, proximal PAD was associated with worse prognosis compared with patients with distal disease.19 Patients in this study were hospitalized, mostly men (77%) and had PAD severe enough to be referred for angiography, in contrast to our cohort of 12 731 consecutive patients with known or suspected PAD referred for non‐invasive evaluation in the outpatient setting. Disease location was based on angiographic appearance whereas we assigned disease location based on Doppler.
We have demonstrated that patients with distal disease have poorer survival than those without distal disease independent of relevant covariates (HR, 1.2; 95% CI, 1.1 to 1.3). A previous study showed that multilevel disease was associated with greater mortality in PAD patients.14 In the present study, patients with multilevel disease had more prevalent CHD and CVD compared with patients with only distal disease, which may have led to earlier treatment in this group. In previous studies, patients with CHD and PAD, when compared with patients with PAD alone, received more cardiovascular medical therapy, reducing their long‐term mortality.24–25 van Kuijk et al26 reported that PAD patients with CHD or/and CVD received better medical treatment compared with patients with PAD alone.
Study Strengths and Limitations
To the best of our knowledge, the study sample (n=12 731) contained the largest cohort of patients with PAD (n=8930) for whom disease location has been ascertained with Doppler. All the patients included underwent Doppler evaluation in an accredited noninvasive vascular laboratory, and disease location was ascertained by uniform methods. Patients in our study were identified and evaluated from the noninvasive vascular laboratory of a tertiary‐care center, and some degree of referral bias may have been present. Use of medical record review to ascertain risk factors may be associated with reporting bias. We used the lower of the two ABIs from each leg whereas conventionally the higher ABI is used. We based our choice on a study that demonstrated that the ABI based on the lower ankle pressure identifies a greater number of patients at risk for adverse cardiovascular events than an ABI based on the higher ankle pressure.28 While the Doppler criteria used to assign disease location in this study are commonly used in clinical vascular laboratories we cannot exclude some degree of misclassification. However, Doppler derived inference of disease location is well correlated with that derived from angiography (see Supplement), and the latter is not practical in large studies given its cost and invasive nature. Missing data were also a limitation, as a number of patients (n=905) had Doppler evaluations performed only at the distal arteries. To address this, results from the proximal and distal regions separately were reported, as well as results using the combined data.
Conclusions
PAD involves >1 arterial location, with the CF being the most commonly affected. Proximal and distal disease was associated with distinctive risk factor and comorbidity profiles. Older age, male sex, diabetes, heart failure, and critical limb ischemia were more significantly associated with distal disease, whereas female sex, smoking, hypertension, dyslipidemia, CHD, CVD, and COPD were more significantly associated with proximal disease. Patients with distal disease had poorer prognosis compared with patients without distal disease, independent of age, sex, comorbid conditions, medication (lipid‐lowering and aspirin) use, and resting ABI, whereas patients with proximal disease showed no difference in prognosis after similar adjustment compared with patients without proximal disease. These findings suggest PAD is complex and heterogeneous and not a uniform entity. The implications for epidemiologic studies, including biomarker and genetic association studies as well as drug trials, are substantial.
Disclosures
None.
Acknowledgments
We thank Vicki Schmidt for help with manuscript preparation.
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd‐Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel‐Smoller S, Hong Y. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007; 115:e69-e171 [DOI] [PubMed] [Google Scholar]
- 2.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006; 114:688-699 [DOI] [PubMed] [Google Scholar]
- 3.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary, a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006; 47:1239-1312 [DOI] [PubMed] [Google Scholar]
- 4.Hills AJ, Shalhoub J, Shepherd AC, Davies AH. Peripheral arterial disease. Br J Hosp Med (Lond). 2009; 70:560-565 [DOI] [PubMed] [Google Scholar]
- 5.Aboyans V, Lacroix P, Criqui MH. Large and small vessels atherosclerosis: similarities and differences. Prog Cardiovasc Dis. 2007; 50:112-125 [DOI] [PubMed] [Google Scholar]
- 6.Diehm N, Shang A, Silvestro A, Do DD, Dick F, Schmidli J, Mahler F, Baumgartner I. Association of cardiovascular risk factors with pattern of lower limb atherosclerosis in 2659 patients undergoing angioplasty. Eur J Vasc Endovasc Surg. 2006; 31:59-63 [DOI] [PubMed] [Google Scholar]
- 7.Begelman SM, Jaff MR. Noninvasive diagnostic strategies for peripheral arterial disease. Cleve Clin J Med. 2006; 73suppl 4:S22-S29 [DOI] [PubMed] [Google Scholar]
- 8.Chan D, Anderson ME, Dolmatch BL. Imaging evaluation of lower extremity infrainguinal disease: role of the noninvasive vascular laboratory, computed tomography angiography, and magnetic resonance angiography. Tech Vasc Interv Radiol. 2010; 13:11-22 [DOI] [PubMed] [Google Scholar]
- 9.Aboyans V, Criqui MH, Denenberg JO, Knoke JD, Ridker PM, Fronek A. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006; 113:2623-2629 [DOI] [PubMed] [Google Scholar]
- 10.Garcia LA. Epidemiology and pathophysiology of lower extremity peripheral arterial disease. J Endovasc Ther. 2006; 13suppl 2:II3-II9 [DOI] [PubMed] [Google Scholar]
- 11.Smith FB, Lee AJ, Fowkes FG, Lowe GD, Rumley A. Variation in cardiovascular risk factors by angiographic site of lower limb atherosclerosis. Eur J Vasc Endovasc Surg. 1996; 11:340-346 [DOI] [PubMed] [Google Scholar]
- 12.Criqui MH, Coughlin SS, Fronek A. Noninvasively diagnosed peripheral arterial disease as a predictor of mortality: results from a prospective study. Circulation. 1985; 72:768-773 [DOI] [PubMed] [Google Scholar]
- 13.Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010; 85:678-692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogt MT, Wolfson SK, Kuller LH. Segmental arterial disease in the lower extremities: correlates of disease and relationship to mortality. J Clin Epidemiol. 1993; 46:1267-1276 [DOI] [PubMed] [Google Scholar]
- 15.Arain FA, Ye Z, Bailey KR, Chen Q, Liu G, Leibson CL, Kullo IJ. Survival in patients with poorly compressible leg arteries. J Am Coll Cardiol. 2012; 59:400-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kullo IJ, Fan J, Pathak J, Savova GK, Ali Z, Chute CG. Leveraging informatics for genetic studies: use of the electronic medical record to enable a genome‐wide association study of peripheral arterial disease. J Am Med Inform Assoc. 2010; 17:568-574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen ME, Valentine RJ, McIntire DD, Myers SI, Chervu A, Clagett GP. Age‐related differences in the distribution of peripheral atherosclerosis: when is atherosclerosis truly premature? Surgery. 1995; 118:834-839 [DOI] [PubMed] [Google Scholar]
- 18.Ozkan U, Oguzkurt L, Tercan F. Atherosclerotic risk factors and segmental distribution in symptomatic peripheral artery disease. J Vasc Interv Radiol. 2009; 20:437-441 [DOI] [PubMed] [Google Scholar]
- 19.Aboyans V, Desormais I, Lacroix P, Salazar J, Criqui MH, Laskar M. The general prognosis of patients with peripheral arterial disease differs according to the disease localization. J Am Coll Cardiol. 2010; 55:898-903 [DOI] [PubMed] [Google Scholar]
- 20.van der Feen C, Neijens FS, Kanters SD, Mali WP, Stolk RP, Banga JD. Angiographic distribution of lower extremity atherosclerosis in patients with and without diabetes. Diabet Med. 2002; 19:366-370 [DOI] [PubMed] [Google Scholar]
- 21.Audet P, Therasse E, Oliva VL, Soulez G, Cote G, Wistaff R, Nguyen PV, Blair JF, Bui BT, Cusson JR. Infrarenal aortic stenosis: long‐term clinical and hemodynamic results of percutaneous transluminal angioplasty. Radiology. 1998; 209:357-363 [DOI] [PubMed] [Google Scholar]
- 22.Barretto S, Ballman KV, Rooke TW, Kullo IJ. Early‐onset peripheral arterial occlusive disease: clinical features and determinants of disease severity and location. Vasc Med. 2003; 8:95-100 [DOI] [PubMed] [Google Scholar]
- 23.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992; 326:381-386 [DOI] [PubMed] [Google Scholar]
- 24.McDermott MM, Mehta S, Ahn H, Greenland P. Atherosclerotic risk factors are less intensively treated in patients with peripheral arterial disease than in patients with coronary artery disease. J Gen Intern Med. 1997; 12:209-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welten GM, Schouten O, Hoeks SE, Chonchol M, Vidakovic R, van Domburg RT, Bax JJ, van Sambeek MR, Poldermans D. Long‐term prognosis of patients with peripheral arterial disease: a comparison in patients with coronary artery disease. J Am Coll Cardiol. 2008; 51:1588-1596 [DOI] [PubMed] [Google Scholar]
- 26.van Kuijk JP, Flu WJ, Welten GM, Hoeks SE, Chonchol M, Vidakovic R, Verhagen HJ, Bax JJ, Poldermans D. Long‐term prognosis of patients with peripheral arterial disease with or without polyvascular atherosclerotic disease. Eur Heart J. 2010; 31:992-999 [DOI] [PubMed] [Google Scholar]


