Abstract
Background
Patients with chronic conditions often use complex medical regimens. A nurse‐led strategy to support medication therapy management incorporated into primary care teams may lead to improved use of medications for disease control. Electronic health record (EHR) tools may offer a lower‐cost, less intensive approach to improving medication management.
Methods and Results
The Northwestern and Access Community Health Network Medication Education Study is a health center–level cluster‐randomized trial being conducted within a network of federally qualified community health centers. Health centers have been enrolled in groups of 3 and randomized to (1) usual care, (2) EHR‐based medication management tools alone, or (3) EHR tools plus nurse‐led medication therapy management. Patients with uncontrolled hypertension who are prescribed ≥3 medications of any kind are recruited from the centers. EHR tools include a printed medication list to prompt review at each visit and automated plain‐language medication information within the after‐visit summary to encourage proper medication use. In the nurse‐led intervention, patients receive one‐on‐one counseling about their medication regimens to clarify medication discrepancies and identify drug‐related concerns, safety issues, and nonadherence. Nurses also provide follow‐up telephone calls following new prescriptions and periodically to perform medication review. The primary study outcome is systolic blood pressure after 1 year. Secondary outcomes include measures of understanding of dosing instructions, discrepancies between patient‐reported medications and the medical record, adherence, and intervention costs.
Conclusions
The Northwestern and Access Community Health Network Medication Education Study will assess the effects of 2 approaches to support outpatient medication management among patients with uncontrolled hypertension in federally qualified health center settings.
Clinical Trial Registration
URL: clinicaltrials.gov. Unique identifier: NCT01578577.
Keywords: adherence, electronic health records, hypertension, medication reconciliation, medication therapy management, nurse educator
Introduction
Patients with chronic conditions are asked to use increasingly complex medical regimens.1 Long‐term proper adherence is essential to reap the health benefits demonstrated in clinical trials; however, nonadherence is widespread and is linked to worse outcomes including increased mortality.2 For major chronic illnesses, common forms of nonadherence include failure to fill new prescriptions,3 incomplete adherence to medications being used,4 and premature discontinuation.5 Complex drug regimens increase the risk for medication errors and adverse drug events. Outpatient adverse drug events are prevalent, and many are either preventable or ameliorable.6 Individuals with chronic illness and the elderly are at greatest risk for unintentional medication errors, failing to properly self‐administer a medication as intended.6–8
For outpatient care, patients (or their surrogates) are primarily responsible for executing medication care plans. The expectations for medication management placed on patients by the healthcare system are considerable, with patients expected to perform multiple steps to adhere to their care plan and gain the benefits of chronic drug therapy. Figure 1 provides a conceptual schema of the tasks involved, how they relate to outcomes, and how the process can break down. Ideally, adherence to the care plan begins with an exchange of information between prescriber and patient followed by their agreement on an appropriate medication plan. As a result of these interactions or through information obtained from other sources, patients acquire the information they will use to administer medications on an ongoing basis. Information about prescription medication may come from sources directly involved in the prescribing and dispensing process (prescriber/other healthcare team member, prescription, pharmacist, medication guides, prescription labels, or auxiliary labels) or from other sources (references, Internet, family, friends, advertising).9 Patients and their healthcare providers must also recognize safety problems or adverse events when they occur and modify the medication care plan when necessary. Patients and providers must also monitor the efficacy and tolerability of prescribed medications. Changes to the medication plan are common in response to changing disease conditions, adverse effects, cost considerations, or lack of efficacy. Patients are the ones who must successfully execute these changes. New medicines may be added, others are discontinued, and dosages may be altered. Errors in any of these steps could lead to adverse drug events or reduced efficacy of treatment.
Figure 1.
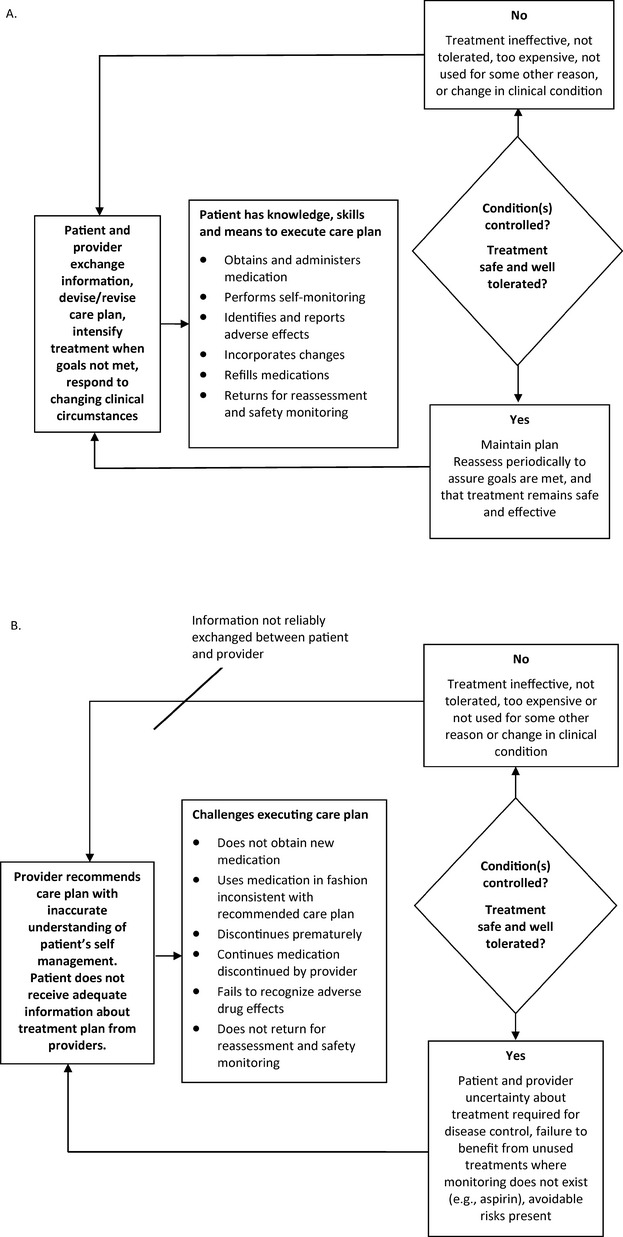
Conceptual schema for executing chronic disease medication care plans. A, Optimal planning and execution of medication care plan for chronic illness care. B, Obstacles to successful medication care plan planning and execution.
A basic understanding of one's medication (the indications for it, how to administer, adverse effects to be aware of) is an essential prerequisite for a safe, successful execution of a medication plan. Unfortunately, evidence indicates that important medication information is delivered to patients in a haphazard way. Multiple studies show that physicians frequently do not adequately counsel patients on safe and appropriate drug use.10–11 Furthermore, physicians rarely assess patient comprehension12 or discuss medication affordability even though out‐of‐pocket cost directly influences adherence.13 In routine outpatient practice, pharmacists seldom provide medication counseling.10
The information patients do receive about their medications may not be provided in a manner that supports prescribed use. Studies indicate that written medication information that accompanies prescription drugs is difficult to understand for many patients.14 Recent studies have repeatedly shown that a large proportion of patients have difficulty performing routine medication management tasks, such as correctly interpreting dosing instructions8,15 and warning labels on prescribed medications,8 accurately and completely self‐reporting currently used medications, and possessing knowledge of the basic indications for prescribed medicine.16–18 In several studies, limited literacy was associated with an increased risk for these medication‐related problems.7–8,15,17
Discrepancies between the medications patients are taking and the medications healthcare providers believe the patients are taking may indicate the presence of ≥1 important problem: (1) patients may unknowingly be out of agreement with the medication care plan intended by their providers (eg, they may have inadvertently not started a medication or failed to realize that their clinicians intended them to stop using a medication); (2) patients may be deliberately not adhering; (3) the healthcare team may have made errors (eg, errors in documentation or actual errors in care); and (4) the healthcare team may be unaware of medications a patient obtained elsewhere. Without systems in place to regularly encourage medication reconciliation, these important problems may go unaddressed.
Multiple recent studies have shown that medication discrepancies are highly prevalent. Physicians rarely perform a comprehensive review of chronic medications19 and therefore may be unaware when patients are not taking essential medications or using medications that were discontinued. Problems result in either scenario, as patients become at risk for potentially dangerous use of medications that were stopped for medical reasons or duplication of medications when a substitution from 1 medication to the next was intended. Medications listed in patients' medical records are frequently discordant with the medications patients report taking.17,20–22 Medication discrepancies are particularly common among patients using multiple medications who have been recently hospitalized.23–25
Among adults receiving care at community health centers in a prior study, patients with low literacy were more likely to have medication discrepancies for their hypertension medications than were patients with adequate literacy.17 Medication reconciliation errors have been associated with worse blood pressure control16 and can curtail patient safety benefits available through the use of the electronic health record (EHR). If medications are not recorded within a patient's EHR, safety features such as the detection of drug–drug interactions or allergies will not function properly.
Improving Medication Use Through Medication Therapy Management
Medication therapy management (MTM) has evolved as a systematic approach to assist patients with many of the medication‐related problems described above. Formally introduced with the implementation of Medicare Part D, MTM now serves as a mandate to Part D insurers to provide qualifying patients with medication assistance. As defined by the American Pharmacists Association, MTM includes medication review, assembly of a personal medication record, development of patient medication‐related action plans, clinical interventions when necessary, and follow‐up.26 The rationale for this program is to provide Medicare beneficiaries who have high drug complexity and high drug cost with additional education and support to improve medication adherence, improve the detection of medication misuse, and reduce adverse drug events.27
Published outcomes from MTM are scant and subject to important methodological limitations. In the Iowa State Medicaid Pharmaceutical Case Management program, MTM reduced the number of potentially inappropriate medications used, but the comparison group was not randomly assigned. In this study, healthcare utilization did not change, and important clinical outcomes were not examined.28 Another report indicated that an MTM intervention increased medication knowledge but not adherence, although there was no control group.29 A Minnesota Blue Cross Blue Shield (BCBS) study done within medical practices compared with historical controls suggested that the MTM program improved the achievement of therapeutic goals and significantly reduced healthcare costs.30 We are not aware of well‐controlled trials performed to examine the impact of a general MTM intervention on clinical outcomes. Rigorously testing viable MTM approaches, particularly among high‐risk groups such as those with limited literacy, is clearly warranted.
In contrast to the limited evidence base for general MTM interventions, multiple disease‐specific interventions that have employed some features of MTM have shown beneficial effects. In the case of hypertension, controlled trials employing pharmacists to perform several hypertension‐related tasks for the most part have shown beneficial effects on blood pressure. A meta‐analysis of 13 controlled trials revealed greater reductions in systolic blood pressure in intervention groups compared with controls (6.9 mm Hg difference). Interpretation of these studies is somewhat limited because of their quality and the possibility of publication bias.31 Other recent trials employing pharmacists have also shown beneficial effects in hypertension.32–33 Studies employing nurses have been mixed, with studies showing positive effects34–36 or no effect.37–38 Similar to hypertension, the findings in interventions aimed at improving diabetes control using pharmacists or nurses have generally been favorable but are similarly heterogeneous.33,39 These disease‐specific studies generally support the notion that the addition of a pharmacist or nurse to the larger care delivery team can produce favorable results. A recent meta‐analysis of interventions to improve adherence among patients with cardiovascular disease or diabetes also supports this conclusion.40 However, it is impossible to apply conclusions from disease‐specific studies (which targeted disease‐specific care processes) to general MTM approaches. Whether non‐disease‐focused MTM interventions change important indicators of chronic disease control is essentially unknown.
A specific limitation to pharmacy‐based MTM (as opposed to MTM delivered by a member of the primary care team) is that medication review performed separately from patients' usual source of care may not effectively detect medication discrepancies, as the third‐party pharmacist would not know firsthand the intent of prescribing clinicians. Also most data on monitoring and disease control would not be available to the pharmacy‐based provider. This study will test the effectiveness of MTM provided by a clinician based within a patient's primary care practice.
MTM has been conceptualized as a task performed by a clinician (typically a pharmacist).26–27 This approach would be costly if widely applied to the large population of patients with chronic disease using multiple chronic medications. Under Medicare Part D, only individuals with anticipated annual drug costs of >$4000 would be eligible for reimbursed MTM, thereby excluding many patients who could potentially benefit from these services and potentially eliminating systemic economic gains. However, health information technology could be leveraged to provide some MTM features at a cost that is not prohibitive. Contemporary EHRs could serve as a platform from which to deliver automated tools to assist with the medication management process for all patients without requiring additional clinical personnel. Once developed, these MTM tools could be widely applied at increasingly diminishing incremental costs.
A substantial portion of medication nonadherence is not a result of a failure to understand basic medication tasks but rather of patients' deliberate choices not to use medications. Research on health behaviors has identified several important determinants of whether an individual performs a specific health behavior: attitudes toward the behavior, the perception of social norms, and one's own sense of self‐efficacy in performing the behavior.41 Patients may have negative attitudes or conflicting beliefs toward the efficacy of certain medications or be influenced by friends or family to not use a prescribed medicine. The limited interactions between patients and their providers and currently used print material may not be sufficient to overcome these negative perceptions of medications. One's sense of self‐efficacy may be reduced if dosing requirements are too burdensome or if cost barriers to obtaining specific medications are not addressed.13,42 The approach we have outlined may help patients to consciously make decisions about their medications that would more likely promote desired health outcomes. For instance, the focus on medication list review will ideally help to initiate provider–patient discussions about medications a patient has chosen not to use so that providers have additional opportunities to engage in discussions about nonadherence that could affect patients' knowledge, attitudes, and behaviors. Similarly, providing simplified medication information sheets should be a more reliable way to give patients salient information about the purpose of using medications that could foster more favorable attitudes at a critical time (point of prescribing).
Although potentially cost‐prohibitive for many primary care settings, the inclusion of a healthcare professional may be necessary to confirm patients receive the EHR‐based MTM materials, subsequently understand how to safely administer their current regimens, and continually adhere to their medicines to achieve optimal health outcomes. A trained clinician who is specifically tasked with helping patients with medication management could use these tools while directly assisting patients with medication list review, information exchange, and dosing simplification. Although prior MTM strategies have used both nurses and pharmacists, availability and cost would likely favor the use of a nurse in primary care settings.
Feasible and sustainable approaches to help patients safely execute complicated outpatient medical regimens are needed. The overall objective of this study is to rigorously evaluate 2 related approaches to improving patients' medication self‐management in primary care settings. We hypothesized that hypertension patients receiving EHR‐based medication management tools alone or EHR tools plus nurse‐led medication management education, compared with usual care, would have lower systolic blood pressure, better understanding of dosing instructions, fewer discrepancies between patient‐reported medications and the medical record, and better medication adherence.
Methods
Health Center Enrollment
Health centers that are part of the Access Community Health Network in the greater Chicago, Illinois, area are eligible for participation if they have completed their implementation of the common EHR shared by the network (EpicCare, Epic Systems Corporation, Verona, WI). Study investigators approached health center leadership, explained the study to them and requested permission to participate. Because the print materials are in English, centers with predominantly non‐English‐speaking patient populations were not approached by study investigators. The institutional review board of Northwestern University reviewed and approved the study.
Randomization
The flow of health centers and patients is depicted in Figure 2. Centers have been enrolled in groups of 3 and randomized to (1) usual care, (2) EHR‐based medication management tools alone, or (3) EHR tools plus nurse‐led medication management education. For the first group of health centers randomized, random numbers were manually selected in a blind fashion by someone not involved with the study, and the allocation of the random number to the study arms was concealed until the randomization was completed. For each subsequent round of randomization of new centers, we use a weighted randomization scheme to increase the likelihood that study arm sizes remained similar. We examined the existing numbers of patients enrolled in each of the 3 study arms to date, and estimated numbers of patients anticipated to be enrolled on the basis of the number of adult hypertension patients who received care at the new health centers (identified through queries of the EHR). For each of the 6 possible random permutations of assigning 3 health centers to 3 arms, the sum of the existing sample size and anticipated sample size was calculated for each arm. The standard deviation of this resulting sample size was calculated across arms. Permutations were weighted by the inverse of these standard deviations. A permutation was then selected at random from the weighted set of 6 permutations. This procedure is more likely to result in similar sample sizes after the new round than a simple random unweighted sampling from the 6 permutations.
Figure 2.
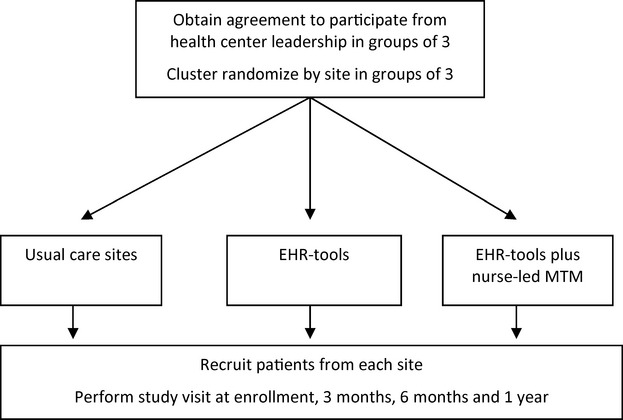
Health center and participant flow diagram. EHR indicates electronic health record; MTM, medication therapy management.
Patient Enrollment
Following health center randomization, individual patients have been recruited using 2 strategies. We generated lists of adult patients with hypertension and ≥3 medications prescribed in the EHR. Access Community Health Network staff have managed all recruitment contact with patients through this list. Primary care physicians have been sent emails within the EHR and have the opportunity to indicate which potentially eligible patients should not be contacted. Unless a physician indicates a patient should not be contacted, patients receive informational letters notifying them about the study. The letter provides a number for patients to call to opt out of the study immediately; the letter also informs patients that they will be called about 10 days after the initial mailing to tell them more about the study and assess their interest in participating.
Patient recruitment also occurs using institutional review board–approved flyers and scripts with research assistants directly approaching patients in each health center. Research assistants explain the study and assess eligibility of interested patients in private areas within the health centers. All patients provide written informed consent before participation.
Eligibility criteria
Patients are eligible if they (1) are ≥18 years, (2) have ≥3 medications of any kind prescribed by their physicians, (3) have mean blood pressure ≥130 mm Hg systolic or ≥80 mm Hg diastolic if they have diabetes or ≥140 mm Hg systolic or ≥90 mm Hg diastolic if they do not have diabetes, (4) have a Mini‐Cog Exam43 score ≥3, (5) are primarily responsible for administering medication, and (6) do not intend to move or change their usual source of medical care during the next year. Patients who are non–English speaking are not eligible.
Interventions
The Electronic Health Record–Based Health Literacy Medication Therapy Management Intervention strategy leverages the Epic EHR platform. EHR interventions are implemented at the health center level and become part of routine work flow for all adult patients. Earlier versions of these interventions were field‐tested previously among patients with varying literacy skills.44 After refinement, final versions of these materials were reviewed and determined to be appropriate by the community health center clinical documentation committee.
Medication list review
As patients arrive, electronic check‐in triggers the printing of their current medication list, accompanied by plain‐language instructions for patients to (1) review medicines; (2) strike out medicines they are not taking; (3) identify if they are taking the remaining medicines as described by the listed instruction (yes, no, only as needed); (4) identify any concerns for each medicine they would like to discuss with their doctors (none, need refill, side effects, cost, other); and (5) add any medicine (prescription, over‐the‐counter, vitamin or other supplement) that they take regularly that is not included in their medical record. A sample of this sheet is provided in Figure 3. These procedures were field‐tested among 150 patients at the Northwestern Medical Faculty Foundation General Medicine health center. Nearly all patients (91%) in the intervention arm receive their medicine list at check‐in, 85% of those receiving lists review them with their physicians, and >90% have discrepancies identified and removed, with an updated list printed and given to patients at discharge. The 20 physicians whose patients received this intervention gave unanimously positive feedback on the value of this process.
Figure 3.
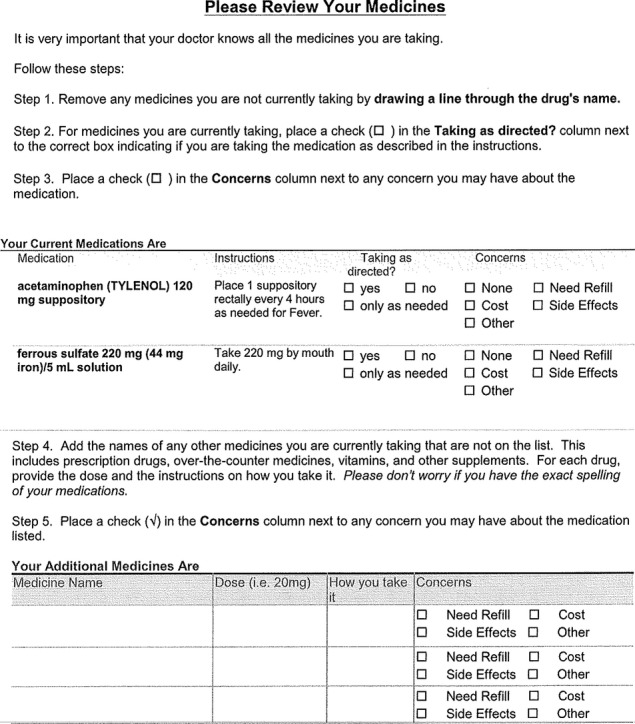
Example of a medication list review sheet.
Med sheets
We created single‐page, plain‐language medication information sheets with content appropriately sequenced from a patient's perspective (drug name, indication, purpose/benefit, how to take and for how long, when to call your doctor, when to stop taking and call your doctor, important information) and following other health literacy best practices. A sample sheet is shown in Figure 4. These sheets are automatically generated for 125 different common chronic disease medications when prescriptions are ordered or refilled and are distributed to patients at checkout along with an after‐visit summary. Lexile analyses on initial information sheets confirmed that each met a <8th‐grade readability standard. The original content was initially developed by 2 pharmacists, supported by an environmental scan of existing tools. Patients, physicians, and health literacy experts reviewed the material and guided revision. We also provide an educational folder that details to patients what they should do before, during, and after a medical encounter.
Figure 4.
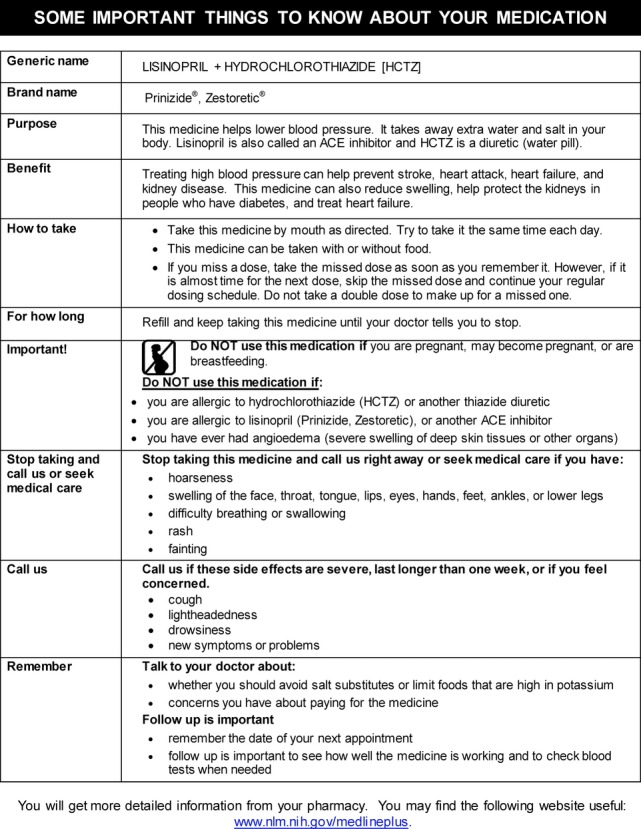
Example of a medication information sheet.
EHR tools plus nurse‐led medication therapy management
Health centers randomized to this study arm have the same implementation of EHR tools above. In addition, patients who enroll in the study receive a medication therapy management intervention delivered by a nurse educator. Nurses receive training from a physician (S.D.P.) regarding the objectives of the intervention, the tasks to be accomplished in advance of and during patient education sessions (listed in Table 1), and methods of communication and collaboration with the rest of the primary care team. During the study, nurses performing medication management consult with patients' primary care providers when patient‐specific questions arise and with the study principle investigator to review general approaches. Before contacting the patient, the nurse educator reviews the EHR medication list and physician notes to identify potential medication errors (duplicates, internal discrepancies) and considers safety monitoring and follow‐up with concern for potential contraindications (eg, renal dysfunction). The nurse will communicate with the treating physician using EHR‐based email (or directly) to clarify questions and will document the review in the medical record.
Table 1.
Nurse‐Led Medication Management Tasks
| Initial chart review (prior to patient contact) | Identify potential reconciliation errors (duplicates within drug class, discrepancies between EHR medication list and clinical notes) |
| Identify gaps in laboratory monitoring (eg, failure to obtain follow‐up labs as instructed by provider, failure to monitor renal and electrolyte function when indicated in prior year, failure to monitor diabetes or lipids when indicated) | |
| Check for potential contraindications due to renal dysfunction or drug–drug interactions | |
| Medication counseling session (general) | Assess medication comprehension and tailor counseling based on patient understanding |
| Use teach‐back | |
| Review patient's medication usage and perform medication reconciliation with EHR medication list | |
| Assist with regimen dosing consolidation when feasible | |
| Help patients maintain their personal medication record | |
| Assess adherence and identify patterns of improper use | |
| When nonadherence is identified, assess reason(s) | |
| Medication counseling session (following medication change) | Determine whether patient obtained new prescription(s), assess use and comprehension, and identify problems or adverse effects |
| Educate about new prescriptions (use medication information sheets) | |
| Assess patient understanding of changes using teach‐back | |
| Assist with medication‐related problem solving (with input from patient's physician when needed) | |
| Recontact patient | Four to 7 days following a new office visit at which medication regimen is changed |
| Within 3 months of prior contact when uncontrolled chronic condition (eg, hypertension, diabetes, asthma) is present | |
| Within 6 months when controlled chronic condition(s) are present | |
| As clinically indicated or as requested by the patient's physician | |
| When contacted by patient |
EHR indicates electronic health record.
Medication counseling sessions can occur in person at a health center or by phone. The initial session includes assessment of medication comprehension and counseling based on patient understanding, review of the patient's medication usage and medication reconciliation with the list in the medical record, assistance with regimen dosing consolidation when feasible, and development of a medication table for complex regimens. Nurses assess adherence and identify patterns of improper use and reasons for nonadherence when present. Education about medication uses teach‐back to confirm understanding. Nurses also assess patients' knowledge of their chronic conditions, address misconceptions, and reinforce the role that proper medication use plays in disease control.
During the intervention year, the nurse attempts to conduct brief medication education and review with patients around the time of scheduled office visits. When new chronic disease medications are prescribed, the nurse telephones patients 4 to 7 days after to determine whether the patient obtained the new prescription(s), to assess use and comprehension, and to identify any problems. A nurse proactively telephones patients who have not returned within 3 months if the patient has an uncontrolled chronic condition (hypertension, diabetes, or other chronic condition [eg, heart failure, asthma]) or within 6 months if chronic conditions are controlled. Nurse‐identified medication problems are conveyed to the patient's primary physician (using EHR email if not urgent and telephone or page if urgent). Other nurse‐initiated actions include prescription refills, directing patients who need renewal of state medical assistance to appropriate staff members, and facilitating appropriate return visits or referrals.
Usual care
Enrolled patients from health centers assigned to usual care will receive study assessments from research assistants at the health centers, but there are changes neither to site EHR functionality nor to health center work flow.
Baseline Measurements
Baseline measures include sociodemographic characteristics, primary language used at home, chronic health conditions, current prescription medication usage, the Patient Activation Measures (13 items).45 Baseline measurements also include the Newest Vital Sign, which is a reliable screening tool to determine risk for limited health literacy.46 It has a high sensitivity for predicting limited literacy skills and is strongly correlated with the short form of the Test of Functional Health Literacy in Adults (r=0.61).46
Outcome Assessments
Trained research assistants conduct outcome assessments 3, 6, and 12 months following enrollment.
Primary Outcome
The primary study outcome is systolic blood pressure. Analysis will be done according to the intention‐to‐treat principle. Research assistants will perform standardized measurements of blood pressures and pulse at baseline and after 3, 6, and 12 months using an automated device (Omron HEM‐907XL). Patients are seated quietly with their feet and back supported for 5 minutes before blood pressure is obtained. Three recordings are to be performed at each visit, and the mean of the second and third readings is used to indicate the blood pressure for that visit. Patient positioning, arm selection, cuff size selection and other techniques follow the procedures for blood pressure measurement of the National Health Examination and Nutrition Survey.47 For the subgroup with diabetes, measures of disease control for hemoglobin A1c, and low‐density lipoprotein cholesterol are obtained from patients' electronic health records. These disease control measures can be scored even when repeat laboratory measurement is not obtained. For diabetes control, the quality measures examined are: (1) an HbA1c test (the most recent measurement recorded within the EHR in the preceding 365 days was >9.0%, poor diabetes control; (2) an alternative measure using the threshold of <8.0% (HbA1c <8.0); and (3) low‐density lipoprotein‐cholesterol obtained in the preceding 365 days and <100 mg/dL.48
Secondary Outcomes
Medication reconciliation
Patients are asked to bring their prescription medications with them to the 3‐, 6‐, and 12‐month study visits. Patients are asked to report on the different prescription medications they are taking and can consult their pill bottles or other aids that they use to keep track of medications. Patients can identify additional medications prescribed that they may not have currently in their possession. We will compare patient‐reported medication regimens with the medical record medication list on the same date.
We will classify patients into 3 primary categories: (1) reconciled—patients name the same medications as recorded in the medical record; (2) reconciliation discrepancies—patients could name ≥1 more medication, but the list was not in full agreement with the medical record; and (3) unable to name any medications—patients provided no recognizable names of prescription medications listed in their medical record. We have already used this classification in 2 recent studies.16–17 We will apply binary classifications (reconciled versus not reconciled) to 5 groups of medications for each patient: (1) all not‐as‐needed prescription medications, (2) antihypertensive medications, (3) diabetes medications, (4) lipid‐lowering medications, and (5) all prescription medications including as needed.
Medication understanding
Understanding of medication instructions and dosing will be assessed through a structured questionnaire. Patients will demonstrate how they take each medication by dose and frequency. We will apply binary classifications (full understanding versus not) to the same 5 groups of medications for each patient. The study team has extensively used these methods in prior studies to evaluate patients' proper understanding and demonstrated use of multidrug regimens.49–50 Patients' knowledge of medication indications will be assessed by a physician, nurse, or pharmacist who is blinded to the patient's study assignment.18
Medication adherence
Adherence is measured for each prescription medication using (1) a 4‐day assessment of pills taken/pills prescribed based on patient self‐report, (2) a 24‐item Patient Medication Adherence Questionnaire51 that will assess individual barriers (eg, cost, adverse effects, salience, stigma, beliefs), and (3) in‐person pill count. The questionnaire developed by Morisky is also used as a general measure of adherence.52 Pill count will be used for antihypertensive, diabetes, and lipid‐lowering medications. Adherence will be treated both continuously (pills taken/pill prescribed) and dichotomously (yes/no—≥80% of expected pills absent from pill bottle). As no single measure may adequately capture a patient's behavior related to taking medicine, we use a diverse set of assessments and will also be able to derive a general factor of adherence using maximum likelihood estimation.
Health‐related quality of life (SF‐12)
We will measure whether the interventions influence health‐related quality of life using the SF‐12.53
Power and Sample Size
The sample size for this study was based on the primary outcome of systolic blood pressure at 12 months. Table 2 shows participants per health center needed to detect a 4‐mm Hg difference in SBP for pair‐wise comparisons of intervention groups to usual care. Required sample sizes are reported for a range of standard deviations, 80% power, 5% type I error, and intra–health center correlation of 0.001. We used this intra–health center correlation based on the very low correlations observed among patients participating in another multiclinic care management study (M. Wolf, personal communication). We will attempt to recruit 140 participants at each health center in order to have ≥105 complete the measurement at 1 year (75% retention). A 4–mm Hg difference in systolic blood pressure was chosen because this difference has been shown to produce a significant 15% reduction in major cardiovascular events among patients randomized to different blood pressure treatment goals.54
Table 2.
Participants Required per Clinic to Detect a 4–mm Hg Difference in Systolic Blood Pressure*
| Standard Deviation of Systolic Blood Pressure | Number of Participants per Health Center Required |
|---|---|
| 14 | 75 |
| 16 | 100 |
| 18 | 130 |
The numbers shown are the number of participants with systolic blood pressure measured at 12 months for each of 12 health centers needed to detect a 4–mm Hg difference in SBP for pair‐wise comparisons of intervention groups with usual care. Required sample sizes are reported for the given range of standard deviations, 80% power, 5% type I error, and intra–health center correlation of 0.001.
Statistical Analysis
This trial uses a cluster‐randomized design in which health center is the unit of randomization. We will randomize health centers in groups of 3 to the 3 arms (usual care, EHR tools only, and EHR tools plus nurse management) and anticipate randomizing 12 health centers.
We will examine for potential confounding factors across the 3 treatment arms. Variables found to have significant differences (P<0.05) across treatment groups will be entered as covariates in the generalized linear mixed models used for formal analyses. We will control for baseline SBP in formal analyses.
SBP at 12 months is the primary outcome. Binary and continuous variables measure secondary outcomes. We will use generalized linear mixed models for analyses of the data. The 3‐category treatment group variable will be the independent variable of primary interest and will be modeled as a fixed effect with the usual care group specified as the reference group. We will also include fixed effects for any potential confounding covariates noted in the descriptive studies. Random effects will be included for each health center to account for intra–health center correlation among participants.
The statistical tests of primary interest will pertain to the fixed main effects comparing EHR tools with usual care and EHR tools plus nurse management with usual care. The beta estimates from the generalized linear mixed models will be deemed statistically significant for P<0.05. Each of these 2 primary comparisons is a specific a priori hypothesis that we are testing at α=0.05. We will examine outcomes separately for subgroups defined by participants' literacy defined as inadequate (Newest Vital Signs score indicating inadequate health literacy versus all others). We will test for differences in intervention effects according to literacy by including a literacy‐intervention interaction term. Statistical significance for the interaction term (P<0.05) will indicate that intervention group differences in SBP at 12 months, as well as the secondary outcomes, vary by literacy level. We do recognize, however, that we are not formally powered to detect such an interaction, and so the analyses will be considered exploratory. Secondarily, we will compare the fixed main effects comparing EHR tools with EHR tools plus nurse management.
Further analyses will compare systolic blood pressure among the study groups using data collected at all postbaseline times and using a patient‐level repeated‐measures linear model that accounts for clustering by health center. The group‐by‐time interaction effect in this model will explore whether and how intervention effects vary over time. In particular, we will explore the hypothesis that the nurse‐led intervention will produce more rapid effects on blood pressure and other outcomes than the EHR‐tools‐only intervention and also examine whether intervention effects appear to wane over time or are sustained.
We will examine loss to follow‐up by intervention group. For the primary outcome, missing data will occur in cases in wich patients complete ≥1 assessments but not the 12‐month assessment. For this type of missing data, bias assessment will be done using either an empirical approach or a model‐based approach. The empirical approach will classify patients as having or not having missing follow‐up data and then compare baseline outcome and mediating variables between these 2 groups. The model‐based approach will try to determine whether the missing data are missing completely at random, missing at random, or are nonignorably missing. To test whether missing data are missing completely at random, we will use a logistic regression analysis adapted by Ridout.55 The probability of no response (ie, data missing) is modeled as a function of previously observed characteristics, and if no association is found between this binary indicator of no response and functions of previously observed characteristics, then the assumption that the data are missing completely at random may be reasonable. In this case, the mixed model using all available data will be used for the intention‐to‐treat analysis. Further assessment of missing data mechanisms may be done using shared parameter models in which serial outcome measurements and missing data indicator variables are jointly modeled under assumptions of different missing data mechanisms.56 The shared parameter model approach to analyze blood pressure over time will be used for the intention‐to‐treat analysis if the Rideout test indicates data are not missing completely at random.
Outcomes for medication reconciliation, medication understanding, and medication adherence will be compared between each of the 3 study groups. We will use generalized linear mixed models for analyses of the data in a fashion similar to the primary analysis using the identity link for continuous data and the logit link for binary data.
Intervention Fidelity
We will use direct observation performed during random clinical sessions at study sites that receive the EHR‐based interventions to determine the fidelity with which medication lists were provided to patients at the beginning of their encounter and medication information print material was provided at the end of visits. Nurses will log all in‐person and telephone encounters with patients.
Cost Analysis
We will calculate the direct and indirect costs of the 2 components of the intervention (implementation of EHR tools and nurse‐led medication therapy management) to inform potential adopters. The primary or direct costs of the nursing intervention will include the costs of the nurse time and estimates of additional office resources consumed (indirect cost). The costs of the EHR tools will include annual maintenance costs for the system but will not include development costs for software that will be available at no additional cost. If these programs lead to reduced blood pressure, we will conduct a cost‐effectiveness analysis to compare the 2 components as a ratio of cost per mean mm Hg systolic blood pressure lowering.57 Alternatively put, the primary outcome measured, change in mean systolic blood pressure, will be compared with the measured intervention costs to obtain a cost per unit change in systolic blood pressure. We will then test the sensitivity of costs to different assumptions about indirect costs and the potential use of less costly staff being substituted for nurse time, assuming less costly staff could have the same impact.
Conclusion
The Northwestern and Access Community Health Network Medication Education Study will test the effects of a higher intensity intervention based on nurse‐led medication therapy management in combination with EHR‐based medication management tools and a lower‐intensity intervention that uses EHR‐based tools only among patients with uncontrolled hypertension and complex drug regimens. Both interventions will be delivered within community health centers and help to inform community practices about methods that may be helpful to assisting patients in their management of complex drug regimens.
Sources of Funding
This work was supported by Award Number R01NR012745 from the National Institute of Nursing Research of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
Disclosures
Dr. Persell has performed paid consulting for the American Board of Internal Medicine, the American Medical Association, and Vimedicus (a health information technology company). Dr. Wolf has received funds personally from Merck Pharmaceuticals and McNeil Consumer Healthcare for service on advisory boards and receives funding through Northwestern University for research grants and contracts from Abbott Labs, Merck Pharmaceuticals, McNeil Consumer Healthcare, and UnitedHealthcare. No other authors have any relevant interests to disclose.
Acknowledgments
The authors thank Ingrid Guzman, Kendra Julion, Berenice Hernandez, Darren Kaiser, MS, Josyln Emerson, PharmD, Anand Reddy, Jennifer Webb, MA, Stacy Bailey, PhD, Daneen Woodard, MD, Jairo Mejia, MD, Larry Manheim, PhD, and Julie Bonello, RN, for their contributions to this study. The authors also acknowledge and thank staff members of participating health centers.
References
- 1.Huang ES, Basu A, Finch M, Frytak J, Manning W. The complexity of medication regimens and test ordering for patients with diabetes from 1995 to 2003. Curr Med Res Opin. 2007; 23:1423-1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho PM, Spertus JA, Masoudi FA, Reid KJ, Peterson ED, Magid DJ, Krumholz HM, Rumsfeld JS. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006; 166:1842-1847 [DOI] [PubMed] [Google Scholar]
- 3.Fischer MA, Stedman MR, Lii J, Vogeli C, Shrank WH, Brookhart MA, Weissman JS. Primary medication non‐adherence: analysis of 195,930 electronic prescriptions. J Gen Intern Med. 2010; 25:284-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choudhry NK, Shrank WH, Levin RL, Lee JL, Jan SA, Brookhart MA, Solomon DH. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009; 15:457-464 [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel D, Lopez J, Meier J. Antihypertensive medication adherence in the Department of Veterans Affairs. Am J Med. 2007; 120:26-32 [DOI] [PubMed] [Google Scholar]
- 6.Gandhi TK, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, Seger DL, Shu K, Federico F, Leape LL, Bates DW. Adverse drug events in ambulatory care. N Engl J Med. 2003; 348:1556-1564 [DOI] [PubMed] [Google Scholar]
- 7.Davis TC, Wolf MS, Bass PF, III, Middlebrooks M, Kennen E, Baker DW, Bennett CL, Durazo‐Arvizu R, Bocchini A, Savory S, Parker RM. Low literacy impairs comprehension of prescription drug warning labels. J Gen Intern Med. 2006; 21:847-851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis TC, Wolf MS, Bass PF, III, Thompson JA, Tilson HH, Neuberger M, Parker RM. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006; 145:887-894 [DOI] [PubMed] [Google Scholar]
- 9.Wolf MS, Parker RM, Clancy C, Frederico F, Ganley C, Shrank WH, Smith S, Williams R, Wood A, Wu A, Harnsberger RL, Jrause JA. Improving prescription drug container labeling in the United States: A health literacy and medication safety initiative. Institute of Medicine (U.S.). Standardizing Medication Labels: Confusing Patients Less, workshop summary. Washington, DC: The National Academies Press; 2008:69–100 [Google Scholar]
- 10.Stevenson FA, Cox K, Britten N, Dundar Y. A systematic review of the research on communication between patients and health care professionals about medicines: the consequences for concordance. Health Expect. 2004; 7:235-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarn DM, Heritage J, Paterniti DA, Hays RD, Kravitz RL, Wenger NS. Physician communication when prescribing new medications. Arch Intern Med. 2006; 166:1855-1862 [DOI] [PubMed] [Google Scholar]
- 12.Schillinger D, Piette J, Grumbach K, Wang F, Wilson C, Daher C, Leong‐Grotz K, Castro C, Bindman AB. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med. 2003; 163:83-90 [DOI] [PubMed] [Google Scholar]
- 13.Tarn DM, Paterniti DA, Heritage J, Hays RD, Kravitz RL, Wenger NS. Physician communication about the cost and acquisition of newly prescribed medications. Am J Manag Care. 2006; 12:657-664 [PubMed] [Google Scholar]
- 14.Wolf MS, Davis TC, Shrank WH, Neuberger M, Parker RM. A critical review of FDA‐approved Medication Guides. Patient Educ Couns. 2006; 62:316-322 [DOI] [PubMed] [Google Scholar]
- 15.Wolf MS, Davis TC, Shrank W, Rapp DN, Bass PF, Connor UM, Clayman M, Parker RM. To err is human: patient misinterpretations of prescription drug label instructions. Patient Educ Couns. 2007; 67:293-300 [DOI] [PubMed] [Google Scholar]
- 16.Persell SD, Bailey SC, Tang J, Davis TC, Wolf MS. Medication reconciliation and hypertension control. Am J Med. 2010; 123:182.e9-182.e15 [DOI] [PubMed] [Google Scholar]
- 17.Persell SD, Osborn CY, Richard R, Skripkauskas S, Wolf MS. Limited health literacy is a barrier to medication reconciliation in ambulatory care. J Gen Intern Med. 2007; 22:1523-1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Persell SD, Heiman HL, Weingart SN, Burdick E, Borus JS, Murff HJ, Bates DW, Gandhi TK. Understanding of drug indications by ambulatory care patients. Am J Health Syst Pharm. 2004; 61:2523-2527 [DOI] [PubMed] [Google Scholar]
- 19.Tarn DM, Paterniti DA, Kravitz RL, Heritage J, Liu H, Kim S, Wenger NS. How much time does it take to prescribe a new medication? Patient Educ Couns. 2008; 72:311-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varkey P, Cunningham J, Bisping DS. Improving medication reconciliation in the outpatient setting. Jt Comm J Qual Patient Saf. 2007; 33:286-292 [DOI] [PubMed] [Google Scholar]
- 21.Nassaralla CL, Naessens JM, Chaudhry R, Hansen MA, Scheitel SM. Implementation of a medication reconciliation process in an ambulatory internal medicine clinic. Qual Saf Health Care. 2007; 16:90-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephens M, Fox B, Kukulka G, Bellamy J. Medication, allergy, and adverse drug event discrepancies in ambulatory care. Fam Med. 2008; 40:107-110 [PubMed] [Google Scholar]
- 23.Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005; 165:1842-1847 [DOI] [PubMed] [Google Scholar]
- 24.Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ. 2005; 173:510-515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kripalani S, Roumie CL, Dalal AK, Cawthon C, Businger A, Eden SK, Shintani A, Sponsler KC, Harris LJ, Theobald C, Huang RL, Scheurer D, Hunt S, Jacobson TA, Rask KJ, Vaccarino V, Gandhi TK, Bates DW, Williams MV, Schnipper JL. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012; 157:1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Medication Therapy Management in community pharmacy practice core elements of an MTM service (version 1.0). J Am Pharm Assoc (2003). 2005; 45:573-579 [DOI] [PubMed] [Google Scholar]
- 27.Pellegrino AN, Martin MT, Tilton JJ, Touchette DR. Medication therapy management services: definitions and outcomes. Drugs. 2009; 69:393-406 [DOI] [PubMed] [Google Scholar]
- 28.Chrischilles EA, Carter BL, Lund BC, Rubenstein LM, Chen‐Hardee SS, Voelker MD, Park TR, Kuehl AK. Evaluation of the Iowa Medicaid pharmaceutical case management program. J Am Pharm Assoc (2003). 2004; 44:337-349 [DOI] [PubMed] [Google Scholar]
- 29.Smith SR, Catellier DJ, Conlisk EA, Upchurch GA. Effect on health outcomes of a community‐based medication therapy management program for seniors with limited incomes. Am J Health Syst Pharm. 2006; 63:372-379 [DOI] [PubMed] [Google Scholar]
- 30.Isetts BJ, Schondelmeyer SW, Artz MB, Lenarz LA, Heaton AH, Wadd WB, Brown LM, Cipolle RJ. Clinical and economic outcomes of medication therapy management services: the Minnesota experience. J Am Pharm Assoc (2003). 2008; 48:203-211 [DOI] [PubMed] [Google Scholar]
- 31.Machado M, Bajcar J, Guzzo GC, Einarson TR. Sensitivity of patient outcomes to pharmacist interventions. Part II: systematic review and meta‐analysis in hypertension management. Ann Pharmacother. 2007; 41:1770-1781 [DOI] [PubMed] [Google Scholar]
- 32.Green BB, Cook AJ, Ralston JD, Fishman PA, Catz SL, Carlson J, Carrell D, Tyll L, Larson EB, Thompson RS. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA. 2008; 299:2857-2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter BL, Bergus GR, Dawson JD, Farris KB, Doucette WR, Chrischilles EA, Hartz AJ. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009; 169:1996-2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill MN, Bone LR, Hilton SC, Roary MC, Kelen GD, Levine DM. A clinical trial to improve high blood pressure care in young urban black men: recruitment, follow‐up, and outcomes. Am J Hypertens. 1999; 12:548-554 [DOI] [PubMed] [Google Scholar]
- 35.Bosworth HB, Olsen MK, Grubber JM, Neary AM, Orr MM, Powers BJ, Adams MB, Svetkey LP, Reed SD, Li Y, Dolor RJ, Oddone EZ. Two self‐management interventions to improve hypertension control: a randomized trial. Ann Intern Med. 2009; 151:687-695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLean DL, McAlister FA, Johnson JA, King KM, Makowsky MJ, Jones CA, Tsuyuki RT. A randomized trial of the effect of community pharmacist and nurse care on improving blood pressure management in patients with diabetes mellitus: study of cardiovascular risk intervention by pharmacists‐hypertension (SCRIP‐HTN). Arch Intern Med. 2008; 168:2355-2361 [DOI] [PubMed] [Google Scholar]
- 37.Jewell D, Hope J. Evaluation of a nurse‐run hypertension clinic in general practice. Practitioner. 1988; 232:484-487 [PubMed] [Google Scholar]
- 38.Bosworth HB, Olsen MK, Dudley T, Orr M, Goldstein MK, Datta SK, McCant F, Gentry P, Simel DL, Oddone EZ. Patient education and provider decision support to control blood pressure in primary care: a cluster randomized trial. Am Heart J. 2009; 157:450-456 [DOI] [PubMed] [Google Scholar]
- 39.Shojania KG, Ranji SR, McDonald KM, Grimshaw JM, Sundaram V, Rushakoff RJ, Owens DK. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta‐regression analysis. JAMA. 2006; 296:427-440 [DOI] [PubMed] [Google Scholar]
- 40.Cutrona SL, Choudhry NK, Stedman M, Servi A, Liberman JN, Brennan T, Fischer MA, Brookhart MA, Shrank WH. Physician effectiveness in interventions to improve cardiovascular medication adherence: a systematic review. J Gen Intern Med. 2010; 25:1090-1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montano DE, Kasprzyk D. In: Glanz K, Rimer BK, Viswanath (eds.). Theory of reasoned, theory of planned behavior, and the integrated behavioral model. Health Behavior and Health Education: Theory, Research, and Practice. 2008San Francisco, CA: Jossey‐Bass; 67-96 [Google Scholar]
- 42.Shrank WH, Hoang T, Ettner SL, Glassman PA, Nair K, DeLapp D, Dirstine J, Avorn J, Asch SM. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med. 2006; 166:332-337 [DOI] [PubMed] [Google Scholar]
- 43.Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini‐cog: a cognitive ‘vital signs’ measure for dementia screening in multi‐lingual elderly. Int J Geriatr Psychiatry. 2000; 15:1021-1027 [DOI] [PubMed] [Google Scholar]
- 44.Webb JR, Feinglass J, Makoul G, Wilkes CL, Dunham DP, Baker DW, Wolf MS. Can electronic health records help improve patients' understanding of medications? Am J Manag Care. 2010; 16:919-922 [PMC free article] [PubMed] [Google Scholar]
- 45.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005; 40:1918-1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss BD, Mays MZ, Martz W, Castro KM, DeWalt DA, Pignone MP, Mockbee J, Hale FA. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005; 3:514-522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Center for Health Statistics. National Health and Nutrition Examination Survey. Physician Examination Procedures Manual, Revised January 2004. Available at: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. Accessed February 13, 2013.
- 48.National Committee for Quality Assurance The State of Health Care Quality 2012. 2012Washington, DC: National Committee for Quality Assurance; 57-59 [Google Scholar]
- 49.Wolf MS, Curtis LM, Waite K, Bailey SC, Hedlund LA, Davis TC, Shrank WH, Parkern RM, Wood AJ. Helping patients simplify and safely use complex prescription regimens. Arch Intern Med. 2011; 171:300-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bailey SC, Chen AH, Sarkar U, Schillinger D, Wolf MS. Evaluation of language‐concordant, patient‐centered drug label instructions. J Gen Intern Med. 2012; 27:1707-1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duong M, Piroth L, Grappin M, Forte F, Peytavin G, Buisson M, Chavanet P, Portier H. Evaluation of the Patient Medication Adherence Questionnaire as a tool for self‐reported adherence assessment in HIV‐infected patients on antiretroviral regimens. HIV Clin Trials. 2001; 2:128-135 [DOI] [PubMed] [Google Scholar]
- 52.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986; 24:67-74 [DOI] [PubMed] [Google Scholar]
- 53.Ware JE, Kosinski M, Dewey JE. How to Score Version 2 of the SF‐36 Health Survey. 2000Lincoln, RI: QualityMetric Incorporated [Google Scholar]
- 54.Turnbull FBlood Pressure Lowering Treatment Trialists' Collaboration Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet. 2003; 362:1527-1535 [DOI] [PubMed] [Google Scholar]
- 55.Ridout MS. Testing for random droupouts in repeated measurement data. Biometrics. 1991; 47:1617-1621 [PubMed] [Google Scholar]
- 56.Follmann D, Wu M. An approximate generalized linear model with random effects for informative missing data. Biometrics. 1995; 51:151-168 [PubMed] [Google Scholar]
- 57.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost‐Effectiveness in Health and Medicine. 1996New York, NY: Oxford University Press [Google Scholar]


