Abstract
Background
Mental stress–induced myocardial ischemia (MSIMI) is associated with adverse prognosis in patients with coronary artery disease (CAD), yet the mechanisms underlying this phenomenon remain unclear. We hypothesized that compared with exercise/pharmacological stress–induced myocardial ischemia (PSIMI) that is secondary to the atherosclerotic burden of CAD, MSIMI is primarily due to vasomotor changes.
Methods and Results
Patients with angiographically documented CAD underwent 99mTc‐sestamibi myocardial perfusion imaging at rest and following both mental and physical stress testing, performed on separate days. The severity and extent of CAD were quantified using the Gensini and Sullivan scores. Peripheral arterial tonometry (Itamar Inc) was used to assess the digital microvascular tone during mental stress as a ratio of pulse wave amplitude during speech compared with baseline. Measurements were made in a discovery sample (n=225) and verified in a replication sample (n=159). In the pooled (n=384) sample, CAD severity and extent scores were not significantly different between those with and without MSIMI, whereas they were greater in those with compared with those without PSIMI (P<0.04 for all). The peripheral arterial tonometry ratio was lower in those with compared with those without MSIMI (0.55±0.36 versus 0.76±0.52, P=0.009). In a multivariable analysis, the peripheral arterial tonometry ratio was the only independent predictor of MSIMI (P=0.009), whereas angiographic severity and extent of CAD independently predicted PSIMI.
Conclusions
The degree of digital microvascular constriction, and not the angiographic burden of CAD, is associated with MSIMI. Varying causes of MSIMI compared with PSIMI may require different therapeutic interventions that require further study.
Keywords: coronary disease, ischemia, mental stress, vasoconstriction
Introduction
A significant proportion of patients with coronary artery disease (CAD) develop myocardial ischemia during mental stress.1–7 Mental stress–induced myocardial ischemia (MSIMI) has been associated with up to 3‐fold higher rate of fatal and nonfatal cardiac events, independent of presence of exercise‐induced ischemia or standard cardiac risk factors.2–5 Although the mechanisms underlying MSIMI remain unclear, its pathophysiology may differ from physical (exercise or pharmacological) stress–induced myocardial ischemia (PSIMI).8–9 For example, compared with PSIMI, MSIMI is more often painless,7,10–12 occurs at lower levels of oxygen demand,7,13–16 and may not be accompanied by PSIMI.17 Notably, while the association between PSIMI and severity of epicardial coronary stenosis is well established, a similar relationship for MSIMI has not been previously investigated.
We and others have previously demonstrated significant coronary vasomotor changes during mental stress testing.18–19 Moreover, MSIMI is associated with a prominent peripheral vasoconstrictor response16,20 that can be measured as a change in digital arterial pulse volume using peripheral arterial tonometry (PAT).21–22 Since changes in peripheral vascular tone may reflect changes in coronary vascular resistance,23–24 peripheral microvascular vasomotor response to mental stress may serve as a surrogate for similar changes in the coronary vasculature. We hypothesized that CAD severity predicts the likelihood of PSIMI, but not of MSIMI, and that increased peripheral vasoconstriction during mental stress is a stronger predictor of MSIMI than CAD severity.
Methods
Patient Population
Patients with clinically stable CAD were recruited directly from clinic or after chart review. Presence of CAD was defined by an abnormal coronary angiogram demonstrating evidence of atherosclerosis ranging from luminal irregularities to 3‐vessel disease, documented previous percutaneous or surgical coronary revascularization, documented myocardial infarction (MI), or a positive nuclear stress test. Patients with an acute coronary syndrome, decompensated heart failure in preceding 2 months, or unstable psychiatric conditions were excluded. Clinical information including previous CAD events, CAD risk factors, and current medications were documented using standardized questionnaires and chart reviews. The research protocols were approved by their respective institutional review board, and all participants provided informed consent.
The discovery sample (group A) included 225 patients recruited in the ongoing Mental Stress Ischemia: Mechanisms and Prognosis study at Emory University. Replication of the findings was performed in 159 patients (group B) enrolled in the Psychological Stress and Risk of Cardiac Events study from the University of Florida, Gainesville. Both studies had a similar inclusion and exclusion criteria and imaging and vascular protocols.
Study Protocol
Patients were tested in the morning after a 12‐hour fast. Antianginal medications (β‐blockers, calcium channel blockers, and long‐acting nitrates) were withheld for 24 to 48 hours, depending on their half‐life, before stress testing (physical and mental).
Mental stress procedure
In a quiet dimly lit, temperature‐controlled (21° to 23°C) room, after a 30‐minute rest period, vital signs were measured and mental stress was induced by a standardized public speaking task, as previously described.25 Briefly, patients were asked to imagine a situation in which a close relative had been mistreated in a nursing home. Patients were given 2 minutes to prepare their speech and 3 minutes to deliver their speech in front of an evaluative audience. Blood pressure (BP) and heart rate (HR) were recorded at 5‐minute intervals during the resting phase and at 1‐minute intervals during and after the mental stress task. The rate–pressure product (RPP) was calculated as peak systolic BP multiplied by peak HR during physical and mental stress, and hemodynamic reactivity to mental stress was calculated as the magnitude of increase in hemodynamic measures from baseline to peak values during the speech task. To ensure adequacy of the mental stress testing, the procedure was administered by trained and experienced staff. Close attention was paid to the psychophysiological stress–provoking elements of the test. All personnel participating in administering the test were white coated, and patients were unaware of the specific contents of the test before they received it.
Myocardial perfusion imaging
Myocardial perfusion imaging with 99mTc‐sestamibi was performed on 2 separate days up to 1 week apart, at rest and during mental and exercise/pharmacological stresses according to standard protocols.26 The sequence of the stressor (mental or physical) was balanced. During mental stress testing, 20 to 30 mCi of 99mTc radioisotope was given at 1 minute into the speech based on previous reports demonstrating that the maximal hemodynamic, neurohormonal, and ischemic responses to mental stress usually occur early.16 Exercise stress testing was performed using the Bruce protocol, and, when contraindicated, pharmacological testing with regadenoson (group A) or adenosine (group B) was performed. Xanthine derivatives and caffeine‐containing products were discontinued 48 and 12 hours before testing, respectively. The radioisotope injection was given at peak exertion during the exercise test, at 3 minutes during the adenosine protocol, or immediately after the regadenoson injection. Exercise was continued for at least 1 minute after the injection. Stress images were acquired 30 to 60 minutes later using conventional methodology with single‐photon emission computed tomography (SPECT).26
Studies were interpreted by 2 experienced nuclear cardiologists blinded to the stressor (mental or exercise/pharmacological) and without prior knowledge of the coronary anatomy. Discrepancies in interpretation of SPECT images with respect to outcome of ischemia were resolved by reaching a consensus between the readers. Rest and stress images were visually compared for number and severity of perfusion defects using a 17‐segment model for group A and a 20‐segment model for group B.27 Each segment was scored from 0 to 4, with 0 being “normal uptake” and 4 being “no uptake,” yielding a total score. A reversible defect score (summed difference score [SDS]) was calculated as the difference between summed stress and summed rest scores. Ischemia was defined as new or worsening perfusion defects during mental, exercise, or pharmacological stress compared with the resting baseline images with an SDS of ≥4. For the purpose of comparison in this study, the same ischemia definition was used for all testing protocols.
Angiographic data
The most recent angiogram before stress testing was used for analysis. The mean duration between the angiogram and stress testing was 37±39 months for group A and 23±20 months for group B. CAD severity and extent were assessed using 2 semiquantitative scoring systems by Gensini28–29 and Sullivan.30 The Gensini score quantifies severity of CAD by a nonlinear points system for the degree of luminal narrowing along with a multiplier for specific coronary tree locations, thereby weighting each lesion score for prognostic significance. The total of the lesion scores is summed to give a final Gensini score. The Sullivan stenosis score quantifies the most severe stenosis observed in each of the main coronary vessels assessed without specific weighting for the territory supplied, and the Sullivan extent score quantifies the percentage of the coronary intimal surface area affected by atheroma without specific weighting for the degree of luminal narrowing.29
Digital blood flow measurement using finger plethysmography
Digital pulse wave amplitude was continuously measured during rest and mental stress using PAT (Itamar‐Medical), as previously described.22 Briefly, the device, which uses a modified form of plethysmography, was applied to the index finger. The pressure changes were fed to a personal computer where the signal is filtered, amplified, stored and analyzed in an operator‐independent manner. The baseline pulse wave amplitude was determined by averaging over the entire rest period. The stress amplitude was determined by averaging over the entire 3‐minute speaking period. The PAT ratio was then calculated as the ratio of pulse wave amplitude during the speaking task compared with the resting baseline, with a ratio <1 signifying a vasoconstrictive response. Decrease in pulse wave amplitude is believed to be due to microvascular constriction.31–33
Statistical Methods
Results for normally distributed variables are summarized as mean±SD values for continuous variables or as proportions for categorical variables. Continuous variables with a non‐normal distribution are shown as the median value with interquartile intervals. The 2‐tailed unpaired Student's t test was used for comparison of normally distributed continuous variables. The Mann–Whitney U test was used to compare the difference in non‐normally distributed variables. The χ2 test was used for comparison of categorical variables. Correlations between continuous variables were assessed with Pearson or Spearman correlation tests, as appropriate. Univariate and multivariable logistic regression models were used to examine the effect of covariates on prediction of the binary outcome of SPECT ischemia. Statistical analysis was initially conducted in the discovery group A, and after the findings were verified in the replication group B, the 2 groups were combined for pooled analysis. Covariates used in the multivariable analysis performed for predictors of MSIMI and PSIMI included age, sex, hypertension, diabetes mellitus, history of ever smoking, prior history of MI, coronary artery bypass graft surgery, percutaneous coronary intervention, depression, medications (aspirin, β‐blockers, angiotensin‐converting enzyme inhibitors, calcium channel antagonists, statins, and nitrates), duration between the angiogram and stress testing, and enrollment group A or B. The Gensini and both Sullivan scores were significantly correlated and thus were entered separately into multivariable models. The Hosmer–Lemeshow test was used to test for model goodness of fit. Considering myocardial perfusion imaging as the gold standard for detection of MSIMI, the diagnostic accuracy of the PAT ratio was evaluated by using the receiver operator characteristic curve. Furthermore, C‐statistic was performed to compare the predictive ability of the PAT ratio over a model based on conventional risk factors for predicting the occurrence of SPECT ischemia. Statistical significance was based on 2‐tailed tests, and P values ≤0.05 were considered significant. Analyses were performed with SPSS (version 20.0, SPSS Inc).
Results
Table 1 summarizes the clinical characteristics of the 2 groups stratified by the presence or absence of both MSIMI and PSIMI. MSIMI was present in 11% and 17% and PSIMI in 27% and 41% of groups A and B, respectively. Of those developing MSIMI, 52% also had PSIMI in group A and 63% in group B. In the combined cohort, patients were further grouped into those who developed ischemia during both stressors (n=30), during neither (n=237), or during 1 stressor only (MSIMI [n=22] or PSIMI [n=95]). Overall, patients with MSIMI were slightly older but were otherwise not significantly different than those without MSIMI in terms of risk factors and medication use. Patients with PSIMI tended to be more frequently male with history of coronary artery bypass graft surgery, hypertension, and diabetes mellitus. Notably, there was no difference in the duration between the most recent angiogram and nuclear stress testing between those with and without MSIMI or PSIMI in all groups (Table 1).
Table 1.
Clinical Characteristics of Study Population
| Total | Physical Stress–Induced Myocardial Ischemia | P Value | Mental Stress–Induced Myocardial Ischemia | P Value | |||
|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | ||||
| Group A | N=225 | n=165 | n=60 | n=200 | n=25 | ||
| Age, y±SD | 64±8 | 64±9 | 65±7 | 0.76 | 64±9 | 66±6 | 0.33 |
| Male sex, n (%) | 159 (71) | 115 (70) | 44 (73) | 0.44 | 143 (72) | 16 (64) | 0.54 |
| Previous MI, n (%) | 78 (35) | 61 (37) | 17 (28) | 0.24 | 69 (35) | 9 (36) | 0.92 |
| Previous PTCA, n (%) | 137 (61) | 97 (59) | 40 (67) | 0.26 | 122 (61) | 15 (60) | 0.85 |
| Previous CABG, n (%) | 71 (32) | 46 (28) | 25 (42) | 0.05 | 60 (30) | 11 (44) | 0.17 |
| Angina in the past 4 weeks, n (%) | 61 (27) | 43 (26) | 18 (30) | 0.55 | 51 (26) | 10 (40) | 0.16 |
| LVEF, % | 59±15 | 60±15 | 59±13 | 0.70 | 60±15 | 58±14 | 0.62 |
| Cardiovascular risk factors | |||||||
| Hypertension, n (%) | 158 (70) | 112 (68) | 46 (77) | 0.18 | 139 (70) | 19 (76) | 0.57 |
| Diabetes, n (%) | 70 (31) | 46 (28) | 24 (40) | 0.08 | 59 (30) | 11 (44) | 0.15 |
| Depression, n (%) | 25 (11) | 19 (12) | 6 (10) | 0.76 | 21 (11) | 4 (16) | 0.43 |
| Current or ex‐smokers, n (%) | 132 (59) | 97 (59) | 35 (58) | 0.99 | 116 (58) | 16 (64) | 0.69 |
| Treatment at study entry | |||||||
| β‐Blocker, n (%) | 157 (70) | 116 (70) | 41 (68) | 0.99 | 140 (70) | 17 (68) | 0.63 |
| ARB, n (%) | 40 (18) | 26 (16) | 14 (23) | 0.16 | 34 (17) | 6 (24) | 0.44 |
| ACEI, n (%) | 78 (35) | 59 (36) | 19 (32) | 0.65 | 65 (33) | 13 (52) | 0.07 |
| Statin, n (%) | 181 (80) | 136 (82) | 45 (75) | 0.34 | 161 (81) | 20 (80) | 0.67 |
| CCB, n (%) | 38 (17) | 29 (18) | 9 (15) | 0.70 | 33 (17) | 5 (20) | 0.72 |
| Duration between angiogram and stress test, mo | 39±42 | 33±32 | 0.31 | 37±39 | 40±40 | 0.71 | |
| Group B | N=159 | n=94 | n=65 | n=132 | n=27 | ||
| Age, y±SD | 64±9 | 64±8 | 64±10 | 0.82 | 63±9 | 67±9 | 0.05 |
| Male sex, n (%) | 109 (69) | 59 (63) | 50 (77) | 0.06 | 92 (70) | 17 (63) | 0.49 |
| Previous MI, n (%) | 23 (15) | 9 (10) | 14 (22) | 0.04 | 18 (14) | 5 (19) | 0.51 |
| Previous PTCA, n (%) | 75 (47) | 43 (46) | 32 (49) | 0.67 | 64 (49) | 11 (41) | 0.46 |
| Previous CABG, n (%) | 54 (34) | 28 (30) | 26 (40) | 0.18 | 45 (34) | 9 (33) | 0.94 |
| Angina in the past 4 weeks, n (%) | 75 (47) | 47 (50) | 28 (43) | 0.55 | 63 (48) | 12 (44) | 0.83 |
| LVEF, % | 55±13 | 56±12 | 53±13 | 0.12 | 56±12 | 51±15 | 0.11 |
| Cardiovascular risk factors, n (%) | |||||||
| Hypertension | 126 (79) | 69 (73) | 57 (88) | 0.008 | 108 (82) | 18 (67) | 0.12 |
| Diabetes | 53 (33) | 28 (30) | 25 (39) | 0.21 | 46 (35) | 7 (26) | 0.38 |
| Depression | 42 (26) | 27 (29) | 15 (23) | 0.43 | 38 (29) | 4 (15) | 0.13 |
| Current or ex‐smokers | 110 (69) | 64 (68) | 46 (71) | 0.51 | 94 (71) | 16 (59) | 0.29 |
| Treatment at study entry, n (%) | |||||||
| β‐Blocker | 121 (76) | 71 (76) | 50 (77) | 0.84 | 102 (77) | 19 (70) | 0.44 |
| ARB | 22 (14) | 13 (14) | 9 (14) | 0.99 | 17 (13) | 5 (19) | 0.44 |
| ACEI | 82 (52) | 47 (50) | 35 (54) | 0.63 | 72 (55) | 10 (37) | 0.09 |
| Statin | 125 (79) | 70 (75) | 55 (85) | 0.13 | 104 (79) | 21 (78) | 0.91 |
| CCB | 36 (23) | 21 (22) | 15 (23) | 0.91 | 30 (23) | 6 (22) | 0.95 |
| Duration between angiogram and stress test, mo | 22±18 | 25±23 | 0.26 | 23±21 | 22±20 | 0.72 | |
| Combined groups A and B | N=384 | n=259 | n=125 | n=332 | n=52 | ||
| Age, y±SD | 64±9 | 64±8 | 64±9 | 0.76 | 64±8 | 67±8 | 0.04 |
| Male sex, n (%) | 268 (70) | 174 (67) | 94 (75) | 0.08 | 235 (71) | 33 (64) | 0.33 |
| Previous MI, n (%) | 101 (26) | 70 (27) | 31 (25) | 0.64 | 87 (26) | 14 (27) | 0.94 |
| Previous PTCA, n (%) | 212 (55) | 140 (54) | 72 (58) | 0.51 | 186 (56) | 26 (50) | 0.38 |
| Previous CABG, n (%) | 125 (33) | 74 (29) | 51 (41) | 0.02 | 105 (32) | 20 (39) | 0.35 |
| Angina in the past 4 weeks, n (%) | 136 (35) | 90 (35) | 46 (37) | 0.59 | 114 (34) | 22 (42) | 0.28 |
| LVEF, % | 58±14 | 58±14 | 56±13 | 0.09 | 58±14 | 55±14 | 0.11 |
| Cardiovascular risk factors, n (%) | |||||||
| Hypertension | 284 (74) | 181 (70) | 103 (82) | 0.003 | 247 (74) | 37 (71) | 0.67 |
| Diabetes | 123 (32) | 74 (29) | 49 (39) | 0.03 | 105 (32) | 18 (35) | 0.67 |
| Depression | 67 (17) | 46 (18) | 21 (17) | 0.82 | 59 (18) | 8 (15) | 0.65 |
| Current or ex‐smokers | 242 (63) | 161 (62) | 81 (65) | 0.47 | 210 (63) | 32 (62) | 0.80 |
| Treatment at study entry, n (%) | |||||||
| β‐Blocker | 278 (72) | 187 (72) | 91 (73) | 0.80 | 242 (73) | 36 (69) | 0.43 |
| ARB | 62 (16) | 39 (15) | 23 (18) | 0.38 | 51 (15) | 11 (21) | 0.32 |
| ACEI | 160 (42) | 106 (41) | 54 (43) | 0.62 | 137 (41) | 23 (44) | 0.78 |
| Statin | 306 (80) | 206 (80) | 100 (80) | 0.78 | 265 (80) | 41 (79) | 0.65 |
| CCB | 74 (19) | 50 (19) | 24 (19) | 0.99 | 63 (19) | 11 (21) | 0.77 |
| Duration between angiogram and stress test, mo | 33±36 | 29±28 | 0.29 | 32±34 | 30±32 | 0.80 | |
P‐value compares subjects with and without ischemia. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft surgery; CCB, calcium channel blocker; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty.
The Gensini score correlated with the Sullivan stenosis and extent scores in group A (r=0.65, P<0.001; r=0.33, P<0.001, respectively), group B (r=0.85, P<0.001; r=0.55, P<0.001, respectively), and in the combined group (r=0.72, P<0.001; r=0.42, P<0.001, respectively). Both Sullivan scores correlated with each other in group A (r=0.78, P<0.001), group B (r=0.85, P<0.001), and in the combined group (r=0.80, P<0.001).
Angiographic Disease Burden and Stress‐Induced Myocardial Ischemia
In pooled analysis, the Gensini, Sullivan stenosis, and Sullivan extent scores correlated with the number of perfusion defects at rest (r=0.18, P<0.001; r=0.18, P<0.001; and r=0.13, P=0.01, respectively), and the SDS during exercise/pharmacological stress (r=0.26, P<0.001; r=0.29, P<0.001; and r=0.17, P=0.001) but not with the SDS during mental stress (r=0.02, P=0.69; r=0.05, P=0.30; and r<0.001, P=0.99, respectively). Moreover, there were no differences in the extent and severity of CAD, measured either by the Gensini or the Sullivan scores, in those with compared with those without MSIMI in both groups and the combined group (Table 2). In contrast, patients with PSIMI had significantly higher scores for angiographic severity (Gensini, Sullivan) and extent (Sullivan) of CAD than those without PSIMI in both groups and in the combined group (Table 2).
Table 2.
Angiographic Coronary Severity and Extent Scores by Ischemia Status for Groups A and B and Pooled Samples
| Physical Stress–Induced Myocardial Ischemia | Mental Stress–Induced Myocardial Ischemia | |||||
|---|---|---|---|---|---|---|
| Negative | Positive | P Value | Negative | Positive | P Value | |
| Group A (n) | 165 | 60 | 200 | 25 | ||
| Gensini score, median (IQR) | 15 (3 to 48) | 44 (15 to 86) | <0.001 | 21 (4 to 56) | 20 (6 to 58) | 0.81 |
| Sullivan stenosis score, median (IQR) | 4 (2 to 6) | 8 (4 to 11) | <0.001 | 5 (2 to 7) | 4 (3 to 9) | 0.25 |
| Sullivan extension score, median (IQR) | 40 (20 to 60) | 58 (40 to 69) | <0.001 | 40 (20 to 60) | 50 (30 to 65) | 0.33 |
| Group B (n) | 94 | 65 | 132 | 27 | ||
| Gensini score, median (IQR) | 14 (4 to 41) | 26 (9 to 81) | 0.007 | 18 (6 to 54) | 11 (4 to 74) | 0.55 |
| Sullivan stenosis score, median (IQR) | 5 (3 to 9) | 7 (4 to 10) | 0.04 | 6 (3 to 9) | 5 (2 to 8) | 0.30 |
| Sullivan extension score, median (IQR) | 40 (25 to 60) | 50 (28 to 65) | 0.03 | 45 (30 to 65) | 40 (20 to 60) | 0.23 |
| Group A+B (n) | 259 | 125 | 332 | 52 | ||
| Gensini score, median (IQR) | 15 (4 to 48) | 39 (10 to 84) | <0.001 | 20 (5 to 54) | 16 (4 to 62) | 0.93 |
| Sullivan stenosis score, median (IQR) | 4 (2 to 7) | 7 (4 to 10) | <0.001 | 5 (2 to 8) | 5 (3 to 8) | 0.74 |
| Sullivan extension score, median (IQR) | 40 (20 to 60) | 55 (40 to 65) | <0.001 | 45 (25 to 60) | 40 (21 to 60) | 0.96 |
IQR indicates interquartile range.
Furthermore, the Gensini and Sullivan scores were higher in subjects developing ischemia regardless of whether patients underwent exercise or pharmacological stress testing. In the combined group of 222 patients undergoing exercise stress, 65 (29%) developed ischemia and had greater severity (Gensini, P=0.025; Sullivan, P=0.002) and extent of CAD (Sullivan, P=0.032) compared with those without ischemia. Similarly, of the 162 patients undergoing pharmacological stress, 60 (37%) developed ischemia and had more severe (Gensini, P<0.001; Sullivan, P<0.001) and extensive CAD (Sullivan, P=0.001) compared with those without ischemia.
Subjects were further divided into those with MSIMI who either had PSIMI (n=30) or not (n=22). There was no difference in the severity and extent of CAD between subjects without ischemia with either stress and those with MSIMI but no PSIMI (Figure 1). Moreover, subjects with MSIMI who also had PSIMI had a greater extent and severity of CAD than those without any ischemia; however, they were not significantly different than those with only PSIMI with respect to extent (P=0.93) and severity (Gensini, P=0.82; Sullivan, P=0.97) of CAD (Figure 1). Hence, the occurrence of MSIMI is not associated with more severe CAD in patients with PSIMI. Moreover, the severity of MSIMI in patients with PSIMI (SDS=6±2) was not different than in those without PSIMI (SDS=6±3; P=0.48).
Figure 1.
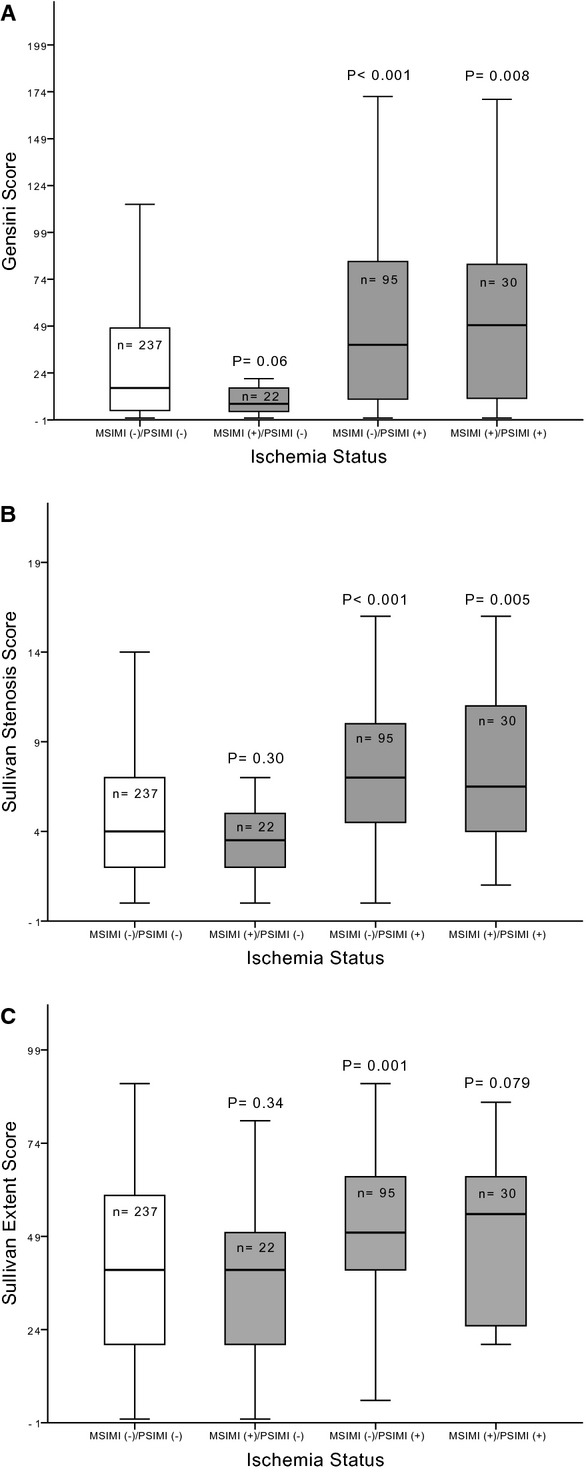
Angiographic burden of coronary artery disease stratified by ischemia status during mental and/or physical stress. P‐value compares the stated group with the group of patients without any ischemia (MSIMI [−]/PSIMI [−]). Panels A and B compare the angiographic severity of coronary artery disease between the groups, assessed by the Gensini and Sullivan coronary scoring systems, respectively. Panel C compares the angiographic extent of coronary artery disease defined by the Sullivan coronary scoring system. MSIMI indicates mental stress–induced myocardial ischemia; PSIMI, exercise or pharmacologic stress–induced myocardial ischemia.
Hemodynamic Responses to Mental and Physical Stress
Table 3 shows hemodynamic measures at rest and in response to mental stress in all groups. There was no difference in the hemodynamic response including RPP in those with or without MSIMI. Similarly, peak RPP during exercise was not significantly different between those with or without a positive exercise stress test in group A (19 042±7109 versus 20 498±6914, P=0.27), group B (22 029±3305 versus 21 563±4458, P=0.64), and combined group (20 134±6174 versus 20 637±6421, P=0.57). Thus, ischemia during mental or exercise stress was not occurring as a result of a greater increase in workload.
Table 3.
Hemodynamic Measures at Baseline and in Response to Mental Stress by Ischemia Status in All Groups
| Mental Stress–Induced Myocardial Ischemia | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study A | Study B | Study A+B | |||||||
| Negative | Positive | P Value | Negative | Positive | P Value | Negative | Positive | P Value | |
| Systolic BP, mm Hg | |||||||||
| Rest | 127±16 | 132±18 | 0.12 | 119±18 | 115±15 | 0.15 | 123±18 | 122±18 | 0.49 |
| Mental stress | 166±22 | 174±25 | 0.08 | 163±27 | 156±20 | 0.15 | 165±24 | 164±24 | 0.75 |
| Diastolic BP, mm Hg | |||||||||
| Rest | 74±12 | 76±14 | 0.53 | 64±9 | 62±7 | 0.11 | 70±12 | 68±10 | 0.21 |
| Mental stress | 98±13 | 101±16 | 0.18 | 92±15 | 92±9 | 0.99 | 95±14 | 97±14 | 0.53 |
| Heart rate, beats/min | |||||||||
| Rest | 61±12 | 58±8 | 0.20 | 59±9 | 60±10 | 0.52 | 60±11 | 59±9 | 0.49 |
| Mental stress | 77±14 | 77±18 | 0.95 | 77±16 | 82±15 | 0.09 | 77±15 | 80±17 | 0.16 |
| RPP, mm Hg×beats/min | |||||||||
| Rest | 7691±1813 | 7545±1318 | 0.70 | 6884±1519 | 6836±1313 | 0.57 | 7397±1739 | 7148±1334 | 0.26 |
| Mental stress | 12 799±3007 | 13 302±3866 | 0.45 | 12 675±3610 | 12 870±3143 | 0.75 | 12 801±3340 | 13 145±3467 | 0.43 |
| Exercise stress | 23 012±3903 | 23 524±4228 | 0.66 | 21 799±4042 | 21 489±4100 | 0.81 | 22 621±3978 | 22 547±4209 | 0.93 |
BP indicates blood pressure; RPP, rate–pressure product (mm Hg×beats per minute).
Interestingly, those with PSIMI had a greater increase in diastolic BP and HR during mental stress compared with those without PSIMI (P=0.006, P=0.042, respectively). Even after adjusting for aforementioned confounders, greater diastolic BP and HR reactivity during mental stress remained independently associated with a greater risk of PSIMI (P=0.018, P=0.025, respectively).
Finger Microvascular Response During MSIMI
Patients with MSIMI had a significantly lower PAT ratio during mental stress testing than those without MSIMI in all groups (Table 4), indicating greater digital microvascular constriction in those with MSIMI. Notably, there was no correlation between the PAT ratio and any of the angiographic scores evaluated in this study, including in subjects with ischemia. The PAT response predicted MSIMI with an area under the curve of 0.66 (95% CI 0.57 to 0.75, P=0.001). The optimal cut‐off value for the PAT ratio determined by the Youden's index was 0.52 for detecting MSIMI. For detection of MSIMI, the PAT ratio had a sensitivity of 61%, specificity of 66%, negative predictive value of 91%, and positive predictive value of 23%.
Table 4.
Digital Microvascular Response During the Speaking Task Assessed as PAT Ratio* in Patients With (Positive) and Without (Negative) Mental Stress–Induced Myocardial Ischemia
| Mental Stress–Induced Myocardial Ischemia | P Value | ||
|---|---|---|---|
| Negative | Positive | ||
| Group A | 0.83±0.50 | 0.63±0.22 | 0.006 |
| Group B | 0.71±0.55 | 0.50±0.39 | 0.026 |
| Groups A and B | 0.76±0.52 | 0.55±0.36 | 0.009 |
PAT indicates peripheral arterial tonometry.
PAT ratio was calculated as the ratio of pulse wave amplitude during the mental stress speaking task compared with resting baseline.
Notably, the association of the PAT response to MSIMI was similar during every phase of the mental stress task. Compared with those without MSIMI, patients with MSIMI demonstrated a greater vasoconstrictor response to the mental stress task as early as the preparation phase of the mental stress task, which also persisted during the post‐speech recovery period (Figure 2).
Figure 2.
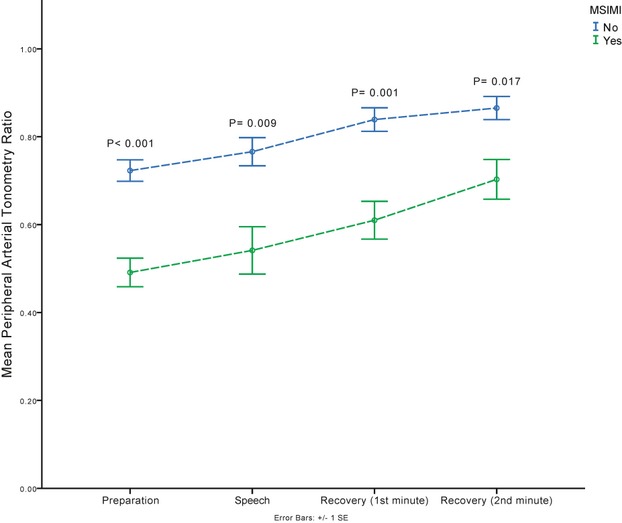
Digital microvascular response during the different phases of the mental stress task assessed as peripheral arterial tonometry (PAT) ratio in patients with and without mental stress–induced myocardial ischemia (MSIMI). The PAT ratio was calculated as the ratio of pulse wave amplitude during the specified phase of the mental stress task compared with the resting baseline. P‐value compares the PAT ratio at each phase between subjects with and without MSIMI.
Interestingly, the PAT ratio during mental stress was also lower in those with PSIMI compared with those without PSIMI (P=0.007), even in those without concomitant MSIMI (P=0.004) (Figure 3). Notably, those with dual ischemia (mental and physical) had greater degree of vasoconstriction during mental stress than those with PSIMI only (P=0.05) (Figure 3).
Figure 3.
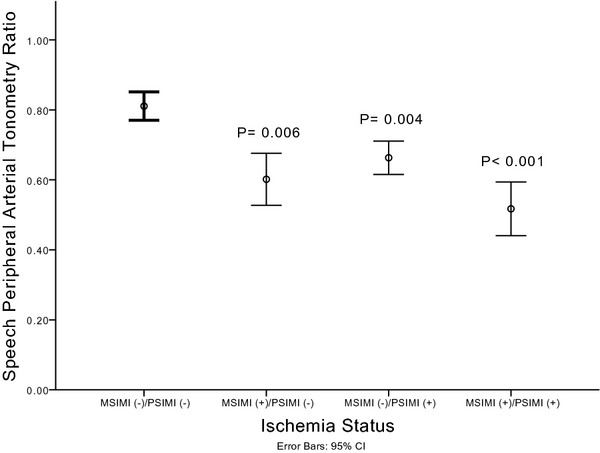
Digital microvascular response stratified by ischemia status during mental and/or physical stress. P‐value compares the stated group to the group of patients without any ischemia (MSIMI [−]/PSIMI [−]). MSIMI indicates mental stress–induced myocardial ischemia; PSIMI, exercise or pharmacologic stress–induced myocardial ischemia.
Predictors of Stress‐Induced Ischemia
Univariate predictors for the development of PSIMI were history of coronary artery bypass graft surgery, hypertension, diabetes mellitus, greater Gensini, Sullivan stenosis, and Sullivan extent scores, and a lower PAT ratio during mental stress (Table 5). In multivariable analysis, independent predictors of PSIMI were greater Gensini score, diabetes mellitus, and a lower PAT ratio during mental stress (Table 5). When the Sullivan stenosis score or the Sullivan extent score were each entered into models instead of the Gensini score, they also remained independent predictors of PSIMI (Table 5). Of note, the C‐statistic for a model predicting PSIMI based on traditional risk factors and CAD severity was 0.66. With the addition of the PAT ratio during mental stress, the model improved to 0.70 (P<0.001) (Figure 4).
Table 5.
Univariate and Multivariable Predictors of Physical Stress–Induced Myocardial Ischemia in the Pooled Group
| OR (95% CI) | P Value | |
|---|---|---|
| Univariate analysis* | ||
| Hypertension | 2.17 (1.24 to 3.80) | 0.007 |
| Diabetes mellitus | 1.63 (1.03 to 2.58) | 0.035 |
| Previous CABG | 1.77 (1.13 to 2.78) | 0.013 |
| Gensini score | 1.012 (1.007 to 1.017) | <0.001 |
| Sullivan stenosis score | 1.167 (1.100 to 1.238) | <0.001 |
| Sullivan extent score | 1.019 (1.009 to 1.030) | <0.001 |
| PAT ratio | 0.41 (0.24 to 0.70) | 0.001 |
| Multivariate analysis | ||
| Model 1 | ||
| Hypertension | 2.07 (1.11 to 3.84) | 0.022 |
| Diabetes mellitus | 1.67 (1.005 to 2.78) | 0.048 |
| Previous CABG | 1.91 (1.15 to 3.16) | 0.012 |
| Model 2+Gensini score | ||
| Gensini score | 1.01 (1.004 to 1.016) | 0.001 |
| Diabetes mellitus | 1.84 (1.09 to 3.11) | 0.020 |
| Model 2+Gensini score+PAT ratio | ||
| Gensini score | 1.01 (1.003 to 1.016) | 0.003 |
| Diabetes mellitus | 2.1 (1.18 to 3.70) | 0.011 |
| PAT ratio | 0.49 (0.26 to 0.91) | 0.025 |
| Model 2+Sullivan stenosis score | ||
| Sullivan stenosis score | 1.13 (1.048 to 1.210) | 0.001 |
| Diabetes mellitus | 1.70 (1.006 to 2.88) | 0.048 |
| Model 2+Sullivan extent score | ||
| Sullivan extent score | 1.012 (1.001 to 1.023) | 0.038 |
| Diabetes mellitus | 1.76 (1.049 to 2.966) | 0.032 |
| Previous CABG | 1.77 (1.048 to 2.98) | 0.033 |
Model 1: age, sex, diabetes mellitus, hypertension, smoking history, previous percutaneous transluminal coronary angioplasty, history of myocardial infarction, CABG, depression, medications (aspirin, β‐blocker, calcium channel inhibitor, angiotensin‐converting enzyme inhibitor, statin, and nitrate), and enrollment group. Model 2: Model 1+duration between angiogram and stress testing. CABG indicates coronary artery bypass graft surgery; PAT, peripheral arterial tonometry.
Adjusted only for enrollment group.
Figure 4.
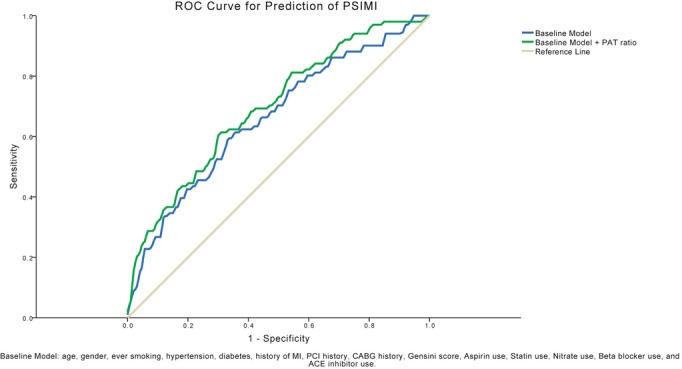
Receiver operating characteristic (ROC) curves for prediction of physical stress–induced myocardial ischemia. The C‐statistic for a model predicting physical stress–induced myocardial ischemia (PSIMI) based on traditional risk factors and CAD severity was 0.66. With the addition of the PAT ratio during mental stress, the model improved to 0.70 (P<0.001). ACE indicates angiotensin‐converting enzyme; CABG, coronary artery bypass graft surgery; CAD, coronary artery disease; MI, myocardial infarction; PAT, peripheral arterial tonometry; PCI, percutaneous coronary intervention.
In contrast, angiographic severity and extent scores were not predictive of MSIMI in either univariate or multivariable analysis (Table S1). However, a lower PAT ratio during mental stress remained an independent predictor for development of MSIMI in both univariate (odds ratio 0.27, 95% CI 0.10 to 0.73, P=0.010) and multivariable analysis (odds ratio 0.24, 95% CI 0.08 to 0.69, P=0.009) adjusting for aforementioned risk factors and medications (Table S1). Similarly, when Gensini, Sullivan stenosis, and Sullivan extent scores were entered separately into the multivariable model, a lower PAT ratio remained as the only independent predictor of MSIMI (P=0.010, P=0.010, and P=0.011, respectively) (Table S1). Finally, the C‐statistics using the PAT ratio during the mental stress task improved the risk prediction of MSIMI based on traditional risk factors and CAD severity from 0.62 to 0.72 (P<0.001) (Figure 5).
Figure 5.
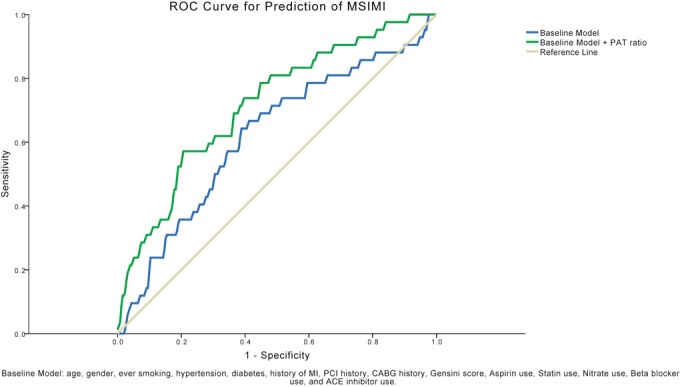
Receiver operating characteristic (ROC) curves for prediction of mental stress–induced myocardial ischemia (MSIMI). The C‐statistics using the PAT ratio during the mental stress task improved the risk prediction of MSIMI) based on traditional risk factors and CAD severity from 0.62 to 0.72 (P<0.001). ACE indicates angiotensin‐converting enzyme; CABG, coronary artery bypass graft surgery; CAD, coronary artery disease; MI, myocardial infarction; PAT, peripheral arterial tonometry; PCI, percutaneous coronary intervention.
Discussion
The major finding of this study is that neither the angiographic extent nor the severity of CAD is associated with the risk of developing MSIMI. However, a greater degree of digital microvascular constriction provoked by mental stress is associated with the likelihood to developing MSIMI, suggesting that vasoconstriction but not the severity of CAD is the predominant underlying mechanism of MSIMI. Interestingly, whereas PSIMI is associated with the severity and extent of CAD, the vasomotor response to mental stress may also help identify those at risk for PSIMI and suggests a contributing role of vasomotor dysregulation in precipitation of PSIMI.
Although it has been suggested that MSIMI is not simply a reflection of coronary atherosclerotic burden, few studies have directly investigated the relationship between MSIMI and angiographic CAD severity, and results have been mixed.6,34,12,35 Modena et al11 reported that MSIMI rates were lower in patients with single‐ compared with those with 3‐vessel disease, a discrepancy that might be due to differences in diagnostic criteria, imaging modalities, and methods of inducing MSIMI, as well as crude assessment of CAD severity compared with the quantitative assessments we used. In our study with exploratory and confirmatory datasets, and using a sensitive imaging modality together with detailed quantitative assessment of disease burden, we have convincingly demonstrated a lack of association between angiographic CAD severity and presence of MSIMI.
Our findings support the concept that the pathophysiology underlying development of MSIMI may differ from that involved in PSIMI.8–9 Arrighi et al8 showed that coronary flow during mental stress was significantly reduced in regions subtended by coronary arteries without compared with those with significant epicardial stenosis, reductions that were proportionately similar in magnitude to those observed in regions with significant epicardial disease in response to dipyridamole vasodilator stress. This supports our current and previous observations that mental stress may induce ischemia even in subjects with negative exercise or pharmacological stress tests.17 Our study also confirmed that MSIMI occurs at a lower RPP or workload than exercise‐induced ischemia.7,13–16 Furthermore, MSIMI was independent of the magnitude of hemodynamic change provoked by mental stress. Thus, factors besides increases in myocardial oxygen demand (RPP) and severity of CAD appear to underlie the phenomenon of MSIMI and are likely due to a concomitant decrease in coronary blood flow during mental stress. In contrast, ischemia during physical stress was directly related to the severity of CAD, largely secondary to demand exceeding reduced supply in a setting of coronary stenosis. Interestingly, we found that the likelihood of PSIMI was greater in those with an exaggerated cardiovascular reactivity during mental stress, manifested by an increased hemodynamic response and greater peripheral microvascular constriction. A similar observation has been reported in asymptomatic individuals at high risk for premature CAD, suggesting that increased sympathetic arousal during mental stress may help identify those at risk for PSIMI.36
In healthy individuals, coronary blood flow increases in response to mental stress as a result of coronary microvascular dilation; however, this response is attenuated or vasoconstrictor in patients with CAD.18–19,18–38 Although epicardial vasospasm has been suggested as a potential cause of MSIMI, this remains a subject of controversy. Coronary angiographic studies have demonstrated responses ranging from no change in epicardial coronary artery diameter,14 constriction in normal segments,19 to constriction of only the diseased segments.39–41 In a study that used speech as the mental stress stimulus, vasoconstriction of non‐diseased coronary artery segments has been reported in patients with and without CAD.39 Yeung et al40 showed that atherosclerotic segments of coronary arteries constricted concomitantly with decreased flow during mental stress, while there was dilation and increased flow in smooth epicardial segments. Kop et al19 found that coronary flow during mental stress increased in healthy individuals but not in those with CAD, where there was a widely variable vasoconstrictor response that was insufficient to explain the decrease in flow. Furthermore, L'Abbate et al14 demonstrated a decrease in coronary blood flow with mental stress in patients with CAD despite no change in lumen diameter at the site of the atherosclerotic lesion. The inconsistent epicardial vasomotor response to mental stress and its discordance with coronary flow suggest that the myocardial ischemic response is predominantly due to microvascular constriction. This is strengthened by our previous data showing that intracoronary phentolamine during mental stress causes a reversal in the attenuation of coronary flow during mental stress despite a lack of effect on epicardial coronary arteries vasomotion.18
We found that digital microvascular constriction, measured as the PAT ratio in response to mental stress, was a reproducible and independent predictor of MSIMI, with a higher likelihood of MSIMI in those with lower PAT ratio. Moreover, neither the PAT ratio nor MSIMI was related to the angiographic severity of CAD, suggesting that the peripheral vasomotor response to mental stress is independent of the factors associated with CAD severity. Our findings are supported by the Psychophysiological Investigations of Myocardial Ischemia (PIMI) investigators, who showed that the most significant hemodynamic feature associated with MSIMI was the increase in systemic vascular resistance, which may be due to increased levels of circulating catecholamines associated with mental stress.16,42 We have previously demonstrated that α‐adrenergic blockade improves coronary microvascular vasodilation during mental stress.18 Whether the peripheral microvascular response to mental stress is a marker of changes also occurring simultaneously in the coronary microvascular bed or whether it plays a direct role in inducing MSIMI by increasing afterload, for example, needs to be determined. Additionally, whether assessment of peripheral microvascular response to mental stress may serve as a diagnostic tool to help identify those at risk for this phenomenon needs further investigation. Our study suggests that the utility of PAT as a diagnostic tool may be greatest in identifying those at low risk for MSIMI given the high negative predictive value of 91%.
Major strengths of our study are the large population investigated and the reproducibility of our findings in both an exploratory and a validation group. Moreover, this is the first study to use a detailed quantitative estimate of CAD extent and severity that also integrates measures of peripheral microvascular function to investigate the mechanisms underlying MSIMI. Limitations are that the angiograms were performed at a variable time interval before the study, thus decreasing the precision of CAD severity at the time of the stress testing. However, the duration between the angiogram and stress testing made no difference to the strong relationship we observed between disease burden and PSIMI in the same subjects, indicating that the angiographic data were reflective of the atherosclerotic burden in our patient population. In addition, we did not assess the peripheral microvascular response during physical stress testing. Therefore, we cannot objectively compare the role of peripheral microvascular vasomotion in the mechanisms of ischemia due to mental versus physical stress.
Conclusions
MSIMI in patients with stable CAD portends a worse prognosis, independent of traditional cardiovascular risk models. Yet, the mechanisms underlying this phenomenon and the clinical characteristics predictive of those at risk remain unclear. Our findings indicate that the angiographic atherosclerotic burden of CAD is not predictive of MSIMI, but its occurrence can be predicted by the digital microvascular constriction in response to mental stress, which may reflect similar changes in the coronary microcirculation due to coronary microvascular dysfunction. Despite current “optimal” medical and revascularization strategies, patients with CAD continue to demonstrate a considerable burden of cardiovascular morbidity and mortality. The differing pathophysiology of ischemia due to mental stress compared with physical stress suggests that therapies targeted specifically at modulating vasomotor changes could potentially be therapeutic and improve outcomes, a hypothesis that warrants further investigation.
Sources of Funding
This study was supported by the National Institutes of Health (grants 5R01HL70265‐5 and 5P01HL101398‐02).
Disclosures
None.
References
- 1.Gullette EC, Blumenthal JA, Babyak M, Jiang W, Waugh RA, Frid DJ, O'Connor CM, Morris JJ, Krantz DS. Effects of mental stress on myocardial ischemia during daily life. JAMA. 1997; 277:1521-1526 [PubMed] [Google Scholar]
- 2.Jain D, Burg M, Soufer R, Zaret BL. Prognostic implications of mental stress‐induced silent left ventricular dysfunction in patients with stable angina pectoris. Am J Cardiol. 1995; 76:31-35 [DOI] [PubMed] [Google Scholar]
- 3.Jiang W, Babyak M, Krantz DS, Waugh RA, Coleman RE, Hanson MM, Frid DJ, McNulty S, Morris JJ, O'Connor CM, Blumenthal JA. Mental stress‐induced myocardial ischemia and cardiac events. JAMA. 1996; 275:1651-1656 [DOI] [PubMed] [Google Scholar]
- 4.Krantz DS, Santiago HT, Kop WJ, Bairey Merz CN, Rozanski A, Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol. 1999; 84:1292-1297 [DOI] [PubMed] [Google Scholar]
- 5.Sheps DS, McMahon RP, Becker L, Carney RM, Freedland KE, Cohen JD, Sheffield D, Goldberg AD, Ketterer MW, Pepine CJ, Raczynski JM, Light K, Krantz DS, Stone PH, Knatterud GL, Kaufmann PG. Mental stress‐induced ischemia and all‐cause mortality in patients with coronary artery disease: results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002; 105:1780-1784 [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal JA, Jiang W, Waugh RA, Frid DJ, Morris JJ, Coleman RE, Hanson M, Babyak M, Thyrum ET, Krantz DS, O'Connor C. Mental stress‐induced ischemia in the laboratory and ambulatory ischemia during daily life. Association and hemodynamic features. Circulation. 1995; 92:2102-2108 [DOI] [PubMed] [Google Scholar]
- 7.Rozanski A, Bairey CN, Krantz DS, Friedman J, Resser KJ, Morell M, Hilton‐Chalfen S, Hestrin L, Bietendorf J, Berman DS. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988; 318:1005-1012 [DOI] [PubMed] [Google Scholar]
- 8.Arrighi JA, Burg M, Cohen IS, Kao AH, Pfau S, Caulin‐Glaser T, Zaret BL, Soufer R. Myocardial blood‐flow response during mental stress in patients with coronary artery disease. Lancet. 2000; 356:310-311 [DOI] [PubMed] [Google Scholar]
- 9.Hassan M, York KM, Li Q, Lucey DG, Fillingim RB, Sheps DS. Variability of myocardial ischemic responses to mental versus exercise or adenosine stress in patients with coronary artery disease. J Nucl Cardiol. 2008; 15:518-525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deanfield JE, Shea M, Kensett M, Horlock P, Wilson RA, de Landsheere CM, Selwyn AP. Silent myocardial ischaemia due to mental stress. Lancet. 1984; 2:1001-1005 [DOI] [PubMed] [Google Scholar]
- 11.Modena MG, Corghi F, Fantini G, Mattioli G. Echocardiographic monitoring of mental stress test in ischemic heart disease. Clin Cardiol. 1989; 12:21-24 [DOI] [PubMed] [Google Scholar]
- 12.Gottdiener JS, Krantz DS, Howell RH, Hecht GM, Klein J, Falconer JJ, Rozanski A. Induction of silent myocardial ischemia with mental stress testing: relation to the triggers of ischemia during daily life activities and to ischemic functional severity. J Am Coll Cardiol. 1994; 24:1645-1651 [DOI] [PubMed] [Google Scholar]
- 13.Schiffer F, Hartley LH, Schulman CL, Abelmann WH. Evidence for emotionally‐induced coronary arterial spasm in patients with angina pectoris. Br Heart J. 1980; 44:62-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.L'Abbate A, Simonetti I, Carpeggiani C, Michelassi C. Coronary dynamics and mental arithmetic stress in humans. Circulation. 1991; 83:II94-II99 [PubMed] [Google Scholar]
- 15.Miller PF, Light KC, Bragdon EE, Ballenger MN, Herbst MC, Maixner W, Hinderliter AL, Atkinson SS, Koch GG, Sheps DS. Beta‐endorphin response to exercise and mental stress in patients with ischemic heart disease. J Psychosom Res. 1993; 37:455-465 [DOI] [PubMed] [Google Scholar]
- 16.Goldberg AD, Becker LC, Bonsall R, Cohen JD, Ketterer MW, Kaufman PG, Krantz DS, Light KC, McMahon RP, Noreuil T, Pepine CJ, Raczynski J, Stone PH, Strother D, Taylor H, Sheps DS. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia study (PIMI). Circulation. 1996; 94:2402-2409 [DOI] [PubMed] [Google Scholar]
- 17.Ramachandruni S, Fillingim RB, McGorray SP, Schmalfuss CM, Cooper GR, Schofield RS, Sheps DS. Mental stress provokes ischemia in coronary artery disease subjects without exercise‐ or adenosine‐induced ischemia. J Am Coll Cardiol. 2006; 47:987-991 [DOI] [PubMed] [Google Scholar]
- 18.Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO., III Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. Am J Cardiol. 1995; 76:125-130 [DOI] [PubMed] [Google Scholar]
- 19.Kop WJ, Krantz DS, Howell RH, Ferguson MA, Papademetriou V, Lu D, Popma JJ, Quigley JF, Vernalis M, Gottdiener JS. Effects of mental stress on coronary epicardial vasomotion and flow velocity in coronary artery disease: relationship with hemodynamic stress responses. J Am Coll Cardiol. 2001; 37:1359-1366 [DOI] [PubMed] [Google Scholar]
- 20.Jain D, Shaker SM, Burg M, Wackers FJ, Soufer R, Zaret BL. Effects of mental stress on left ventricular and peripheral vascular performance in patients with coronary artery disease. J Am Coll Cardiol. 1998; 31:1314-1322 [DOI] [PubMed] [Google Scholar]
- 21.Burg MM, Graeber B, Vashist A, Collins D, Earley C, Liu J, Lampert R, Soufer R. Noninvasive detection of risk for emotion provoked myocardial ischemia. Psychosom Med. 2009; 71:14-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassan M, York KM, Li H, Li Q, Lucey DG, Fillingim RB, Sheps DS. Usefulness of peripheral arterial tonometry in the detection of mental stress‐induced myocardial ischemia. Clin Cardiol. 2009; 32:E1-E6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995; 26:1235-1241 [DOI] [PubMed] [Google Scholar]
- 24.Neunteufl T, Katzenschlager R, Hassan A, Klaar U, Schwarzacher S, Glogar D, Bauer P, Weidinger F. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis. 1997; 129:111-118 [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann PG, McMahon RP, Becker LC, Bertolet B, Bonsall R, Chaitman B, Cohen JD, Forman S, Goldberg AD, Freedland K, Ketterer MW, Krantz DS, Pepine CJ, Raczynski J, Stone PH, Taylor H, Knatterud GL, Sheps DS. The Psychophysiological Investigations of Myocardial Ischemia (PIMI) study: objective, methods, and variability of measures. Psychosom Med. 1998; 60:56-63 [DOI] [PubMed] [Google Scholar]
- 26.Walkers F, Soufer R, Zaret BL. In: Braunwald E, Zipes D, Libby P. (eds.). Nuclear cardiology. Heart Disease: A Text Book of Cardiovascular Medicine. 2000Philadelphia, PA: W.B. Saunders; 273-323 [Google Scholar]
- 27.Berman DS, Hachamovitch R, Kiat H, Cohen I, Cabico JA, Wang FP, Friedman JD, Germano G, Van Train K, Diamond GA. Incremental value of prognostic testing in patients with known or suspected ischemic heart disease: a basis for optimal utilization of exercise technetium‐99m sestamibi myocardial perfusion single‐photon emission computed tomography. J Am Coll Cardiol. 1995; 26:639-647 [DOI] [PubMed] [Google Scholar]
- 28.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983; 51:606. [DOI] [PubMed] [Google Scholar]
- 29.Patel RS, Su S, Neeland IJ, Ahuja A, Veledar E, Zhao J, Helgadottir A, Holm H, Gulcher JR, Stefansson K, Waddy S, Vaccarino V, Zafari AM, Quyyumi AA. The chromosome 9p21 risk locus is associated with angiographic severity and progression of coronary artery disease. Eur Heart J. 2010; 31:3017-3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan DR, Marwick TH, Freedman SB. A new method of scoring coronary angiograms to reflect extent of coronary atherosclerosis and improve correlation with major risk factors. Am Heart J. 1990; 119:1262-1267 [DOI] [PubMed] [Google Scholar]
- 31.Burton A. The range and variability of the blood flow in the human fingers and the vasomotor regulation of body temperature. Am J Physiol. 1939; 127:437-453 [Google Scholar]
- 32.Schnall RP, Shlitner A, Sheffy J, Kedar R, Lavie P. Periodic, profound peripheral vasoconstriction—a new marker of obstructive sleep apnea. Sleep. 1999; 22:939-946 [PubMed] [Google Scholar]
- 33.Lavie P, Schnall RP, Sheffy J, Shlitner A. Peripheral vasoconstriction during rem sleep detected by a new plethysmographic method. Nat Med. 2000; 6:606. [DOI] [PubMed] [Google Scholar]
- 34.Strike PC, Steptoe A. Systematic review of mental stress‐induced myocardial ischaemia. Eur Heart J. 2003; 24:690-703 [DOI] [PubMed] [Google Scholar]
- 35.Kuroda T, Kuwabara Y, Watanabe S, Nakaya J, Hasegawa R, Shikama T, Matsuno K, Mikami Y, Fujii K, Saito T, Masuda Y. Effect of mental stress on left ventricular ejection fraction and its relationship to the severity of coronary artery disease. Eur J Nucl Med. 2000; 27:1760-1767 [DOI] [PubMed] [Google Scholar]
- 36.Kral BG, Becker LC, Blumenthal RS, Aversano T, Fleisher LA, Yook RM, Becker DM. Exaggerated reactivity to mental stress is associated with exercise‐induced myocardial ischemia in an asymptomatic high‐risk population. Circulation. 1997; 96:4246-4253 [DOI] [PubMed] [Google Scholar]
- 37.Schoder H, Silverman DH, Campisi R, Sayre JW, Phelps ME, Schelbert HR, Czernin J. Regulation of myocardial blood flow response to mental stress in healthy individuals. Am J Physiol Heart Circ Physiol. 2000; 278:H360-H366 [DOI] [PubMed] [Google Scholar]
- 38.Schoder H, Silverman DH, Campisi R, Karpman H, Phelps ME, Schelbert HR, Czernin J. Effect of mental stress on myocardial blood flow and vasomotion in patients with coronary artery disease. J Nucl Med. 2000; 41:11-16 [PubMed] [Google Scholar]
- 39.Lacy CR, Contrada RJ, Robbins ML, Tannenbaum AK, Moreyra AE, Chelton S, Kostis JB. Coronary vasoconstriction induced by mental stress (simulated public speaking). Am J Cardiol. 1995; 75:503-505 [DOI] [PubMed] [Google Scholar]
- 40.Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ, Jr, Ganz P, Selwyn AP. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991; 325:1551-1556 [DOI] [PubMed] [Google Scholar]
- 41.Boltwood MD, Taylor CB, Burke MB, Grogin H, Giacomini J. Anger report predicts coronary artery vasomotor response to mental stress in atherosclerotic segments. Am J Cardiol. 1993; 72:1361-1365 [DOI] [PubMed] [Google Scholar]
- 42.Becker LC, Pepine CJ, Bonsall R, Cohen JD, Goldberg AD, Coghlan C, Stone PH, Forman S, Knatterud G, Sheps DS, Kaufmann PG. Left ventricular, peripheral vascular, and neurohumoral responses to mental stress in normal middle‐aged men and women. Reference group for the Psychophysiological Investigations of Myocardial Ischemia (PIMI) study. Circulation. 1996; 94:2768-2777 [DOI] [PubMed] [Google Scholar]


