Abstract
Background
In Fontan and atrial switch patients, transcatheter ablation is limited by difficult access to the pulmonary venous atrium. In recent years, transbaffle access (TBA) has been described, but limited data document its safety and utility.
Methods and Results
All ablative electrophysiological study cases of this population performed between January 2006 and December 2010 at Boston Children's Hospital were reviewed. Pre‐case and follow‐up clinical characteristics were documented. Adverse events were classified by severity and attributability to the intervention. We included 118 cases performed in 90 patients. TBA was attempted in 74 cases and was successful in 96%: in 20 via baffle leak or fenestration and in 51 (94%) of 54 using standard or radiofrequency transseptal techniques. There were 10 procedures with adverse events ranked as moderate or more severe. The event rate was similar in both groups (TBA 8% versus non‐TBA 9%, P=1), and no events were directly attributable to TBA. There was a trend to higher proportion of cases having a >5‐point drop in saturations from baseline in the TBA group versus the non‐TBA group in Fontan cases (15% vs 0%, P=0.14). When cases with follow‐up >90 and >365 days were analyzed, the median initial arrhythmia score of 5 significantly changed −3 points in both time periods (P≤0.001).
Conclusions
TBA is feasible in this population; its use was not associated with a higher incidence of adverse events; and changes in clinical scores support its efficacy. Desaturation observed in some patients is of uncertain significance but warrants postablation monitoring and prospective study.
Keywords: ablation, catheterization, Fontan, Mustard, Senning, transbaffle
Introduction
The incidence of atrial tachyarrhythmias in patients who have undergone atrial switch operation or Fontan palliation is high, occurring in as many as 48% of patients over time.1–2 In this population, therapy with transcatheter ablative techniques frequently is limited due to difficulties accessing the common pulmonary venous atrium (PVA) from the systemic veins.3–4 Several approaches have been used to access the PVA in these procedures, including retrograde via systemic artery, direct via sternotomy, and transthoracic via percutaneous access.5 Using these techniques can result in poor stability and flexibility of the ablation catheter, hemodynamic instability, and risk of valvar dysfunction and/or injury, as is the case with retrograde approach, or can be associated with a high incidence of complications or technical limitations, as is the case for transthoracic or via sternotomy access.5–8
In recent years, an alternative solution with puncture or access across the baffle that divides the systemic with the pulmonary venous atrium has been described and is now more commonly used. There are limited data in the literature in regard to this new technique consisting mainly of case reports and small case series with encouraging results.9–12 The goals of this study are to find the frequency of the use of transbaffle access (TBA) for mapping or ablation of atrial tachycardias in this patient population, the safety of this procedure along with the acute success rate, and an initial report of a longer‐term success rate.
Methods
Population and Baseline Characteristics
A retrospective chart review of all electrophysiological study (EPS) cases of patients with Mustard, Senning, or Fontan procedures performed at Boston Children's Hospital from January 2006 to December 2010 was performed. Intended atrial ablative procedures were included, excluding cases in which the objective was exclusively a diagnostic evaluation or assessment of a ventricular arrhythmia. Pre‐EPS demographic characteristics, oxygen saturations during the preoperative evaluation, and relevant details on cardiac surgical and arrhythmia history were collected. An assessment of the arrhythmia burden was performed using a multiaxis severity score previously described by Triedman et al.13
Arrhythmia Score
Four subscores were summed to yield a clinical arrhythmia score that ranged from 0 (no arrhythmia activity) to 12 points (severe, incessant, and life‐threatening arrhythmia activity) (Table 1). Each subscore was calculated to reflect the poorest clinical outcomes in the preceding 3 months to each of the encounters and included items attempting to quantify the duration of the arrhythmia, associated symptoms, and the medical and electrical therapies provided for it.
Table 1.
Arrhythmia Score
| Documented Arrhythmia | Frequency of Cardioversion | ||
|---|---|---|---|
| None | 0 | None | 0 |
| Non sustained only | 1 | One cardioversion | 1 |
| Sustained arrhythmia | 2 | AAIT cardioversions | 2 |
| Incessant | 3 | Two or more cardioversions | 3 |
| Arrhythmia Severity | Antiarrhythmic Medications | ||
|---|---|---|---|
| Asymptomatic | 0 | None or digoxin only | 0 |
| Palpitations only | 1 | Class II or class IV | 1 |
| Syncope/CHF/thrombosis | 2 | Class I or class III | 2 |
| Cardiac arrest | 3 | Amiodarone toxicity | 3 |
Score of 0 to 12 points is calculated as the sum of the highest score achieved in each of the 4 categories on the prior 3 months of the scoring date. Thrombosis determined by either echocardiographic evidence of intracardiac thrombosis or clinical evidence of thromboembolic event. AAIT cardioversions are defined as 1 or more automatic or manual cardioversions performed using an implanted atrial pacemaker and not requiring any additional intervention. Amiodarone toxicity includes documented abnormalities of thyroid, hepatic or pulmonary function attributed by clinician to amiodarone administration, whether necessitating discontinuation of medication. CHF indicates congestive heart failure, determined by review of clinical records and/or hemodynamic measurement.
EPS Reports
The EPS reports were used to obtain the baseline rhythm, procedure difficulty factors, mechanisms of arrhythmia, and location of the substrates. We documented the interventions performed including attempted ablations, TBA, and any other non‐EP procedures during the case.
We defined TBA as crossing from the systemic venous atrium (SVA) into the pulmonary venous atrium (PVA) using a fenestration, a baffle leak, or a new puncture access. A successful TBA was considered to have been achieved when mapping in the PVA could be completed using that technique. An ablative case was considered to have partial success if at least 1 of the targeted arrhythmia mechanisms was ablated with demonstrable block and/or noninducibility and was classified as having complete success when the same applied for all the relevant arrhythmia substrates.
Complications after the procedure were recorded and further classified by attributability to EPS, anesthesia, access, TBA, or ablation procedure and were also subdivided by severity using a standard definition into trivial, minor, moderate, major, and catastrophic (Table 2).
Table 2.
Adverse Event Classification
| Low | Trivial | No change in condition, may require monitoring with no intervention |
| Minor | Transient change in condition, not life threatening, condition returns to baseline, requires monitoring, minor intervention as holding a medication or a laboratory test | |
| High | Moderate | Transient change in condition may be life threatening if not treated, requires intervention as medication, ICU monitoring or moderate transcatheter procedure |
| Major | Change in condition that may be permanent, life threatening if not treated, may require ICU, emergent readmit to the hospital, invasive monitoring or interventions such as DCCV, intubation or major transcatheter interventions to correct condition | |
| Catastrophic | Any death and emergent surgery or heart lung bypass support to prevent death with failure to wean from bypass support |
DCCV indicates direct current cardioversion; ICU, intensive care unit.
Statistical Analysis
We considered that the acute procedural outcomes, attempt, and success at TBA and case‐related complications after each EPS were independent results in patients who had >1 EPS. For this reason, we regarded all cases as single independent events for statistical analysis. Our primary outcome was the rate of successful TBA, and our secondary outcomes included the rate of successful ablation, change in the arrhythmia score, and postprocedure desaturation defined as a decrease in the baseline oxygen saturation >5 points after the EPS. We used the Fisher exact test for the categorical variables and the Wilcoxon rank sum test for the continuous variables. We divided the cases between 2 time periods (2006–2008 and 2009–2011) and used the Fisher exact test to provide an analysis of the proportions of times a TBA was used during these 2 time periods. We determined the success and complication rates as described earlier along with their 95% CI.
Results
Study Population
During the study period, 134 EP cases were performed in eligible patients with atrial baffles. Excluding 16 who had planned only diagnostic studies or ventricular tachycardia ablations, 118 procedures in 90 patients were identified. The majority had Fontan palliation (75%), with the remainder having atrial switch procedures (Table 3). The 118 cases were performed in 90 different patients with a median age of 26.4 years (range 1.6 to 55 years). In the majority (91%), the indications for the procedure were associated symptoms with a prior documented atrial or supraventricular tachycardia, of which the most common was intra‐atrial reentry tachycardia (IART), identified clinically in 80% of the cases before the EPS.
Table 3.
Baseline Characteristics of Cases
| All Cases | TBA | No TBA | P Value | |
|---|---|---|---|---|
| Total cases | n=118 | n=74 | n=44 | |
| Median age, y (range) | 26.3 (1.6 to 54.9) | 26.7 (1.8 to 54.9) | 24.8 (1.6 to 47) | 0.28 |
| Median weight, kg (range) | 63 (9.4 to 165) | 63 (11 to 121) | 64.5 (9.4 to 165) | 0.8 |
| Median follow‐up, d (range) | 353 (1 to 1862) | 394 (1 to 1862) | 247 (1 to 1343) | 0.06 |
| Atrial anatomy and palliation | ||||
| Classic or modified Fontan | 52 (44%) | 24 (32%) | 28 (64%) | 0.001 |
| Lateral tunnel Fontan | 34 (29%) | 24 (32%) | 10 (23%) | 0.3 |
| Extracardiac Fontan | 3 (3%) | 2 (3%) | 1 (2%) | |
| Mustard | 16 (14%) | 13 (18%) | 3 (7%) | 0.16 |
| Senning | 13 (11%) | 11 (15%) | 2 (5%) | 0.12 |
| Arrhythmia history | ||||
| IART | 94 (80%) | 61 (82%) | 33 (74%) | 0.34 |
| Atrial fibrillation | 22 (19%) | 12 (16%) | 10 (23%) | 0.46 |
| Unclassified SVT | 22 (19%) | 10 (14%) | 12 (27%) | 0.09 |
| Other | 27 (23%) | 17 (23%) | 10 (23%) | 1 |
| Indications for EPS | ||||
| Symptoms and ECG documentation | 101 (86%) | 65 (88%) | 36 (82%) | 0.42 |
| Arrest or syncope and ECG documentation | 6 (5%) | 5 (7%) | 1 (2%) | 0.4 |
| Symptoms only | 4 (3%) | 1 (1%) | 3 (7%) | |
| Asymptomatic ECG documentation | 6 (5%) | 2 (3%) | 4 (9%) | 0.19 |
| Preexitation only | 1 (1%) | 1 (1%) | 0 (0%) | |
ECG indicates echocardiogram; EPS, electrophysiology study; IART, intra‐atrial reentry tachycardia; SVT, supraventricular tachycardia; TBA, transbaffle access.
Diagnostic Assessment
After atrial stimulation and mapping protocols as clinically indicated, arrhythmia substrates were identified during the EPS in 116 cases. There were 2 patients with negative atrial stimulation, and they were on antiarrhythmic drugs at the time of the study for control of the clinical tachycardia. The most common identified arrhythmia substrate was IART, which was induced in 66% of the cases, this was followed in frequency by focal tachycardias, atrioventricular nodal reentry tachycardia, atrioventricular reentry tachycardia, and twin node reentry tachycardias, which in combination were present in 36% of all cases (Table 4).
Table 4.
Arrhythmia Substrates on EPS Cases and TBA
| Substrate | Total of Cases (n=118) | TBA (n=74) | No TBA (n=44) |
|---|---|---|---|
| IART | 78 (66%) | 54 (73%) | 24 (55%) |
| Undefined | 46 (39%) | 23 (31%) | 23 (52%) |
| Focal | 22 (18%) | 9 (12%) | 13 (30%) |
| AVNRT | 11 (9%) | 6 (8%) | 5 (11%) |
| AVRT | 7 (6%) | 5 (7%) | 2 (5%) |
| Twin nodes | 6 (5%) | 4 (5%) | 2 (5%) |
AVNRT indicates atrioventricular nodal reentry tachycardia; AVRT, atrioventricular reentry tachycardia; IART, intra‐atrial reentry tachycardia; TBA, transbaffle access.
TBA
A general technical approach to ablation including TBA is described next, with the understanding that specific procedural details varied to meet the challenges of each case. Patients were studied when under general anesthesia. Electroanatomical mapping (Biosense) was used for the majority of the cases. The electrophysiological stimulation and mapping of the substrates were performed initially on the SVA. If the assessment of the EPS data was consistent with an ablatable PVA arrhythmia substrate, a TBA was considered. An angiogram of the SVA was performed and recorded to levophase to delineate the anatomy and identify the presence of baffle leaks. If a leak was present, its suitability to support mapping and ablation was assessed by its location and size, and an attempt to cross it was made accordingly. In the absence of an adequate baffle leak, a transbaffle puncture was performed. The default approach was to first make a transseptal attempt using a standard Brockenbrough needle (St Jude, Inc) with a Mullins sheath (Cook Inc). If this technique was unsuccessful, the use of alternative sheaths and/or a radiofrequency needle was considered as described by Esch et al.14 Most procedures were guided fluoroscopically, but when intracardiac echocardiography was used, it provided useful information in some cases (Videos S1 and S2).
Access to the PVA was attempted in 74 (63%) cases. In 20, a fenestration or a baffle leak was used to gain access to this location, and in 54, a new puncture was performed: in 34 using a transseptal needle, in 8 using radiofrequency, and in 12 using both techniques. The procedure was considered successful in 96% of the attempts, providing adequate access for mapping across the baffle, with 3 instances in which the TBA attempt failed. When comparing type of repair, arrhythmia history, and indications for ablation, there was a higher proportion of Fontan cases in the non‐TBA group specifically with underlying modified or classic‐type Fontan palliations (Table 3). Access to the PVA was attempted via a retrograde approach in 13 cases, and it was considered adequate in 6 of them.
We compared the frequency of TBA use by dividing the cases into 2 time periods. During 2006 to 2008, TBA was attempted in 29 of 52 cases (56%), and from 2009 to 2011, it was attempted in 45 of 66 cases (68%). The relative frequency of attempted TBA in these 2 groups was not significantly different (P=0.18).
Failed TBA
The 3 cases who had a failed attempt at TBA were all Fontan patients. In 2 instances, inability of the transseptal needle to cross the baffle possibly related to a suboptimal angle and patch calcifications were the attributable causes. In the last case, a sheath was advanced across the baffle, but limited mobility impaired acceptable mapping. Comparing those 3 cases with successful TBAs, no associations were observed by univariate analysis relating to patient demographics, type of repair, main anatomical problem, sheath used, TBA difficulty factors, patch material, or date of the procedure.
Ablation Outcomes
Ablation was attempted in 108 of 118 cases (92%) during the EPS, and 95 (81%) had either a complete or partial acute successful ablation (TBA 84% versus no TBA 75%, P=0.33). In 68 cases (58%), success was classified as complete (TBA 61% versus no TBA 52%, P=0.44).
The median follow‐up duration post procedure was 12 months (1 day to 61 months) with a total follow‐up of 156 patient‐years. During the follow‐up period, 8 patients died, 3 within the first 3 months and an additional patient between 3 and 12 months after the EPS; 2 deaths were considered related or possibly related to the EP procedure. Thirty‐two cases (27%) underwent either a repeat EPS or a surgical maze procedure (Table 5), 11 within 90 days of the index intervention and 21 before the end of the first postablation year.
Table 5.
Outcomes in TBA and No TBA Groups
| Total | TBA (%) | No TBA (%) | P Value | |
|---|---|---|---|---|
| Atrial switch patients | n=29 | n=24 | n=5 | — |
| Successful TBA | — | 24 (100%) | — | — |
| Retrograde PVA access | 2 (7%) | 0 (0%) | 2 (40%) | |
| Repeat EPS/maze | 6 (21%) | 4 (17%) | 2 (40%) | 0.5 |
| Subsequent desaturation* | 0 (0%) | 0 (0%) | 0 (0%) | — |
| ≥Moderate complications* | 1 (3%) | 1 (4%) | 0 (0%) | — |
| Ablation success | 25 (86%) | 22 (92%) | 3 (60%) | 0.13 |
| Fontan patients | N=89 | N=50 | N=39 | — |
| Successful TBA | — | 47 (94%) | — | — |
| Retrograde PVA access | 11 (12%) | 5 (10%) | 6 (15%) | 0.5 |
| Repeat EPS/maze | 26 (29%) | 12 (24%) | 14 (36%) | 0.24 |
| Subsequent desaturation* | 5 (6%) | 5 (10%) | 0 (0%) | 0.06 |
| ≥Moderate complications* | 9 (10%) | 5 (10%) | 4 (10%) | 1 |
| Ablation success | 68 (76%) | 38 (76%) | 30 (77%) | 1 |
EPS indicates electrophysiological study; PVA, pulmonary venous atrium; TBA, transbaffle access.
Desaturation defined as oximetry saturation measured in a follow up clinic appointment 5 points lower than on the pre‐EPS visit.
Complications that were considered either related or possibly related and classified as moderate, severe, or catastrophic.
Arrhythmia Score
For the total cohort, the initial median arrhythmia score was 6, similar for the TBA and no‐TBA groups. A total of 89 (75% of total) cases had available arrhythmia scores 90 days after the EPS: 59 (79%) in the TBA and 30 (68%) in the no‐TBA groups. In each of these groups, the median initial arrhythmia score was 5 and the median score change was −3 points, reaching statistical significance in all of them. Similarly, when only cases with >365 days of follow‐up were selected, 58 (49%) of the total were included: 40 in the TBA and 18 in the no‐TBA group (54% and 41%, respectively). The median initial scores for the TBA and no‐TBA groups were 6 and 4 (P=0.41), and their median final arrhythmia scores were 2 and 3.5, respectively, reaching statistical significance in the TBA group (P<0.001) (Figure 1).
Figure 1.
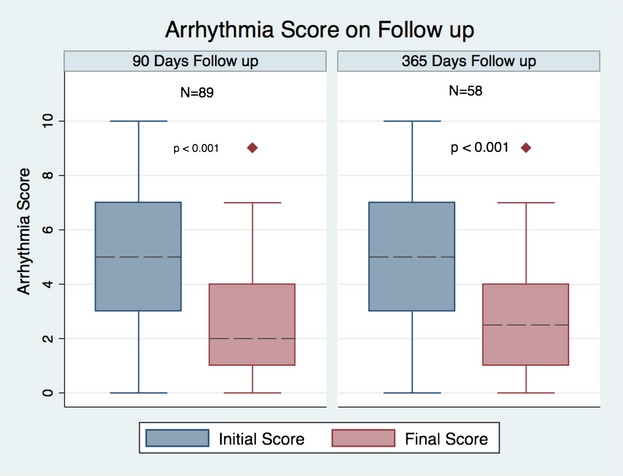
Follow‐up of arrhythmia score.
The median change in arrhythmia score at 90 days in the group of patients with any type of success was −3 versus 0 for the nonsuccessful cases (P=0.004). A similar result was found when the analysis was done with cases who had a follow‐up >12 months, with score changes of −3 and −1 for the groups with any success and no success, respectively (P=0.009) (Figures 2 and 3).
Figure 2.
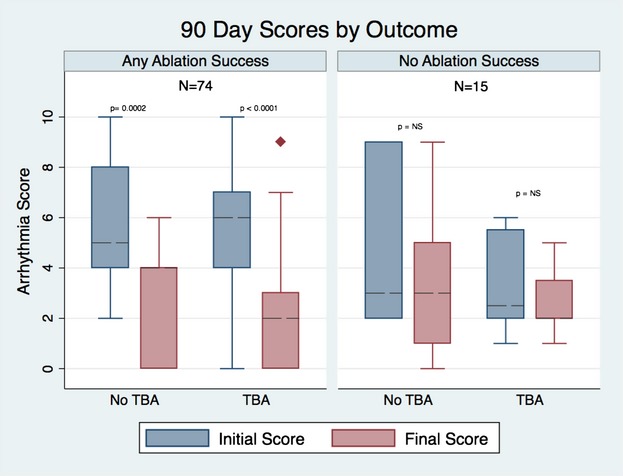
Follow‐up arrhythmia score at 90 days by outcome and transbaffle access (TBA).
Figure 3.
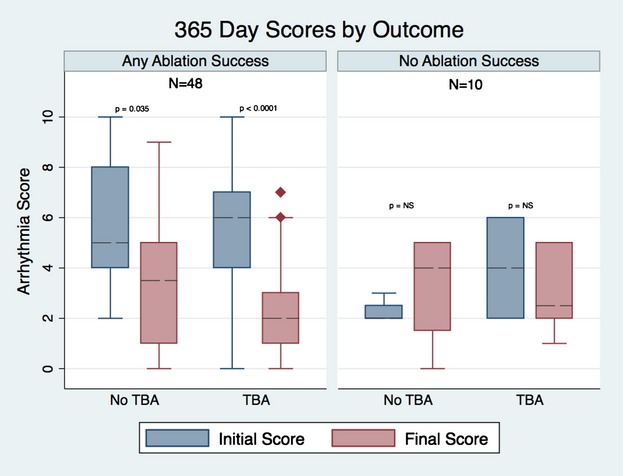
Follow arrhythmia score at 365 days by outcome and transbaffle access (TBA).
Adverse Events
There were 10 (8.5%) cases in which moderate or more severe adverse events were encountered that were classified as related or possibly related to the electrophysiological procedure (TBA 6 [8%] versus no TBA 4 [9%], P=1). Two of the TBA cases with complications had 2 separate adverse events each for a total of 12 different such occurrences. Two of them were catastrophic, and 5 were classified as major and 5 as moderate in severity.
Deaths
A 46‐year‐old man with lateral tunnel Fontan underwent a partially successful EPS for IART ablation with TBA and fenestration closure. He had amiodarone‐related lung disease, elevated Fontan pressures, and low cardiac index. Five days after the procedure while being loaded with propafenone, he developed wide‐complex tachycardia with no response to resuscitation efforts. This event was likely related to drug‐induced proarrhythmia but was classified as possibly attributable to the EPS.
A 37‐year‐old woman with a fenestrated Fontan, amiodarone‐induced lung disease, a preexisting baffle leak, and a history of a cardiac arrest underwent left atrial maze. Due to persistent cyanosis, she had repeat catheterization 3 days later with baffle leak closure. She died 1 month later secondary to renal failure, incessant atrial arrhythmias, respiratory failure, and infection. This event was deemed possibly attributable to the EPS with unclear association with the TBA.
Other Adverse Events
Two of the major complications (in a single patient who underwent 2 different EPSs) were documented persistent cyanosis. These occurred in conjunction with demonstration of larger leaks across an atrial defect associated with worsening cyanosis and were deemed attributable to ablation lesions to this region. We also observed the development of permanent complete heart block during a cavotricuspid isthmus–dependent IART in a classic Fontan patient, as well as the finding of a large coronary sinus thrombus seen on echocardiography 4 weeks after IART ablation, for which surgical debulking and a surgical maze were performed and a retroperitoneal hematoma resulting from a right iliac venous tear. This same patient later developed a pseudoaneurysm on the left femoral vein that was managed with compression therapy and was classified as a separate moderate complication. There were 3 cases of acute kidney injury extending the intensive care unit support in this moderate category, attributed to low cardiac output and contrast toxicity. The last of the moderate complications was the development of hemoptysis during endotracheal tube manipulation that extended the intubation time overnight after the EPS (Table 6). None of these adverse events were deemed attributable to TBA.
Table 6.
Adverse Events
| Severity | Adverse Event | TBA | No TBA | P Value |
|---|---|---|---|---|
| Catastrophic | Death | 2 | 0 | — |
| Major | Shunts and cyanosis | 2 | 0 | — |
| Permanent AV block | 1 | 0 | — | |
| CS thrombosis | 0 | 1 | — | |
| Retroperitoneal bleed | 1 | 0 | — | |
| Moderate | Pseudoaneurysm | 1 | 0 | — |
| Acute kidney injury | 0 | 3 | 0.05 | |
| Hemoptysis | 1 | 0 | — | |
| All | Total>minor adverse events | 8 | 4 | 1 |
AV indicates atrioventricular; CS, coronary sinus; TBA, transbaffle access.
Post‐EPS Oxygen Saturation
There were 5 cases in which postprocedure oxygen saturation was ≥5 points lower than the baseline recorded before the EPS. All 5 cases were Fontan patients in whom a TBA was attempted. In 2 of these cases, there was clinician concern of cyanosis, and a right‐to‐left shunt was demonstrated by the use of different diagnostic modalities. One was presumed to be associated with the TBA puncture site with saturations that improve spontaneously few weeks after the procedure with no additional interventions, and the other, described earlier as an adverse event, was thought to be due to a worsening coronary sinus leak after radiofrequency ablation in this region. In the other 3 cases, no clinical concerns of desaturation were documented and no evidence of a new shunt was reported on routine diagnostic modalities or interventions for this indication. These 5 cases were part of the group of 54 Fontan patients with oxygen saturations measured at clinic visits available for analysis, and although there was a trend toward higher frequency of post‐EPS desaturation in comparison to no‐TBA cases, this was not statistically significant (P=0.14, Fisher's exact test).
Discussion
In this study, we report a large series of cases in patients with atrial switches or Fontan palliations in whom a TBA was used for mapping and ablation of atrial arrhythmias. Prior publications include case reports and small series exhibiting the potential uses of this technique but showed little evidence of its reliability to reach and support catheter manipulation to allow mapping and ablation on the PVA of these patients. Attempts at TBA in our cohort were highly successful, with the majority using a new puncture with a transseptal needle or the addition of radiofrequency energy but also using residual baffle leaks or patent fenestrations. The procedure proved to be generally safe in our group, although we identified some cases with potentially significant postprocedure cyanosis, which appeared in some patients to be possibly related to the intervention. Our analysis continues to support the concept that catheter ablation is a reasonable approach for effectively improving the clinical implications of atrial arrhythmias in this complex population.
Efficacy of TBA
After atrial switch operation, the cavotricuspid isthmus is frequently included in the critical conduction corridor for IART circuits, this is also the region where the anatomical substrates for many other supraventricular tachycardias are located and, as a result of the Mustard and Senning operations in a large proportion of cases, is located on the PVA side of the baffle. This makes finding a reliable access to reach it for mapping and ablation essential for the majority of these patients.4,15
The high frequency with which access to the PVA is deemed necessary for atrial arrhythmia ablation in the atrial switch population is supported by our finding that 83% of the patients with this anatomy had a TBA attempted, a proportion that was significantly higher compared with the Fontan patients. The lateral tunnel and extracardiac Fontan population is growing, and even though the incidence of atrial arrhythmias is lower compared with the nonclassic and modified Fontans, the majority of the atrial tissue with arrhythmogenic potential is on the pulmonary venous side of the baffle. The challenges of retrograde access include crossing the systemic semilunar and the atrioventricular valve, with the risk of injuring them or temporarily disrupting their function, which could be poorly tolerated in patients with single ventricle physiology. Additionally, catheter manipulation is frequently limited in the pulmonary venous atrium with the tortuous retrograde course. A combination of these factors explains why, in our analysis, of the 13 patients in whom a retrograde access was attempted, in only 6 was it considered adequate for completing the mapping and ablation. In a small series, Ernst et al16 reported better ablation outcomes with retrograde access using remote‐controlled magnetic navigation in this population, but this technology is not widely available at present.
Other approaches to the PVA have been described. Nehgme et al reported a percutaneous transthoracic access used successfully for 6 EPS in 5 Fontan patients with no long‐term adverse events but with acute hemothorax and pneumothorax complicating 2 of them.7 Khairy et al5 described a “hybrid” approach for an EPS in a 6‐year‐old patient who had incessant supraventricular tachycardia early in the postoperative period of an extracardiac Fontan. Direct atrial access through a medial sternotomy was obtained and a successful ablation was performed.
Recently, the use of a TBA approach for hemodynamic evaluation, dilation of pulmonary venous obstructions, and creation of fenestrations has been reported by El‐Said et al9 in 39 Fontan, atrial switch, and atrial septal defect repair patients. Comparable to our numbers, they had a successful TBA in 97% of the cases with no complications related to the procedure. The first report of TBA after an atrial switch for electrophysiological mapping and ablation was by Perry et al10 in 2 patients after Mustard and Senning operations, and they concluded this approach allowed easy access to the PVA. Since that report, only case reports or very small series have published results of this technique in EPS with Fontan and atrial switch patients.11,17–18
Our analysis with a larger number of cases in a heterogeneous group of patients supports the efficacy of this intervention. The TBA attempts achieved an acceptable mapping and ablative access to the PVA in 96% of all the instances and in 94% when only new puncture sites in 54 cases are included. We only had 3 nonsuccessful TBA cases, 1 due to poor mobility of the PVA catheter for mapping and ablation and 2 due to failure of the transseptal needle to cross into it.
Safety of TBA
Our analysis supports the notion that TBA in this population is generally safe. When we analyzed the causes of moderate to catastrophic adverse events that were related or possibly related to the EPS, we did not find any that were clearly caused by the TBA.
One concern over the use of TBA in this population is the creation of a persistent shunt and the substrate for worsening cyanosis or paradoxical embolism. There has been so far no strong evidence to support the risk or safety of the intervention in this regard. In the series by El‐Said et al,9 after the 38 cases with successful TBA, they had follow‐up echocardiograms on 30 with the only 2 who had evidence of a persistent shunt being those who had the procedure with the goal of creating a fenestration.
In an attempt to address the possibility of persistent shunts or worsening cyanosis after TBA, we compared oxygen saturations during clinic visits before and after EPS and found that 5 patients experienced a drop in the saturation of ≥5 points, all of them in the TBA group. This difference did not reach statistical significance, and given the low frequency, it is difficult to make definitive conclusions but highlights the possibility of this undesirable outcome and suggests that patients who undergo TBA may benefit from focused postprocedure investigation for the presence of a residual iatrogenic shunt. An additional prospective study would be needed to elucidate whether the use of TBA is associated with lower oxygen saturation and its clinical implications.
Ablation Outcomes
Our analysis supports the clinical utility of ablation therapies in this population and, in contrast to prior studies, it includes a large proportion of cases in which TBA was used. In our center, the use of TBA is commonly considered after preliminary mapping has raised the suspicion of clinically significant arrhythmia substrates that can be ablated on that side of the baffle. With this in mind, the fact that the follow‐up arrhythmia scores and acute success rates on the TBA and no‐TBA populations are similar is indicative that ablation of such substrates can be an effective tool to improve the arrhythmia burden in this population.
We found that 81% of the cases in our series had an ablation success, and in 58% it was complete. These figures are comparable to the outcomes reported by other groups for catheter‐based ablative procedures after congenital heart disease surgery. Yap et al3 published their IART ablation experience with 130 patients with congenital heart disease. They found an overall an acute complete success rate of 69% with a multivariate analysis showing as negative predictors having had a Fontan palliation (19 patients) or a Mustard operation (21 patients). Similarly, Kanter et al15 reported a complete success rate for IART ablations in 11 patients with d‐transposition of the great arteries and Mustard or Senning operations of 67%, and Yap et al19 published this rate as 54% for the 11 patients with Fontan palliation who were included in their series of 41 patients with congenital heart disease and underwent EPS for IART ablation. Finally, Triedman et al,13 in their experience of 134 patients with congenital heart disease (including 63 with Fontan palliations and 22 with Mustard and Sennings) who underwent EPS for IART ablation, also found a comparable limited and complete procedural success rates of 79% and 66%, respectively. They also used the same clinical arrhythmia score applied to IART and found a mean preablation score of 6.2 that decreased to 3.1 after a mean follow‐up of 25 months, numbers that are in agreement with our findings of a median arrhythmia score change of −3 after 12 months of the procedure. This suggests an improvement in the overall clinical impact of the arrhythmia, supporting the acute ablation efficacy with improvements that persists at least during the short‐ and mid‐term follow‐up. There was a nonstatistically significant trend toward improvement in the median arrhythmia score change in the group with nonsuccessful ablations after 12 months of follow‐up. There were only 10 cases in this group, and this statistical finding can be explained by some arrhythmia substrate modification despite no meeting criteria of success, by chance only, or be secondary to optimization in the medical management after the EPS.
Limitations
This study is retrospective, and its subjects include a wide spectrum of underlying anatomical substrates and surgical interventions complicating direct comparison of TBA and no‐TBA groups. A substantial proportion of cases also reached an end point of a repeat EPS, death, or surgical maze within the first 12 months of the procedure in addition to the cases that did not have available data after this same period of time, limiting observations of follow‐up outcomes. Finally, percutaneous oxygen saturation changes may be an insensitive or inaccurate test for detection of residual anatomic defect. However, it was widely available in this retrospective data set and addresses our main goal of describing clinically relevant acute safety concerns around this procedure.
Conclusions
In Fontan and atrial switch patients, TBA can be safely and effectively performed in a high proportion of cases, providing an acceptable access to the PVA for mapping and ablation with desired changes in clinical arrhythmia scores supporting its efficacy in ablative interventions. Increased postprocedure cyanosis observed in a small number of patients indicates the need for further study of this procedure.
Sources of Funding
.Pediatric Electrophysiology Department, Children's Hospital Boston.
Disclosures
Rafael Correa, MD, Edward P. Walsh, MD, Mark E. Alexander, MD, Douglas Y. Mah, MD, Frank Cecchin, MD: None. Dominic J. Abrams, MD, MRCP, is a stock owner in Johnson & Johnson. John K. Triedman, MD, has been a consultant for Biosense Webster.
References
- 1.Flinn CJ, Wolff GS, Dick M, II, Campbell RM, Borkat G, Casta A, Hordof A, Hougen TJ, Kavey RE, Kugler J, Liebman J, Greenhouse J, Hess P. Cardiac rhythm after the mustard operation for complete transposition of the great arteries. N Engl J Med. 1984; 310:1635-1638 [DOI] [PubMed] [Google Scholar]
- 2.Puley G, Siu S, Connelly M, Harrison D, Webb G, Williams WG, Harris L. Arrhythmia and survival in patients >18 years of age after the mustard procedure for complete transposition of the great arteries. Am J Cardiol. 1999; 83:1080-1084 [DOI] [PubMed] [Google Scholar]
- 3.Yap SC, Harris L, Silversides CK, Downar E, Chauhan VS. Outcome of intra‐atrial re‐entrant tachycardia catheter ablation in adults with congenital heart disease: negative impact of age and complex atrial surgery. J Am Coll Cardiol. 2010; 56:1589-1596 [DOI] [PubMed] [Google Scholar]
- 4.Collins KK, Love BA, Walsh EP, Saul JP, Epstein MR, Triedman JK. Location of acutely successful radiofrequency catheter ablation of intraatrial reentrant tachycardia in patients with congenital heart disease. Am J Cardiol. 2000; 86:969-974 [DOI] [PubMed] [Google Scholar]
- 5.Khairy P, Fournier A, Ruest P, Vobecky SJ. Transcatheter ablation via a sternotomy approach as a hybrid procedure in a univentricular heart. Pacing Clin Electrophysiol. 2008; 31:639-640 [DOI] [PubMed] [Google Scholar]
- 6.Olsson A, Darpo B, Bergfeldt L, Rosenqvist M. Frequency and long term follow up of valvar insufficiency caused by retrograde aortic radiofrequency catheter ablation procedures. Heart. 1999; 81:292-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nehgme RA, Carboni MP, Care J, Murphy JD. Transthoracic percutaneous access for electroanatomic mapping and catheter ablation of atrial tachycardia in patients with a lateral tunnel fontan. Heart Rhythm. 2006; 3:37-43 [DOI] [PubMed] [Google Scholar]
- 8.Minich LL, Snider AR, Dick M., II Doppler detection of valvular regurgitation after radiofrequency ablation of accessory connections. Am J Cardiol. 1992; 70:116-117 [DOI] [PubMed] [Google Scholar]
- 9.El‐Said HG, Ing FF, Grifka RG, Nihill MR, Morris C, Getty‐Houswright D, Mullins CE. 18‐year experience with transseptal procedures through baffles, conduits, and other intra‐atrial patches. Catheter Cardiovasc Interv. 2000; 50:434-439 [DOI] [PubMed] [Google Scholar]
- 10.Perry JC, Boramanand NK, Ing FF. “Transseptal” technique through atrial baffles for 3‐dimensional mapping and ablation of atrial tachycardia in patients with d‐transposition of the great arteries. J Interv Card Electrophysiol. 2003; 9:365-369 [DOI] [PubMed] [Google Scholar]
- 11.Dave AS, Aboulhosn J, Child JS, Shivkumar K. Transconduit puncture for catheter ablation of atrial tachycardia in a patient with extracardiac fontan palliation. Heart Rhythm. 2010; 7:413-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Justino H, Benson LN, Nykanen DG. Transcatheter creation of an atrial septal defect using radiofrequency perforation. Catheter Cardiovasc Interv. 2001; 54:83-87 [DOI] [PubMed] [Google Scholar]
- 13.Triedman JK, Alexander ME, Love BA, Collins KK, Berul CI, Bevilacqua LM, Walsh EP. Influence of patient factors and ablative technologies on outcomes of radiofrequency ablation of intra‐atrial re‐entrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 2002; 39:1827-1835 [DOI] [PubMed] [Google Scholar]
- 14.Esch JJ, Triedman JK, Cecchin F, Alexander ME, Walsh EP. Radiofrequency‐assisted transseptal perforation for electrophysiology procedures in children and adults with repaired congenital heart disease. Pacing Clin Electrophysiol. 2013; 36:607-611 [DOI] [PubMed] [Google Scholar]
- 15.Kanter RJ, Papagiannis J, Carboni MP, Ungerleider RM, Sanders WE, Wharton JM. Radiofrequency catheter ablation of supraventricular tachycardia substrates after mustard and senning operations for d‐transposition of the great arteries. J Am Coll Cardiol. 2000; 35:428-441 [DOI] [PubMed] [Google Scholar]
- 16.Ernst S, Babu‐Narayan SV, Keegan J, Horduna I, Lyne J, Till J, Kilner PJ, Pennell D, Rigby ML, Gatzoulis MA. Remote‐controlled magnetic navigation and ablation with 3D image integration as an alternative approach in patients with intra‐atrial baffle anatomy. Circ Arrhythm Electrophysiol. 2012; 5:131-139 [DOI] [PubMed] [Google Scholar]
- 17.Pass RH, Nappo L, Eugenio P, Lopez L. A transbaffle approach to ablation in a child with an extracardiac fontan. Pacing Clin Electrophysiol. 2010; 33:368-371 [DOI] [PubMed] [Google Scholar]
- 18.McCanta AC, Kay JD, Collins KK. Cryoablation of the slow atrioventricular nodal pathway via a transbaffle approach in a patient with the mustard procedure for d‐transposition of the great arteries. Congenit Heart Dis. 2011; 6:479-483 [DOI] [PubMed] [Google Scholar]
- 19.Yap SC, Harris L, Downar E, Nanthakumar K, Silversides CK, Chauhan VS. Evolving electroanatomic substrate and intra‐atrial reentrant tachycardia late after fontan surgery. J Cardiovasc Electrophysiol. 2012; 23:339-345 [DOI] [PubMed] [Google Scholar]


