Introduction
Stem cell therapy aimed at restoring organ function, notably myocardial repair and regeneration postmyocardial infarction (MI), is one of the most exciting and promising frontiers of medical research. While new pharmacotherapies and advances in interventional cardiology have significantly reduced the mortality of ischemic heart disease and heart failure, there remains an ongoing need for innovative cell‐based therapies that can prevent or reverse cardiac ventricular remodeling post‐MI. Although questions remain on how to best implement cell‐based interventions, a growing number of preclinical studies and clinical trials have demonstrated the safety of a variety of adult stem cell types. This review will focus on the collective progress in cardiovascular regenerative medicine, with particular emphasis on the findings from the most recently published or announced clinical trials: the PercutaneOus StEm Cell Injection Delivery Effects On Neomyogenesis (POSEIDON),1 the Stem Cell Infusion in Patients with Ischemic cardiomyopathy (SCIPIO),2–4 Cardiosphere‐Derived aUtologous stem Cells to reverse ventricUlar dySfunction (CADUCEUS),5 the Swiss Multicenter Intracoronary Stem Cells Study in Acute Myocardial Infarction (SWISS‐AMI),6–7 the AutoLogous Human Cardiac‐Derived Stem Cell to Treat Ischemic cArdiomyopathy (ALCADIA)8 (NCT00981006), the Cardiovascular Cell Therapy Research Network (CCTRN) trials, the Transplantation In Myocardial Infarction Evaluation (TIME),9 LateTIME,10 the First Mononuclear Cells injected in the United States conducted by the CCTRN (FOCUS‐CCTRN),11 and the Cardiopoietic stem Cell therapy in heart failURE (C‐CURE) trial.12 These trials illustrate how a novel intervention like stem cell therapy requires innovative evaluation and assessment tools that place an emphasis on clinical parameters and imaging techniques. From dosing and delivery to evaluating efficacy, stem cell therapy provides not only opportunities but also challenges in our quest to develop an effective and sustainable therapeutic intervention for cardiomyopathies.
To date, researchers have experimented with multiple cell types in preclinical and clinical studies to determine which cell lines prove most safe and efficacious. At first, embryonic stem cells (ESC) and skeletal myoblasts were evaluated as viable options, but the most promising results have recently become evident from bone marrow‐derived mesenchymal stem cells, cardiac stem cells, and cardiospheres.13–14
Studies Employing Pluripotent Stem Cells and Skeletal Myoblasts
Initial studies with ESCs reported surprisingly low rates of cardiac differentiation, and high rates of teratoma formation, immunologic responses, and cell rejection.13 Additionally, the ethical concerns surrounding their use have impaired their development into clinical trials. A major scientific advance that circumvented the ethical concerns was the discovery of methods to reprogram adult somatic cells (ie, fibroblast and epithelial cells) into a pluripotent state, termed inducible pluripotent stem (iPS) cells. While iPS cells may serve as an alternative to ESCs, many questions and concerns remain regarding tumorigenicity, durability, and viability of this approach.13–15
Skeletal myoblasts are a cluster of quiescent stem cells found in muscle fibers that have demonstrated the ability to regenerate after muscle tissue damage. Research groups led by Taylor et al and Menashe et al demonstrated experimentally that skeletal myoblast injections into infarcted cardiac muscle resulted in improved contractility.15–17 However, it was later demonstrated that skeletal myoblasts do not express connexin 43 and cannot electrically couple with endogenous cardiac myocytes, increasing risk for ventricular tachyarrhythmias.17–18
Clinical Trials Employing Bone Marrow‐Derived Mononuclear Cells (BM‐MNCs)
Acute Myocardial Infarction
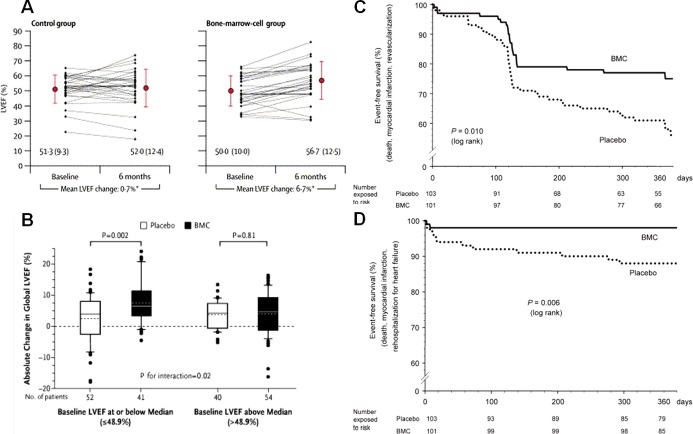
Adult bone marrow is a source of heterogeneous stem cells and precursor lineage cells that are hypothesized to have the potential to differentiate into cardiac cellular elements and/or provide paracrine or miracrine support to the healing heart.14,19–20 Because of the easy accessibility of whole bone marrow, clinical trials began immediately in the early 2000s following provocative findings obtained in animal models that bone marrow cells could reduce infarct size and improve left ventricular (LV) function following MI. The 2 most influential early clinical trials were the BOne marrOw transfer to enhance ST‐elevation infarct regeneration (BOOST)21 and the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR‐AMI).22 Data from the BOOST and REPAIR‐AMI clinical trials demonstrated that intracoronary BM‐MNC delivery led to a 6.7% point improvement in left ventricular ejection fraction (LVEF) at 6 months and a 5.5% point improvement in LVEF at 4 months, respectively (Figure 1A and 1B). In addition, the REPAIR‐AMI showed increased event‐free survival at 12 months after treatment (Figure 1C and 1D).
Figure 1.
Benefits of bone marrow mononuclear cell (BM‐MNC) therapy. A and B, Intracoronary BM‐MNC delivery led to a 6.7% point improvement in left ventricular ejection fraction (LVEF) at 6 months in the BOOST clinical trial and to 5.5% improvement in LVEF at 4 months in the REPAIR‐AMI clinical trial, respectively. C, Kaplan–Meier event‐free survival analysis in the REPAIR‐AMI at 12 months showed better survival from death, recurrence of myocardial infarction, or revascularization procedures and (D) death, recurrence of myocardial infarction, or rehospitalization for heart failure in the BM‐MNC group. Panel A was reproduced with permission from Wollert et al, Lancet, 2004,21 panel B from Schachinger et al, New England Journal of Medicine, 2006,22 and panels C and D from Schachinger et al, European Heart Journal, 2006.23 BOOST indicates BOne marrOw transfer to enhance ST‐elevation infarct regeneration; REPAIR‐AMI, Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction.
Many clinical trials explored timing of intracoronary delivery. REPAIR AMI suggested that 5 to 7 days after acute MI produced a superior improvement in global cardiac function (LVEF) when compared to an earlier delivery time.23 Several recent trials led by the National Heart, Lung, and Blood Institute (NHLBI) CCTRN, devoted major attention to the timing of intracoronary bone marrow therapy following MI. The TIME trial conducted by Traverse et al9 enrolled 120 patients with LV dysfunction (ejection fraction [EF] <45%) who had undergone a successful percutaneous coronary intervention (PCI) for anterior wall STEMI and assessed whether delivery of autologous BM‐MNC at 3 or 7 days would improve global LVEF and regional LV function. Autologous BM‐MNCs (150 million) were administered via the intracoronary route. Contrary to the REPAIR‐AMI findings, at the 6‐month time point there was no significant improvement in global or regional LV function, as measured by cardiac magnetic resonance imaging (MRI), in the BM‐MNC group when compared to the controls, regardless of which day (3 or 7) the cells were given to the patient. The recently published LateTIME trial tested whether delaying BM‐MNC delivery for 2 to 3 weeks following MI and primary PCI improves global and regional LV function.10 Similar to the TIME trial results, in LateTIME there were no significant changes between baseline and 6‐month measures in LVEF, wall motion of the infarct zone, and wall motion of the border zone, as measured by cardiac MRI, in the BM‐MNC group compared to placebo. Although the results of the TIME and LateTIME trials demonstrated that intracoronary autologous BM‐MNC delivery at the 3 or 7 day and 2 to 3 week time point after MI was not effective for improving LV function, long‐term follow‐up and designation of new endpoints may reveal previously hidden benefits of this type of cell therapy. However, it has become increasingly evident that stem cell and patient characteristics influence therapeutic effect.24–25 In this regard, BM‐MNCs secrete lower amounts of angiogenic and antiapoptotic growth factors than other cell types.24 The percentage of CD34+ and CD133+ cells in BM‐MNCs is important because these cell populations secrete factors that recruit cells, promote cell survival, increase microvascular density, and rescue cardiomyocytes from hibernation.26–27 Moreover, a beneficial effect of infarct‐related artery infusion of autologous CD34 cells after STEMI on perfusion and infarct size was shown in the AMR‐01 clinical trial.28 In TIME and LateTIME, the percentage of CD34+ and CD133+ cells was low, ≈2% and 1%, respectively. There is also substantial evidence that ischemic heart disease and aging negatively impact the regenerative capacity of autologous stem cells,25,29–31 thus supporting the use of allogeneic cell therapy.1
Similar results to TIME and LateTIME were seen in the SWISS‐AMI, a more recent study investigating autologous BM‐MNCs in patients with STEMI.6–7 Sürder et al enrolled 67 patients and aimed to improve LV dysfunction after acute MI by utilizing intracoronary infusion of BM‐MNCs cells at 5 to 7 days or 3 to 4 weeks after primary PCI. At the 4‐month time point, there was no significant improvement in LVEF in either the early or late infusion times.7 Additionally, there was no evidence of significant improvement in endpoints including LV volume, scar size, and myocardial thickness in the infarct region. Although there was no demonstrated efficacy at the 4‐month time point, the researchers showed that the use of BM‐MNCs was safe, as there was no significant difference in adverse events among the groups. Patients will continue to be followed until the 12‐month time point in order to determine if there is a delayed benefit from the BM‐MNC therapeutic intervention.
Chronic Ischemic Cardiomyopathy
BM‐MNCs have also been tested for chronic ischemic heart failure. The FOCUS‐CCTRN was a phase II trial that investigated the efficacy of transendocardial delivery of BM‐MNCs on LV performance and perfusion at 6 months in patients with chronic ischemic cardiomyopathy.11 Similar to BM‐MNC trials in AMI, this study showed no significant effect on LV end‐systolic volume, maximal oxygen consumption, or myocardial perfusion. However, exploratory analyses demonstrated significant improvement in stroke volume and LVEF that was associated with higher bone marrow CD34+ and CD133+ progenitor cell counts, suggesting the highly important concepts that the cellular composition of the bone marrow determines clinical efficacy and that certain cell populations provide greater regenerative benefit.
One of the challenging aspects of stem cell efficacy is poor cell retention, especially in the chronic heart failure setting. Preclinical studies have demonstrated that extracorporeal shock wave treatment, similar to that used to treat nephrolithiasis, increases homing factors and chemokines such as stromal cell‐derived factor 1 (SDF‐1),32 which translated into a higher retention of BM‐MNCs in the myocardium.33 In the recently published phase I/II placebo‐controlled CELLWAVE (NCT NCT00326989) trial, a total of 103 patients with chronic heart failure were randomized into 5 different arms that were designed to assess dose‐responsiveness (low and high) of shock wave pretreatment with or without subsequent intracoronary BM‐MNC delivery.34 Assmus et al showed that patients receiving shock wave treatment prior to intracoronary BM‐MNC infusion had a modest but significant improvement in LVEF (absolute change of 3.2% when compared to shock wave/placebo infusion group) and improvement in wall thickening of the infarcted segments at the 4‐month time point when compared to the placebo group. This new approach to enhance homing can be translated to subpopulations of BM‐MNCs as well as other stem cells that express the SDF‐1 receptor. While these findings are promising, a larger clinical trial is necessary to thoroughly evaluate the safety of the approach and potential improvements in clinical outcomes.
Future Direction of BM‐MNCs
Together the aforementioned studies support the safety profile of BM‐MNCs in acute and chronic ischemic cardiomyopathy. Some of the trials7,9–10 also highlight the need for more optimization studies to determine the ideal time for cell delivery after acute MI. Interestingly, 2 meta‐analyses35–36 have provided evidence of efficacy. Indeed, their findings suggest that BM‐MNC therapy prevents remodeling by reducing infarct size and LV chamber enlargement and these benefits persisted during long‐term follow‐up. Importantly, one of these meta‐analyses confirmed a long‐observed clinical benefit that is out of proportion to increases in cardiac function: BM‐MNC therapy reduced the incidence of death, recurrent MI, and stent thrombosis in patients with ischemic heart disease.35
The field of BM‐MNC cell therapy is at a crucial crossroads. The benefits of BM‐MNCs include their accessibility, ability to obtain large quantity of cells without a need for ex vivo expansion, and vast clinical experience with bone marrow transplantation.19 Additionally, multiple phase I clinical trials have established the safety profile of this cell type35–36; however, the recent results from TIME, LateTIME, and FOCUS have not demonstrated the efficacy of BM‐MNCs.9–11 The completed phase I trials were primarily focused on establishing the safety profile of BM‐MNCs, and efficacy results were limited by the small number of patients. To address the limitations of the smaller studies, a multicenter, randomized, controlled, phase III study, The Effect of Intracoronary Reinfusion of BM‐MNC on All Cause Mortality in Acute Myocardial Infarction (BAMI) trial (NCT01569178) is underway and is adequately powered to detect evidence of efficacy. Regardless of outcome, this trial will be the first with adequate power and rigorous design that addresses clinical outcomes as opposed to surrogates for patients with acute MI. Meanwhile, investigators have placed increasing emphasis and focus on other well understood and potentially promising cell types, such as mesenchymal stem cells, cardiac stem cells, and cell combinations.
Clinical Trials Employing Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs), which were first identified over 4 decades ago, are under active investigation for their regenerative potential and are thought to be a possible lead candidate for the active constituent in bone marrow.20 MSCs, initially described as plastic‐adherent stromal cells, are a multipotent cell line capable of forming colonies and differentiating into mesodermal (bone, fat, cartilage) and nonmesodermal lineages.20 Originally thought to reside in bone marrow, they have now been isolated from diverse tissue sources.37 Results from large‐animal models demonstrate that MSCs have the ability to engraft, differentiate into myocytes, vascular smooth muscle and endothelial cells, and enhance cardiovascular hemodynamic parameters.20,38–41 The ease in preparation, immunoprivilege properties, and multipotent nature have made MSCs very promising for cardiac cell therapy,14–15,20,42 as recently shown in the Transendocardial Autologous Cells in Ischemic Heart Failure (TAC‐HFT) and POSEIDON clinical trials.1,43
MSCs have immunosuppressive and immunomodulatory properties. They do not express Major Histocompatibility Class II antigens or the B7 and CD40 ligand costimulatory molecules.44–45 Additionally, they can suppress innate immunity, making allogeneic transplantation of MSCs feasible.45
MSCs for Acute Myocardial Infarction
The ability to collect and store allogeneic stem cells from healthy donors has advantages over the harvesting and expansion of a patient's autologous cells, particularly their availability for timely transplant in the setting of acute MI.46 The first major study of allogeneic MSCs was a phase I, multi‐center, randomized, double‐blinded, placebo‐controlled study46 in patients with acute myocardial infarction. Patients (n=53) were treated 3 to 10 days post‐MI with 1 of 3 cell‐dose levels (0.5, 1.6, and 5.0 mol/L allogeneic MSCs/kg) or placebo, administered intravenously, and followed for 6 months. With regard to safety, there were no deaths, toxicity, or serious adverse events following the administration of the allogeneic MSCs. Patients in the MSC‐treated group experienced fewer arrhythmic events and an improvement in their overall clinical status at 6 months as compared to those receiving placebo. In addition, patients in the MSC group with major anterior wall MI had a significant improvement in EF at 3 months and 6 months over baseline, while similar patients receiving placebo did not have significant improvement. These provocative results led to a phase II, 220 patient study in the setting of acute MI with depressed EF. It was preliminarily reported by Osiris in July 2012 (unpublished findings) that a single intravenous infusion of either allogeneic MSCs or placebo within 7 days of an acute MI significantly reduced cardiac hypertrophy, stress‐induced ventricular arrhythmia, heart failure, and rehospitalization for cardiac complications compared to patients receiving placebo. The full impact of this study awaits publication of the data.
MSCs for Chronic Ischemic Cardiomyopathy
MSCs are also thought to have a role for established ischemic cardiomyopathy. While allogeneic MSCs appeared safe, a direct comparison with their autologous counterparts had never been conducted. To address this, our group performed a phase I/II randomized trial to compare the safety and efficacy of allogeneic and autologous MSCs delivered via transendocardial injection in patients with chronic ischemic cardiomyopathy. In the POSEIDON trial, 30 patients were assigned to 6 subgroups based on type of MSC (allogeneic or autologous) and dose (20, 100, and 200 million).1,47 At 13‐month follow‐up, the results showed that both allogeneic and autologous MSCs were safe and efficacious. MSC therapy was associated with reversal of LV remodeling (Figures 2 through 4), reduction in myocardial scar size (measured by CT imaging as early enhancement defect [EED] (Figures 5 and 6C),48–49 and improvement in patient functional capacity and quality of life (Figure 7). Neither cell type showed a significant improvement in EF. Although this initial study was limited by lack of a placebo group, it validated the safety profile of allogeneic MSCs and encourages a larger phase II trial to establish efficacy of MSC therapy. Importantly, this trial provided crucial immunologic data, showing that the allogeneic cell recipients did not mount anti‐donor antibodies that could potentially limit subsequent organ transplantation options.
Figure 2.
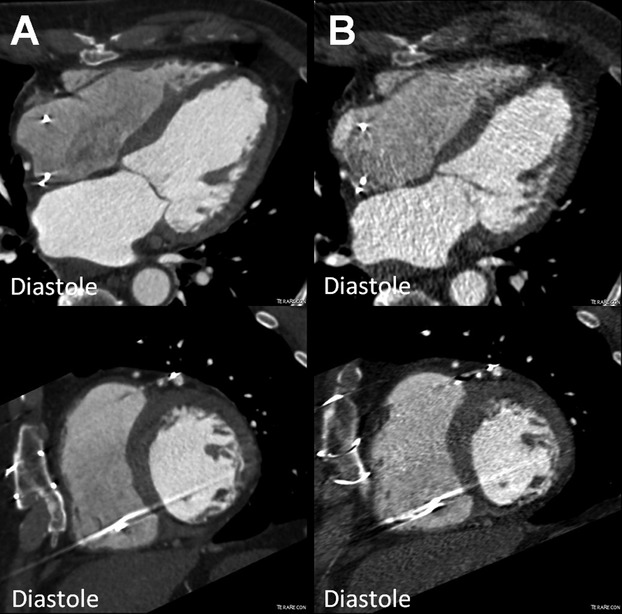
Reduction of left ventricular end‐diastolic volume (EDV) in the POSEIDON study. MDCT images (4 chamber and short axis view) of a patient with chronic ischemic cardiomyopathy (A) before (EDV 176.1 mL) and (B) after transendocardial stem cell injection (TESI) with 20 million autologous mesenchymal stem cells (EDV 136.8 mL). MDCT indicates multi‐detector computer tomography; POSEIDON, PercutaneOus StEm Cell Injection Delivery Effects On Neomyogenesis.
Figure 4.
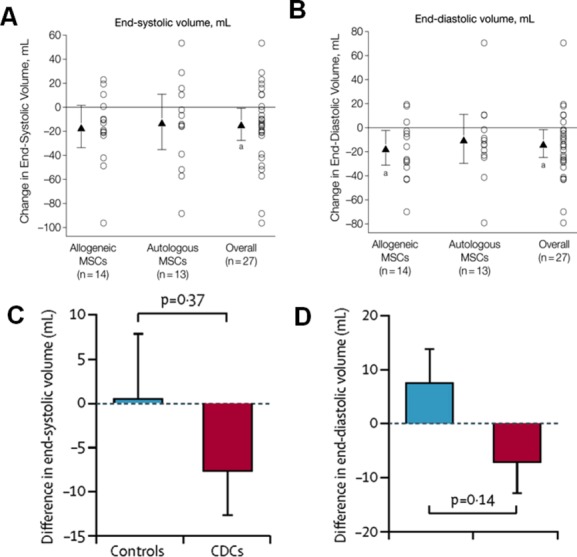
Comparison of end‐systolic and end‐diastolic volumes in POSEIDON and CADUCEUS trials. A and B, Chamber volumes in POSEIDON trial. Mean changes from baseline to 13 months are noted by triangles and depict changes in cardiac phenotype assessed by cardiac MDCT scan. Error bars indicate 95% CIs. Individual patient changes from baseline are shown as circles. Shown are changes in cardiac volumes from baseline to 13‐month follow‐up in allogeneic, autologous, and overall patient groups. Within‐group P values are noted as a P<0.05. C and D, Chamber volumes in CADUCEUS study participants. C, Treatment effects (baseline vs 6 months) for end‐systolic volume. D, Treatment effects (baseline vs 6 months) for end‐diastolic volume. Panels A and B are reproduced with permission from Hare et al, JAMA, 2012.1 Panels C and D are reproduced with permission from Makkar et al, Lancet, 2012.5 CADUCEUS indicates Cardiosphere‐Derived aUtologous stem Cells to reverse ventricUlar dySfunction; CDCs, cardiosphere‐derived cells; CIs, confidence intervals; MDCT, multi‐detector computer tomography; MSCs, mesenchymal stem cells; POSEIDON, PercutaneOus StEm Cell Injection Delivery Effects On Neomyogenesis.
Figure 5.
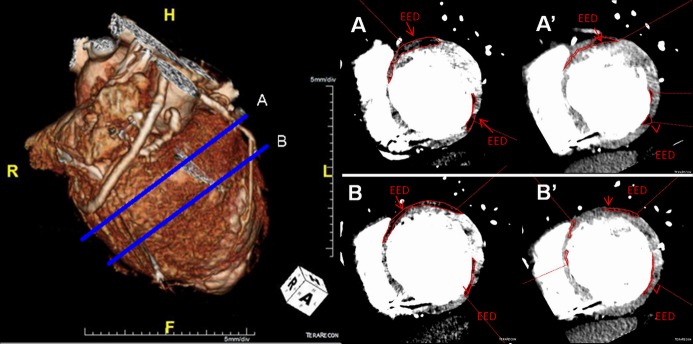
Reduction of scar size (POSEIDON clinical trial). (Left) MDCT 3D reconstruction image with (Right) respective (A) basal and (B) midventricular short axis images of a patient with chronic ischemic cardiomyopathy with a scar size (MDCT‐EED outlined in red) of (A and B) 40.34 g and its reduction to (A' and B') 26.35 g after transendocardial stem cell injection (TESI) with allogeneic MSCs (100 million). EED indicates early enhancement defect; MDCT, multi‐detector computer tomography; MSCs, mesenchymal stem cells; POSEIDON, PercutaneOus StEm Cell Injection Delivery Effects On Neomyogenesis.
Figure 6.
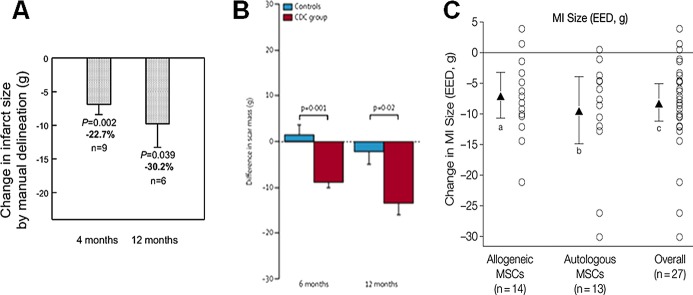
Comparison of scar size reduction in (A) SCIPIO, (B) CADUCEUS, and (C) POSEIDON trials. A, SCIPIO Trial 1‐year follow‐up shows infarct size reduction at 4 and 12 months after CSC therapy. B, CADUCEUS trial showed decreases in scar mass and increases in viable mass on MRI in patients treated with CDCs but not controls. C, Myocardial infarct (MI) size reduction shown in POSEIDON trial. Panel A reproduced with permission from Chugh et al, Circulation, 20124; panel B from Makkar et al, Lancet, 20125; and panel C from Hare et al, JAMA, 2012.1 CADUCEUS indicates Cardiosphere‐Derived aUtologous stem Cells to reverse ventricUlar dySfunction; CDC, cardiosphere‐derived cell; CSCs, cardiac stem cells; EED, early enhancement defect; MRI, magnetic resonance imaging; MSCs, mesenchymal stem cells; POSEIDON, PercutaneOus StEm Cell Injection Delivery Effects On Neomyogenesis; SCIPIO, Stem Cell Infusion in Patients with Ischemic cardiomyopathy.
Figure 7.
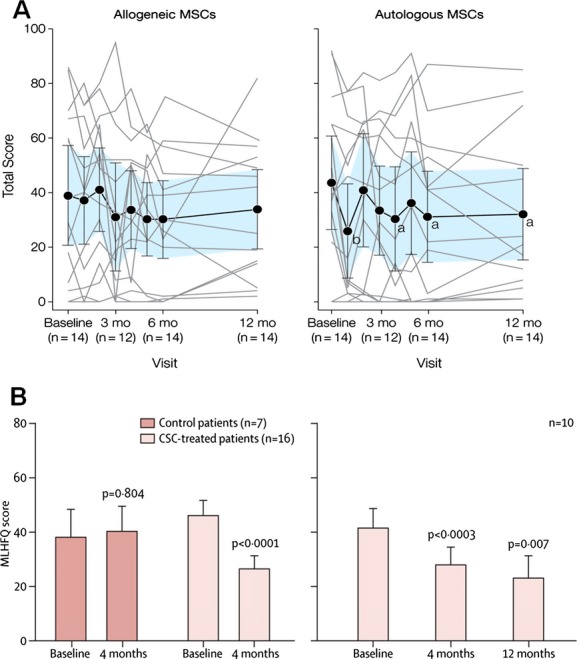
Comparison of MLHFQ scores in POSEIDON and SCIPIO trials. A, POSEIDON trial showed improvement in MLHFQ score in the autologous MSC group at the 6‐ and 12‐month time points. B, SCIPIO trial demonstrated continuous improvement in MLHFQ score at 4 and 12 months. Panel A reproduced with permission from Hare et al, JAMA, 20121 and panel B from Chugh et al, Circulation, 2012.4 CSCs indicates cardiac stem cells; MLHFQ, Minnesota Living with Heart Failure Questionnaire; MSC, mesenchymal stem cell; POSEIDON, PercutaneOus StEm Cell Injection Delivery Effects On Neomyogenesis; SCIPIO, Stem Cell Infusion in Patients with Ischemic cardiomyopathy.
The results from the POSEIDON study also provide a potentially attractive alternative cell source for patients who may have limited regenerative potential with their own cells. For example aging or prior chemotherapy may adversely affect the function and capabilities of MSCs,50–52 and this may contribute to impaired healing and recovery in aging populations. Thus the findings from POSEIDON supporting transplantation of allogeneic MSCs hold promise for the availability of cell‐based therapies for individuals with impaired endogenous cells.
MSCs are currently among the most promising source for successful cell therapy in ischemic cardiomyopathy, but concerns have been raised regarding tumorigenicity and ectopic tissue formation. This would be a serious complication53 that was suggested by studies using murine MSCs, which after certain number of passages were at a higher risk for mutations.54–55 However, this complication has not materialized in the POSEIDON1 population and that of other studies testing human MSCs in humans,46,55–56 likely due to a limited differentiation capacity, since MSCs are multipotent and not pluripotent like embryonic stem cells. Moreover, MSCs appear to have a low level of sustained engraftment in humans,56 which reduces the long‐term risks of this therapy.
Bone marrow‐derived MSC precursors,57–61 cardiopoietic MSCs,12,62 umbilical cord,63–64 placenta,65 and adipose‐derived MSCs66–67 are all currently under preclinical or clinical investigation for therapeutic potential (Table). These approaches provide additional translational opportunities for MSCs.
Table 1.
Future and Ongoing MSC Clinical Trials
| Study Name | NCT # Clinicaltrials.gov | Description | Potential Advantages | Status |
|---|---|---|---|---|
| Adipose‐Derived MSCs | ||||
| APOLLO Trial: A Randomized Clinical Trial of AdiPOse‐derived Stem ceLLs in the Treatment of Patients With ST‐elevation myOcardial Infarction | NCT00442806 | Phase I trial to establish the safety profile of adipose‐derived stem and regenerative cells (ADRCs) in patients who have suffered ST‐elevation AMI | Ease of access to cells | Active Phase I, not recruiting |
| ADVANCE Study: ADRCs Delivered Via the Intracoronary Route in the Treatment of Patients With ST‐elevation Acute Myocardial Infarction | NCT01216995 | A double‐blind, prospective, randomized, placebo controlled safety and efficacy trial to evaluate the intracoronary delivery of ADRCs in patients with ST‐elevation AMI. Will enroll 216 patients | Ease of access to cells Large patient population Multi‐center |
Phase II, recruiting |
| MyStromalCell Trial: MesenchYmal STROMAL CELL Therapy in Patients With Chronic Myocardial Ischemia | NCT01449032 | After promising pilot study that demonstrated safety of adipose‐derived MSCs, this will be a double‐blind, placebo‐controlled phase II to evaluate if adipose derived MSCs are able to improve cardiac tissue perfusion, exercise capacity and reduce symptoms in patients with CAD | Ease of access to cells | Phase II, recruiting |
| MSC Precursor Types | ||||
| AMICI Trial: Safety Study of Allogeneic Mesenchymal Precursor Cell Infusion in MyoCardial Infarction | NCT01781390 | Double‐blind, randomized, placebo‐controlled trial that will enroll 225 patients with AMI due to lesion in LAD. They will undergo revascularization with PCI followed by intracoronary delivery of placebo or Stro3 MPC cells. Conducted by Angioblast systems | Cell type that improves paracrine activity and engraftment rates Demonstrates multilineage potential | Phase II, not yet open for recruiting |
| Safety Study of Allogeneic Mesenchymal Precursor Cells (MPCs) in Subjects With Recent Acute Myocardial Infarction | NCT00555828 | This trial will evaluate the safety and efficacy of dose‐dependent (25, 75, 150 mol/L) transendocardial injections of allogeneic stro3 MPCs using the Biosense NogaStarTM Mapping Catheter in patients with AMI. | Assess optimal dose for stro3 MPCs Explore late‐term dose related tolerance at days 90, 180, 360 |
Phase Ib/IIa, recruiting |
| Umbilical Cord‐Derived MSCs | ||||
| Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy (RIMECARD) | NCT01739777 | This trial will evaluate safety and efficacy of umbilical cord derived mesenchymal stem cells (ucMSC) in patients with compensated dilated cardiomyopathy. Aims to have 30 patients with 2 arms; the first group will receive intravenous injection of ucMSC and other group will serve as control. | Ease of Access to MSCs Does not require invasive procedures to isolate MSCs |
Phase I/II, recruiting |
| Intracoronary Human Wharton's Jelly‐ Derived Mesenchymal Stem Cells (WJ‐MSCs) Transfer in Patients With Acute Myocardial Infarction (AMI) (WJ‐MSC‐AMI) | NCT01291329 | This is a double‐blind, placebo‐controlled, multicenter trial that involved 160 patients with acute STEMI. Patients received intracoronary infusion of WJ‐MSCs or placebo medium into infarct artery 4 to 7 days after a successful PCI therapy. | WJ‐MSCs have short doubling time which allows them to be quickly produced in large numbers. | Study has been completed |
| Allogeneic Bone Marrow‐Derived MSCs | ||||
| The POSEIDON‐DCM Study PercutaneOus StEm Cell Injection Delivery Effects On Neomyogenesis in Dilated CardioMyopathy | NCT01392625 | This is a pilot study with 36 patients that will evaluate the safety and efficacy of transendocardial injections of allogeneic and autologous MSCs in patients with non‐ischemic dilated cardiomyopathy. | Allogeneic MSCs offer the potential “off the shelf” therapy | Phase I/II, active recruiting |
| STEMI: A Study of Allogeneic Mesenchymal Bone Marrow Cells in Subjects With ST Segment Elevation Myocardial Infarction | NCT01770613 | This trial will evaluate the safety and efficacy of allogeneic mesenchymal bone marrow cells that will be administered intravenously to patients who have experienced STEMI. Estimated enrollment will be 40 subjects. | Allogeneic MSCs offer the potential “off the shelf” therapy | Phase II, not yet open for recruiting |
| MSCs and other Stem Cell Combinations | ||||
| AIRMID Trial: Autologous Cardioblasts for Reverse Remodeling in Ischemic Dilated Cardiomyopathy | Not Registered | Planning Phase | ||
AMI indicates acute myocardial infarction; CAD, coronary artery disease LAD, left anterior descending; MSCs, mesenchymal stem cells; PCI, percutaneous coronary intervention.
MSC Precursors for Acute Myocardial Infarction
It is now appreciated that bone marrow‐derived MSC precursors (MPCs) can be identified based upon cell surface marker expression. Notable examples include stromal precursor antigens (STRO‐1, STRO‐3) and CD271.57–61 After promising preclinical experimentation, STRO‐selected cells have progressed into phase II trials, including the Safety Study of Allogeneic Mesenchymal Precursor Cell (MPC) Infusion in Myocardial Infarction (AMICI Trial; NCT01781390). This is an ongoing, double‐blind trial conducted by Angioblast Systems that will assess safety and feasibility of intracoronary STRO‐3 MPC injections into patients who undergo left anterior descending revascularization after acute MI. Allogeneic STRO‐3 MPCs will also be studied in the Safety Study of Allogeneic Mesenchymal Precursor Cells (MPCs) in Subjects With Recent Acute Myocardial Infarction (NCT00555828), which is evaluating the safety and efficacy of transendocardial injections of MPCs. In vitro studies have shown that the STRO‐1 and STRO‐3 enriched populations exhibit a higher level of paracrine activity as well as proliferative and multilineage regenerative capacity compared to plastic‐adherent MSCs.57,60–61 These qualities could improve engraftment rates and provide us with a more effective type of stem cell. With respect to CD271+ cells, preclinical studies are currently ongoing where selection of CD271+ cells from bone marrow of humans and pigs significantly increased clonogenic outgrowth of MSCs.58 By selectively choosing MSCs with antigens like STRO and CD271, researchers hope to improve the migratory, immunologic, and engraftment properties of MSCs.
Cardiopoietic MSCs for Chronic Ischemic Cardiomyopathy
Growth factor‐guided cardiopoietic specification of human bone marrow‐derived autologous MSCs has also shown efficacy in preclinical studies.62 These studies tested a recombinant cocktail consisting of transforming growth factor‐β‐1, bone morphogenetic protein‐4, activin A, retinoic acid, insulin‐like growth factor‐1, fibroblast growth factor‐2, α‐thrombin, and interleukin‐6 to induce cardiogenic differentiation of the harvested MSCs prior to transplantation. The recently published multicenter C‐CURE clinical trial assessed feasibility and safety of cardiopoietic MSCs therapy in patients with chronic ischemic cardiomyopathy.12 Forty‐five patients were randomized to standard medical care or autologous cardiopoietic MSC (600 to 1200 million) treatment delivered into viable but dysfunctional LV segments using electromechanical guidance. At 6 months follow‐up, there was improvement of clinical performance as assessed by improved 6‐minute walk‐test, decreased end‐systolic volumes, and increased LVEF in cell‐treated patients compared to controls (Figure 8). While these investigations into the therapeutic efficacy of MPCs and cardiopoietic MSCs are in the early stages, these lineage‐selected subpopulations of MSCs hold tremendous promise for regenerative medicine. Safety of MSCs has already been established, but now lineage‐selected subpopulations allow us to optimize the cell phenotype with the goal of increasing cell engraftment and retention.
Figure 8.
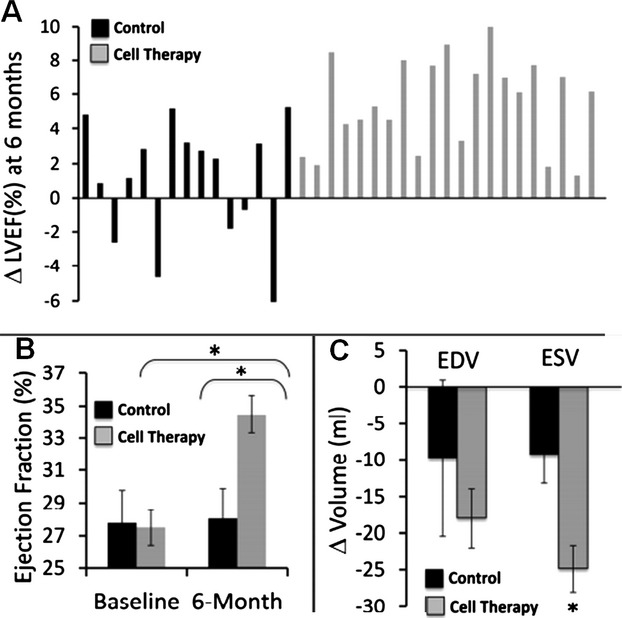
Greater reversal of remodeling after autologous cardiopoietic stem cell therapy at 6‐month follow‐up (C‐CURE trial). A, Changes in left ventricular ejection fraction (LVEF) for each individual patient. Control group (black) includes patients that received standard of care alone. Cell therapy group (gray) includes patients that received standard of care plus cardiopoietic stem cells. B, Median values for LVEF prior to and 6‐months following treatment. C, Changes in left ventricular end‐diastolic (ED) and end‐systolic (ES) volumes. Reproduced with permission from Bartunek et al, JACC, 2013.12
Adipose‐Derived MSCs for Acute Myocardial Infarction
Preclinical studies have demonstrated therapeutic potential for adipose‐derived MSCs, laying the foundation for clinical trials.66–67 In a porcine acute MI model, intracoronary delivery of adipose tissue‐derived stem cells (ADSCs) or bone marrow‐derived MSCs similarly improved cardiac function after 4 weeks compared to control.66 This study demonstrated that not only bone marrow‐derived MSCs but also ADSCs engrafted into the infarct region 4 weeks after intracoronary cell transplantation improved cardiac function and perfusion via angiogenesis.
The noncultured adipose stromal vascular fraction, a heterogeneous population of cells with multilineage differentiation potential, is being tested in 2 clinical trials: The APOLLO Trial (NCT00442806; A Randomized Clinical Trial of AdiPOse‐derived Stem ceLLs in the Treatment of Patients With ST‐elevation myOcardial Infarction) and the ADVANCE study (NCT01216995; ADRCs Delivered Via the Intracoronary Route in the Treatment of Patients With ST‐elevation Acute Myocardial Infarction). Preliminary data from the APOLLO trial showed that the intracoronary infusion of autologous adipose‐derived stem and regenerative cells (ADRCs) in acute MI patients after successful revascularization resulted in improvement in LVEF and myocardial perfusion and reduction in infarct size.68 The ADVANCE study is a phase II/III trial that has been initiated to further evaluate the efficacy of this approach defined as reduction in infarct size at 6 months.
Adipose‐Derived MSCs for Chronic Ischemic Cardiomyopathy
There is also one ongoing clinical trial using culture‐expanded adipose tissue‐derived MSCs, the MesenchYmal STROMAL CELL Therapy in Patients With Chronic Myocardial Ischemia (NCT01449032; MyStromalCell Trial), that is investigating the safety and efficacy of intramyocardial delivery of VEGF‐A165‐stimulated autologous adipose tissue‐derived MSCs to improve myocardial perfusion and exercise capacity and reduce symptoms in patients with chronic ICM. The PRECISE trial (NCT00426868; A Randomized Clinical Trial of Adipose‐Derived Stem and Regenerative Cells In the Treatment of Patients With Non Revascularizable Ischemic Myocardium) is using noncultured adipose stromal vascular fraction cells to test the effect of intramyocardial delivery in patients with chronic ICM. The preliminary data showed a reduction in infarct size and an improvement in maximum oxygen consumption and exercise capacity.69
In summary, the ease in preparation, immunoprivilege properties, multipotent nature, safety, and positive efficacy findings have made MSCs very promising for cardiac cell therapy.1,14,43 Cells can be collected using the most stringent and optimal criteria to improve efficacy and exclude comorbidities. The findings from POSEIDON serve as the first step to developing a cell therapy intervention that is truly practical in the clinical setting. Given that the allogeneic MSC phenotype offers major advantages in terms of availability, such as for time sensitive situations such as acute MI, much future research is expected to focus upon allografting for regenerative medicine. The possibility of having an effective “off the shelf” cell‐based intervention with regenerative effects could be transformative in the management of ischemic heart disease.
Clinical Trials Employing Cardiac Stem Cells
While the above‐mentioned sources of stem cells originate from extra‐cardiac locations, it is now apparent that tissue‐specific cardiac stem cells (CSCs) reside in niches (Figure 9) within the heart where they participate in cardiomyocyte turnover and contribute to myocardial recovery after injury.41,70–71 This discovery represents a paradigm shift from the traditional concept that the heart had a finite number of myocytes from birth and that the only way to increase myocardial mass was by hypertrophy of existing myocytes.71 An impressive array of preclinical studies performed in multiple species has demonstrated that injection of CSCs into animal models with ischemic cardiomyopathy slows the progression of LV remodeling and improves LV function.72–76
Figure 9.
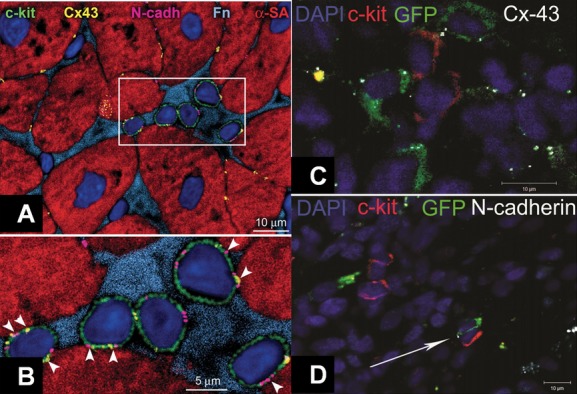
Human cardiac stem cell (CSC) niche in the myocardium. A, c‐kit+ (green) human cardiac stem cells (hCSCs) are nested in the interstitium and are surrounded by fibronectin (Fn, bright blue). These hCSCs are connected by gap junctions represented by connexin 43 (Cx43, yellow dots) and adherens junctions shown by N‐cadherin (N‐cadh, magenta) to cardiomyocytes (α‐sarcomeric actin, α‐SA, red). B, These cell‐to‐cell communications between CSCs and cardiomyocytes are indicated by arrowheads. C, Cell–Cell interactions between endogenous and exogenous stem cells. C and D, Immature mesenchymal stem cells (MSCs, green) are found within the swine myocardium to interact with resident c‐kit+ CSCs (red) by connexin‐43 (C, white) and N‐cadherin (D, white arrow) connections, closely resembling CSC niches. Panels A and B reproduced with permission from Leri et al, Circulation, 2013,70 panels C and D from Hatzistergos et al, Circulation Research 2010.41DAPI indicates 4',6‐diamidino‐2‐phenylindole; GFP, green fluorescent protein.
The promising preclinical studies coupled with technologic advancements facilitating isolation and ex vivo amplification of autologous c‐kit+ CSCs from heart biopsies of atrium or right ventricular septum set the stage for translational advancement of this field. Bolli et al built on the promising preclinical results and conducted a first‐in‐human clinical trial testing the therapeutic potential of cardiac c‐kit+ CSCs. The SCIPIO trial was the first randomized phase I study using c‐kit‐positive, lineage‐negative autologous CSCs in patients with LV dysfunction (EF <40%).2–4 The CSCs were extracted from right atrial surgical appendage biopsies obtained during coronary artery bypass graft (CABG) surgery, and patients were treated with a single dose intracoronary injection of CSCs at 4±1 months after the surgery. Results from patients who finished a 2‐year follow up were recently published.36–37 At the 4‐month time point, 20 patients who had received CSC therapy demonstrated via 3‐dimensional (3D) echo an increase in LVEF from a baseline of 29.0±1.7% to 36.0±2.5% (P<0.001). Additionally, these patients also had an improvement in regional wall motion score in all segments assessed by echocardiography. It is even more promising and encouraging to note that the improvements from CSC therapy were not only sustained but also increased at the 1‐ and 2‐year time points. The LVEF improved by 8.1% at 1 year (P<0.001, n=17) and 12.1% at 2 years (P<0.008, n=8). MRI analysis on 9 CSC patients demonstrated a reduction in scar size at 4 months (34.9±2.3 to 21.6±2.7 g, P<0.001) and at 1 year (33.9±3.0 to 18.7±3.6 g, P=0.003, n=6) (Figure 6). Additionally, the viable LV tissue mass increased at 4 months (+11.6±5.2 g, P=0.055) and at 1 year (+31.5±11.0 g, P=0.035). In contrast, the control group of 13 patients demonstrated no significant improvement in LVEF (baseline: 29.2±1.9% to 4 months: 29.4±1.8%) or in patient quality of life, assessed by the Minnesota Living with Heart Failure Questionnaire (MLHFQ) (Figure 7).
This phase I trial established the safety profile of CSCs as no adverse effects were reported up to 2 years after therapy. Additionally, it demonstrated that interventions with autologous CSCs in patients with ischemic cardiomyopathy have a promising and beneficial impact that continued to increase until the 2‐year follow‐up time point.
Similar to the SCIPIO trial, the ALCADIA8 (NCT00981006) trial represents a second phase I CSC trial conducted by Takehara et al at the Kyoto Prefectural University of Medicine in Japan to evaluate safety and efficacy of using autologous CSCs with a controlled release of basic fibroblast growth factor (bFGF) in patients with ischemic cardiomyopathy with LV dysfunction (15%<EF<35%). Preliminary results of this nonrandomized, open label study that enrolled 6 patients were presented at the 2012 American Heart Association Scientific Session.8 Autologous CSCs, after proper isolation and harvest, were coinjected with bFGF directly into the infarct zone concomitantly with CABG. bFGF exerts stem cell proliferative properties and, in addition, the microvascular fabric weaved by bFGF enhances engraftment and long‐term survival of transplanted CSCs.8,77–78 Cardiac MRI imaging showed that LVEF increased 12.1%, 6 months after CSC therapy. Additionally, cardiac MRI revealed a reduction in infarct size and significant improvement in wall motion score. The findings from SCIPIO and ALCADIA, albeit preliminary, offer promising results and encourage additional and larger clinical trials with CSCs.
Cell Combination Therapy
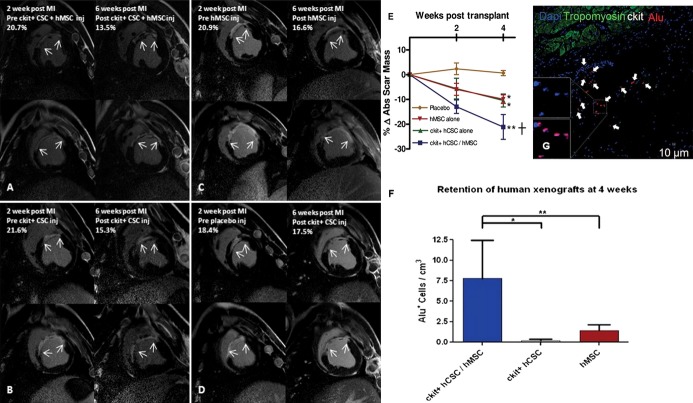
One of the newest concepts in cardiac cell therapy is that of combining cells to leverage complementary or synergistic interactions between cells types. In preclinical models, Hatzistergos et al41 demonstrated that intramyocardial injection of bone marrow‐derived MSCs stimulated a substantial proliferation of endogenous cardiac c‐kit+ cells. Moreover, ex vivo mixing of the 2 cell types enhanced c‐kit+ cell proliferation and lineage commitment towards a cardiac phenotype. The established safety profile of MSCs and CSCs from POSEIDON and SCIPIO, respectively, created an opportunity for exploring therapeutic enhancement of combination cell therapy. Based on our findings that MSCs induce proliferation and differentiation of c‐kit CSCs,41 we investigated whether the combination of both cell types produced a greater reduction in MI size and improvement in LV function than each cell type alone.76 In a recent preclinical xenotransplantation study employing a myocardial ischemia‐reperfusion model, swine were administered either human combination CSCs/MSCs (1/200 mol/L, n=5), human CSCs alone (1 mol/L, n=5), human MSCs alone (200 mol/L, n=5), or placebo (PBS, n=5) intramyocardially to the infarct and border zones at 14 days post‐MI. Each cell therapy group reduced MI size relative to placebo (P<0.05), but the MI size reduction was 2‐fold greater in combination versus either cell therapy alone (Figure 10A through 10E), P<0.05). There was also significant improvement in LV chamber compliance and contractility in the combination‐treated swine. EF was restored to baseline in all the cell therapy groups, whereas placebo pigs had no improvement in LV function (P<0.05). The engraftment of stem cells was 7‐fold greater in the combination group versus either cell type alone (Figure 10F and 10G, P<0.001). In summary, combining human MSCs and human CSCs as a cell therapy enhanced MI size reduction and restored diastolic and systolic function toward normal after MI.
Figure 10.
Human c‐kit+ cardiac stem cells (CSCs) and bone marrow mesenchymal stem cells (MSCs) reduce infarct size and enhance engraftment in a porcine model. A through D, Delayed enhancement CMR images showing pre‐injection scar and 4‐weeks post‐injection scar changes (white arrows indicate infarct extension). E, Reduction in absolute infarct size from preinjection to 4 weeks post‐injection (*P<0.05 within group ANOVA; †P<0.05 vs placebo at 4 weeks post‐injection by Bonferroni posttest). F, Immunohistochemical‐stained images showing clusters of Alu‐positive human stem cells (white arrows) engrafted in the infarct territory. G, Retention of Alu+ human stem cells was 7‐fold higher when hCSCs and hMSCs were injected together compared with either cell type administered alone (n=3 analyzed per treatment group). Graphs represent mean±SEM *P<0.001 and **P<0.05, between‐group 1‐way ANOVA. Reproduced with permission from Williams et al, Circulation, 2013.76 ANOVA indicates analysis of variance; CMR, cardiovascular magnetic resonance; MI, myocardial infarction; SEM, standard error of the mean.
Together with the demonstrated safety of cell‐based therapy using MSCs1 and c‐kit+ CSCs2–3 in patients with ischemic cardiomyopathy (ICM), these preclinical findings illustrate important biological interactions between c‐kit+ CSCs and MSCs that enhance cell therapeutic responses and support the next phase in the concept of clinical trials for stem cells, a clinical trial that has received regulatory approval from the FDA. From the mechanistic perspective, a trial testing combination therapy will add to our fundamental understanding of regenerative medicine and basic stem cell biology. Should this trial show safety and efficacy results, we will have effectively capitalized on the synergistic behavior of 2 different cell types, which will in turn possibly advance therapeutic opportunities using cell‐based therapy.
Clinical Trials Employing Cardiospheres
Another new approach for cell therapy is to employ culture‐expanded cellular products obtained from the heart itself. As initially described by Messina et al,79 under appropriate culture conditions, cells will emanate from pieces of myocardial tissue, expand, and form aggregates of cell clusters that have become known as cardiospheres. These floating spheres form in nonadhesive substrates. Essentially, they are a conglomerate of both early‐stage committed and primitive cells that in earlier descriptions contained a central core of c‐kit+ stem cells (≈15%), layers of differentiating cells, and an outside cell layer of mesenchymal stromal cells.80 More recent studies, however, have shown that the percentage of c‐kit+ cells in cardiospheres is as low as 3% to 7%.24,81–82 The presence of connexin 43 and gap junctions between undifferentiated and differentiated cells plays a crucial role in the proliferation of the undifferentiated lineage and also functions as an avenue for electrical coupling in cells committed to forming myocytes. These findings have led to the proposal that cardiospheres could potentially recapitulate cardiac niche biology in the in vitro environment.14
Preclinical models have demonstrated that autologous as well as allogeneic cardiosphere‐derived cells (CDCs) reduce scar size and improve cardiac function after MI through mostly indirect (paracrine) but also direct mechanisms.24,81–83 Recently, there have been several evolutions in the interpretation of the active constituent cell component within the cardiosphere. Initially, it had been proposed that the cardiac stemness properties of the cardiosphere were central to the activity of the cell therapeutic resulting in true cardiac regeneration. More recently, however, c‐kit depletion of the cardiosphere has been reported to not reduce the regenerative response.84 Moreover, recent studies have shown little‐to‐no medium‐ or long‐term engraftment of cardiospheres in myocardial injury models.81,85 Together these findings support an interpretation that cardiospheres do not constitute a formulation of cardiac “stem cells” but are more likely to be cardiac stromal cells stimulating secondary endogenous repair.
Recent evidence from in vitro studies has also substantiated the potential use of allogeneic cardiac‐derived stem/progenitor cells.81,86 Human cardiac stem/progenitors cells express very low levels of human leukocyte antigen (HLA) class II, do not express costimulatory molecules, do not trigger allogeneic T‐cell responses, and activate regulatory T cells in vitro.86 This immunomodulatory capacity, similar to that of MSCs, supports the feasibility of using allogeneic cardiac stem/progenitor cells for cardiac regeneration, although preclinical studies in large animal models are needed to confirm these findings.
CADUCEUS is the first randomized prospective phase I trial that assessed the safety of intracoronary administration of autologous CDCs to 31 patients 1.5 to 3 months after an MI.5 They demonstrated that the use of autologous CDCs after MI was safe. There was no significant difference in end‐diastolic volume and end systolic volume seen in either the CDC or control groups (Figure 4). However, compared to the control group of patients, those treated with CDCs showed increase in viable myocardium, regional contractility, and reductions in scar mass at the 6‐month time point. Clinical trials with larger numbers of patients and longer follow up are necessary to establish efficacy of CDCs in ischemic cardiomyopathy.
Evaluation of Stem Cell Therapy Efficacy by Cardiac Phenotyping and Clinical Parameters
The trials discussed above demonstrated the safety of a variety of adult stem cells. In order to compellingly and accurately demonstrate efficacy of stem cell therapies, innovative evaluation and assessment tools need to be investigated. Mechanistically, stem cell therapy is different than other currently available pharmaceutical interventions and therefore cannot be evaluated in the same manner. The purpose of cell therapy is to prevent or reverse ventricular remodeling, a pathophysiological process of MI that results in ventricular wall thinning and collagenous scar formation.87 Regeneration of the injured myocardial regions via cell therapy improves regional contractility, which only translates into a minor or insignificant improvement of LVEF, a measure of global cardiac function.
Until recently, EF was the primary endpoint to evaluate therapeutic interventions. While EF is a good measure of global cardiac function, it is load‐dependent and influenced by preload, afterload, and contractility.87–88 Additionally, in a post‐MI state, the neurohormonal system will induce a vasoconstrictive state that also results in a slightly reduced LVEF. The inconsistency between EF and efficacy of cell therapy is carefully highlighted in the phase I TAC‐HFT89 (NCT00768066) trial, where Williams et al43 showed statistically significant parallel decreases in end‐diastolic volume and end‐systolic volume at the 12‐month endpoint. These findings not only suggest the regeneration of myocardial tissue, but also translate into a prominent increase in regional function and contractility in the infarct zone. The true value and importance of cell therapies would be missed if EF was the sole endpoint utilized when evaluating ventricular remodeling. Variables indicative of LV remodeling, such as decrease in chamber volumes (Figures 2 and 3), sphericity index (Figure 11), and scar size reduction (Figures 5 and 6) are proving to be more clinically meaningful and informative measures to elucidate the efficacy of stem cell therapies.1,87–88 Therefore, the TAC‐HFT study, along with similar results seen in the POSEIDON study, reaffirms the need to focus less on global EF as a primary endpoint in evaluating the efficacy of cell‐based therapy.1,20
Figure 3.
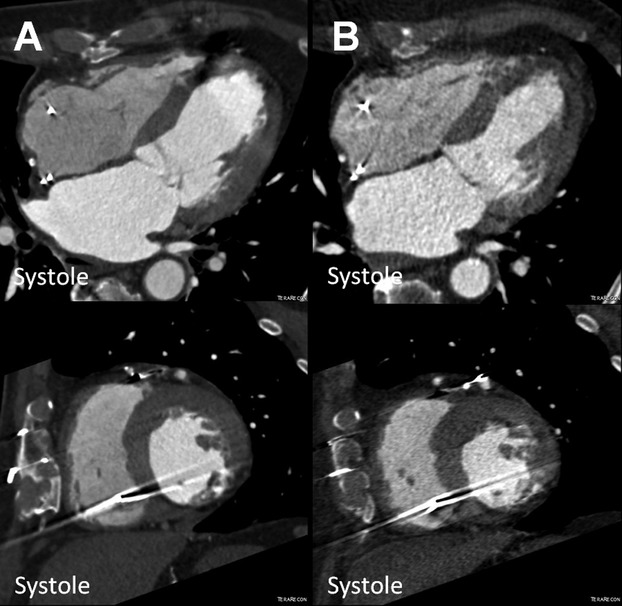
Reduction of left ventricular end‐systolic volume (ESV) in the POSEIDON study. MDCT images (4 chamber and short axis view) of a patient with chronic ischemic cardiomyopathy (A) before (ESV 126.9 mL) and (B) after transendocardial stem cell injection (TESI) with 20 million autologous MSCs (ESV 77.1 mL). MDCT indicates multi‐detector computer tomography; MSCs, mesenchymal stem cells; POSEIDON, PercutaneOus StEm Cell Injection Delivery Effects On Neomyogenesis.
Figure 11.
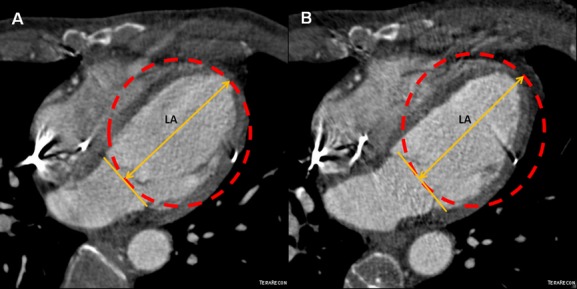
Reversal of remodeling as reduction of sphericity index in the POSEIDON trial. A, Four chamber MDCT images of a chronic ischemic cardiomyopathy patient with a sphericity index of 0.48 and (B) its reduction to 0.41 after transendocardial stem cell injection (TESI) with allogeneic mesenchymal stem cells (MSCs, 20 million). Sphericity index was calculated as: EDV/volume of sphere using long axis (LA) as diameter. EDV indicates end‐diastolic volume; MDCT, multi‐detector computer tomography; POSEIDON, PercutaneOus StEm Cell Injection Delivery Effects On Neomyogenesis.
Whether it is stem cell differentiation into beating cardiomyocytes or the stimulation of endogenous progenitors that results in regeneration of the myocardial wall remains controversial. Various preclinical and clinical studies support potentially meaningful reductions of infarct size in patients with acute and chronic ischemic cardiomyopathy. It is certain that an imaging tool that can provide a detailed report on myocardial viability will provide a valuable tool for planning appropriate therapeutic strategies. Consequently, evaluation of myocardial viability has turned into one of the most important endpoints in assessing stem cell therapy efficacy. Imaging modalities such as cardiovascular magnetic resonance (CMR),49,90–91 multi‐detector computer tomography (MDCT),48 and single‐photon emission computed tomography (SPECT) have been the focus of such an assessment.87,92
Clinical trials like CADUCEUS,5 POSEIDON,1 ALCADIA,8 and SCIPIO2,4 are now starting to measure efficacy of cell therapy via clinical parameters such as the 6‐minute walk test, peak VO2, and MLHFQ. The 2‐year results from SCIPIO and the autologous group in POSEIDON both demonstrated improvement in MLHFQ scores (Figure 7). The 6‐minute walk test has been proven to be a reliable and valid measure that can independently predict morbidity and mortality in patients with LV dysfunction.93
Our lab measured all 3 values in the comparison between autologous and allogeneic MSCs in the POSEIDON study. The findings indicate that allogeneic MSCs were the only group that resulted in improvement from baseline in the 6‐minute walk test and the MLHFQ questionnaire, but neither group improved Peak VO2. When compared to the hemodynamic and structural improvements, both cell groups demonstrated a reduction in both mean early enhancement defect (EED; infarct size) by −33.21% and sphericity index.1 Additionally, the improvement in end‐diastolic and end‐systolic volumes was similar between both groups (see online CINE CT supplementary video). This study was a good example of how the hemodynamic and structural improvements indicated by imaging may not always directly correlate to improvements in the clinical parameters.
The CADUCEUS,5 SCIPIO,2–4 and ALCADIA8 studies also found improvement in clinical parameters. In the CADUCEUS trial, patients injected with CDCs showed a mean increase of 33.0 m at 12 months in the 6‐minute walk test as compared to a 9.6 m decrease at 12 months in the control group. The peak VO2 also improved in the CDC injected group by 2.6 mL/kg per minute at the 6‐month time point and was stable in the control group, but the values were not significant. The MLHFQ scores declined equally in both the control and CDC groups, from 24.9 at baseline to 14.1 at 6 months in the CDC group and from 35.4 to 25.1, respectively, in the control group. Likewise, the patients who received CSCs in the SCIPIO trial also reported quality of life improvements using the MLHFQ. The ALCADIA trial also demonstrated improvement in clinical parameters as 5 of the 6 patients displayed improvements in New York Heart Association classification of III/IV to I/II and in maximal O2 from baseline value of 12.2 to 16.7 at the 6‐month time point.8
Conclusion
The POSEIDON,1 SCIPIO,2 SWISS‐AMI,6 CADUCEUS,5 CCTRN trials, TIME,9 LateTIME,10 and FOCUS11 were crucial in establishing the safety profiles for bone marrow‐derived mononuclear cells, mesenchymal stem cells, and cardiac‐derived stem cells. Also emerging from the new wave of small clinical trials is a greater sense of the efficacy of cell therapy. Most importantly, these trials can also be evaluated from a mechanistic perspective as they have employed sophisticated imaging modalities that allow the evaluation of scar size and remodeling parameters in addition to measures of global and regional cardiac function. Additionally, the latest findings support an earlier idea that cell therapies may have a disproportionately greater impact on clinical outcomes relative to increase in EF, as shown by improvements in measures of patient functional status and quality of life. These latest findings from clinical trials of stem cell therapy bring us another step closer to a future with cell‐based therapeutic interventions for ischemic cardiomyopathies. There is substantial ongoing activity in planning new trials designed to evaluate the efficacy of various adult stem cells and also the combination of cell types such as the c‐kit+ CSC and MSC mixture that will be evaluated in AIRMID. With a substantial number of studies demonstrating safety, the stage is set for the field to contemplate pivotal clinical trials designed to demonstrate efficacy of cell therapies.
Acknowledgments
The authors would like to thank Vasileios Karantalis, MD and Wayne E. Balkan, PhD for their assistance in critical review and editing.
Sources of Funding
This work is supported by NIH grants: RO1 HL094849, P20 HL101443, RO1 HL084275, RO1 HL107110, and U54 HL081028 to Dr Hare.
Disclosures
Dr Hare is listed on a patent for cardiac cell‐based therapy, receives research support from Biocardia, and reports an equity interest and being a board member of Vestion, Inc. Dr Hare is a consultant to Kardia. The other authors report no conflict of interest relevant to this work.
References
- 1.Hare JM, Fishman JE, Gerstenblith G, Difede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis‐Sproul J, Byrnes J, George R, Lardo A, Schulman IH, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW. Comparison of allogeneic vs autologous bone marrow‐derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012; 308:1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011; 378:1847-1857 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Wagner SG, Beache GM, Leri A, Hosoda T, Goihberg P, Fiorini C, Solankhi N, Fahsah I, Elmore JB, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Effect of cardiac stem cells in patients with ischemic cardiomyopathy: interim results of the SCIPIO trial up to 2 years after therapy. Circulation. 2012; 126:2776-2799 [Google Scholar]
- 4.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012; 126:S54-S64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere‐derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012; 379:895-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surder D, Schwitter J, Moccetti T, Astori G, Rufibach K, Plein S, Lo Cicero V, Soncin S, Windecker S, Moschovitis A, Wahl A, Erne P, Jamshidi P, Auf der Maur C, Manka R, Soldati G, Buhler I, Wyss C, Landmesser U, Luscher TF, Corti R. Cell‐based therapy for myocardial repair in patients with acute myocardial infarction: rationale and study design of the swiss multicenter intracoronary stem cells study in acute myocardial infarction (SWISS‐AMI). Am Heart J. 2010; 160:58-64 [DOI] [PubMed] [Google Scholar]
- 7.Surder D. Results of the swiss multicenter intracoronary stem cells study in acute myocardial infarction (SWISS AMI) trial. Circulation. 2012; 126:2776-2799 [Google Scholar]
- 8.Takehara N, Ogata T, Nakata M, Kami D, Nakamura T, Matoba S, Gojo S, Sawada T, Yaku H, Kyoto HM. The ALCADIA (Autologous Human Cardiac‐Derived Stem Cell to Treat Ischemic Cardiomyopathy) Trial. Circulation. 2012; 126:2776-2799 [Google Scholar]
- 9.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, Perin EC, Chambers J, Baran KW, Raveendran G, Lambert C, Lerman A, Simon DI, Vaughan DE, Lai D, Gee AP, Taylor DA, Cogle CR, Thomas JD, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Kappenman C, Westbrook L, Piller LB, Simpson LM, Baraniuk S, Loghin C, Aguilar D, Richman S, Zierold C, Spoon DB, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012; 308:2380-2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS, Perin EC, Baran KW, Chambers J, Lambert C, Raveendran G, Simon DI, Vaughan DE, Simpson LM, Gee AP, Taylor DA, Cogle CR, Thomas JD, Silva GV, Jorgenson BC, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Smith DX, Baraniuk S, Piller LB, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011; 306:2110-2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopoulos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, Handberg E, Olson RE, Geither C, Bowman S, Francescon J, Baraniuk S, Piller LB, Simpson LM, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS‐CCTRN trial. JAMA. 2012; 307:1717-1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, Nakadi BE, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo‐Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure the C‐CURE multicenter randomized trial with lineage‐specified biologics. J Am Coll Cardiol. 2013; 61:2329-2338 [DOI] [PubMed] [Google Scholar]
- 13.Boudoulas KD, Hatzopoulos AK. Cardiac repair and regeneration: the Rubik's cube of cell therapy for heart disease. Dis Model Mech. 2009; 2:344-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karantalis V, Balkan W, Schulman IH, Hatzistergos KE, Hare JM. Cell‐based therapy for prevention and reversal of myocardial remodeling. Am J Physiol Heart Circ Physiol. 2012; 303:H256-H270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazhari R, Hare JM. Translational findings from cardiovascular stem cell research. Trends Cardiovasc Med. 2012; 22:1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998; 4:929-933 [DOI] [PubMed] [Google Scholar]
- 17.Menasche P. Skeletal myoblasts for cardiac repair: Act II? J Am Coll Cardiol. 2008; 52:1881-1883 [DOI] [PubMed] [Google Scholar]
- 18.Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagege AA. The myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial: first randomized placebo‐controlled study of myoblast transplantation. Circulation. 2008; 117:1189-1200 [DOI] [PubMed] [Google Scholar]
- 19.Malliaras K, Marban E. Cardiac cell therapy: where we've been, where we are, and where we should be headed. Br Med Bull. 2011; 98:161-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011; 109:923-940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wollert KC, Meyer GP, Lotz J, Ringes‐Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone‐marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004; 364:141-148 [DOI] [PubMed] [Google Scholar]
- 22.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow‐derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006; 355:1210-1221 [DOI] [PubMed] [Google Scholar]
- 23.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Werner N, Haase J, Neuzner J, Germing A, Mark B, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Improved clinical outcome after intracoronary administration of bone‐marrow‐derived progenitor cells in acute myocardial infarction: final 1‐year results of the REPAIR‐AMI trial. Eur Heart J. 2006; 27:2775-2783 [DOI] [PubMed] [Google Scholar]
- 24.Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marban L, Marban E. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere‐derived cells. J Am Coll Cardiol. 2012; 59:942-953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004; 109:1615-1622 [DOI] [PubMed] [Google Scholar]
- 26.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. CD34‐positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006; 114:2163-2169 [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S, Shibata T, Suehiro S, Asahara T. Dose‐dependent contribution of CD34‐positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006; 113:1311-1325 [DOI] [PubMed] [Google Scholar]
- 28.Quyyumi AA, Waller EK, Murrow J, Esteves F, Galt J, Oshinski J, Lerakis S, Sher S, Vaughan D, Perin E, Willerson J, Kereiakes D, Gersh BJ, Gregory D, Werner A, Moss T, Chan WS, Preti R, Pecora AL. CD34(+) cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J. 2011; 161:98-105 [DOI] [PubMed] [Google Scholar]
- 29.Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer‐Rasokat U, Heeschen C, Spyridopoulos I, Dimmeler S, Zeiher AM. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol. 2007; 49:2341-2349 [DOI] [PubMed] [Google Scholar]
- 30.Zhuo Y, Li SH, Chen MS, Wu J, Kinkaid HY, Fazel S, Weisel RD, Li RK. Aging impairs the angiogenic response to ischemic injury and the activity of implanted cells: combined consequences for cell therapy in older recipients. J Thorac Cardiovasc Surg. 2010; 139:1286-1294 [DOI] [PubMed] [Google Scholar]
- 31.Kinkaid HY, Huang XP, Li RK, Weisel RD. What's new in cardiac cell therapy? Allogeneic bone marrow stromal cells as “universal donor cells”. J Card Surg. 2010; 25:359-366 [DOI] [PubMed] [Google Scholar]
- 32.Aicher A, Heeschen C, Sasaki K, Urbich C, Zeiher AM, Dimmeler S. Low‐energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation. 2006; 114:2823-2830 [DOI] [PubMed] [Google Scholar]
- 33.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal‐cell‐derived factor 1 on stem‐cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003; 362:697-703 [DOI] [PubMed] [Google Scholar]
- 34.Assmus B, Walter DH, Seeger FH, Leistner DM, Steiner J, Ziegler I, Lutz A, Khaled W, Klotsche J, Tonn T, Dimmeler S, Zeiher AM. Effect of shock wave‐facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. JAMA. 2013; 309:1622-1631 [DOI] [PubMed] [Google Scholar]
- 35.Jeevanantham V, Butler M, Saad A, Abdel‐Latif A, Zuba‐Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long‐term improvement in cardiac parameters: a systematic review and meta‐analysis. Circulation. 2012; 126:551-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, Martin‐Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012; 2:CD006536. [DOI] [PubMed] [Google Scholar]
- 37.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post‐natal organs and tissues. J Cell Sci. 2006; 119:2204-2213 [DOI] [PubMed] [Google Scholar]
- 38.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci USA. 2009; 106:14022-14027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009; 30:2722-2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002; 73:1919-1925 [DOI] [PubMed] [Google Scholar]
- 41.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010; 107:913-922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008; 8:726-736 [DOI] [PubMed] [Google Scholar]
- 43.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011; 108:792-796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003; 31:890-896 [DOI] [PubMed] [Google Scholar]
- 45.Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005; 12:47-57 [DOI] [PubMed] [Google Scholar]
- 46.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double‐blind, placebo‐controlled, dose‐escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009; 54:2277-2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marban E, Malliaras K. Mixed results for bone marrow‐derived cell therapy for ischemic heart disease. JAMA. 2012; 308:2405-2406 [DOI] [PubMed] [Google Scholar]
- 48.Lessick J, Dragu R, Mutlak D, Rispler S, Beyar R, Litmanovich D, Engel A, Agmon Y, Kapeliovich M, Hammerman H, Ghersin E. Is functional improvement after myocardial infarction predicted with myocardial enhancement patterns at multidetector CT? Radiology. 2007; 244:736-744 [DOI] [PubMed] [Google Scholar]
- 49.Mahnken AH, Koos R, Katoh M, Wildberger JE, Spuentrup E, Buecker A, Gunther RW, Kuhl HP. Assessment of myocardial viability in reperfused acute myocardial infarction using 16‐slice computed tomography in comparison to magnetic resonance imaging. J Am Coll Cardiol. 2005; 45:2042-2047 [DOI] [PubMed] [Google Scholar]
- 50.Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006; 441:1080-1086 [DOI] [PubMed] [Google Scholar]
- 51.Ballard VL. Stem cells for heart failure in the aging heart. Heart Fail Rev. 2010; 15:447-456 [DOI] [PubMed] [Google Scholar]
- 52.Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S. Young adult bone marrow‐derived endothelial precursor cells restore aging‐impaired cardiac angiogenic function. Circ Res. 2002; 90:E89-E93 [DOI] [PubMed] [Google Scholar]
- 53.Hatzistergos KE, Blum A, Ince T, Grichnik JM, Hare JM. What is the oncologic risk of stem cell treatment for heart disease? Circ Res. 2011; 108:1300-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez R, Rubio R, Masip M, Catalina P, Nieto A, de la Cueva T, Arriero M, San Martin N, de la Cueva E, Balomenos D, Menendez P, Garcia‐Castro J. Loss of p53 induces tumorigenesis in p21‐deficient mesenchymal stem cells. Neoplasia. 2009; 11:397-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casiraghi F, Remuzzi G, Abbate M, Perico N. Multipotent mesenchymal stromal cell therapy and risk of malignancies. Stem Cell Rev. 2013; 9:65-79 [DOI] [PubMed] [Google Scholar]
- 56.von Bahr L, Batsis I, Moll G, Hagg M, Szakos A, Sundberg B, Uzunel M, Ringden O, Le Blanc K. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long‐term engraftment and no ectopic tissue formation. Stem Cells. 2012; 30:1575-1578 [DOI] [PubMed] [Google Scholar]
- 57.Psaltis PJ, Paton S, See F, Arthur A, Martin S, Itescu S, Worthley SG, Gronthos S, Zannettino AC. Enrichment for STRO‐1 expression enhances the cardiovascular paracrine activity of human bone marrow‐derived mesenchymal cell populations. J Cell Physiol. 2010; 223:530-540 [DOI] [PubMed] [Google Scholar]
- 58.Noort WA, Oerlemans MI, Rozemuller H, Feyen D, Jaksani S, Stecher D, Naaijkens B, Martens AC, Buhring HJ, Doevendans PA, Sluijter JP. Human versus porcine mesenchymal stromal cells: phenotype, differentiation potential, immunomodulation and cardiac improvement after transplantation. J Cell Mol Med. 2012; 16:1827-1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Psaltis PJ, Carbone A, Nelson AJ, Lau DH, Jantzen T, Manavis J, Williams K, Itescu S, Sanders P, Gronthos S, Zannettino AC, Worthley SG. Reparative effects of allogeneic mesenchymal precursor cells delivered transendocardially in experimental nonischemic cardiomyopathy. JACC Cardiovasc Interv. 2010; 3:974-983 [DOI] [PubMed] [Google Scholar]
- 60.Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003; 116:1827-1835 [DOI] [PubMed] [Google Scholar]
- 61.Gronthos S, Fitter S, Diamond P, Simmons PJ, Itescu S, Zannettino AC. A novel monoclonal antibody (STRO‐3) identifies an isoform of tissue nonspecific alkaline phosphatase expressed by multipotent bone marrow stromal stem cells. Stem Cells Dev. 2007; 16:953-963 [DOI] [PubMed] [Google Scholar]
- 62.Behfar A, Yamada S, Crespo‐Diaz R, Nesbitt JJ, Rowe LA, Perez‐Terzic C, Gaussin V, Homsy C, Bartunek J, Terzic A. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol. 2010; 56:721-734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van de Ven C, Collins D, Bradley MB, Morris E, Cairo MS. The potential of umbilical cord blood multipotent stem cells for nonhematopoietic tissue and cell regeneration. Exp Hematol. 2007; 35:1753-1765 [DOI] [PubMed] [Google Scholar]
- 64.Nishiyama N, Miyoshi S, Hida N, Uyama T, Okamoto K, Ikegami Y, Miyado K, Segawa K, Terai M, Sakamoto M, Ogawa S, Umezawa A. The significant cardiomyogenic potential of human umbilical cord blood‐derived mesenchymal stem cells in vitro. Stem Cells. 2007; 25:2017-2024 [DOI] [PubMed] [Google Scholar]
- 65.Roy R, Kukucka M, Messroghli D, Brodarac A, Becher M, Choi YH, Tschope C, Stamm C. Placenta‐derived mesenchymal stem cells for allogenic cardiac cell therapy. Thorac Cardiovasc Surg. 2012; 60:28 [Google Scholar]
- 66.Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue‐derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007; 28:2667-2677 [DOI] [PubMed] [Google Scholar]
- 67.Li B, Zeng Q, Wang H, Shao S, Mao X, Zhang F, Li S, Guo Z. Adipose tissue stromal cells transplantation in rats of acute myocardial infarction. Coron Artery Dis. 2007; 18:221-227 [DOI] [PubMed] [Google Scholar]
- 68.Duckers E. Freshly adipose‐derived stem cell in acute myocardial infarction. The APOLLO trial. 7th International Symposium on Stem Cell Therapy and Cardiovascular Innovation. Madrid, Spain, 2010 [Google Scholar]
- 69.Perin E, Fernandez‐Aviles F. The PRECISE trial. 7th International Symposium on Stem Cell Therapy and Cardiovascular Innovation. Madrid, Spain, 2010 [Google Scholar]
- 70.Leri A, Anversa P. Stem cells and myocardial regeneration: cooperation wins over competition. Circulation. 2013; 127:165-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res. 2011; 109:941-961 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa‐Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007; 104:14068-14073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal‐Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003; 114:763-776 [DOI] [PubMed] [Google Scholar]
- 74.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self‐renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA. 2005; 102:8966-8971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rota M, Padin‐Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira‐Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008; 103:107-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013; 127:213-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang YH, Zhang GW, Gu TX, Li‐Ling J, Wen T, Zhao Y, Wang C, Fang Q, Yu L, Liu B. Exogenous basic fibroblast growth factor promotes cardiac stem cell‐mediated myocardial regeneration after miniswine acute myocardial infarction. Coron Artery Dis. 2011; 22:279-285 [DOI] [PubMed] [Google Scholar]
- 78.Takehara N, Tsutsumi Y, Tateishi K, Ogata T, Tanaka H, Ueyama T, Takahashi T, Takamatsu T, Fukushima M, Komeda M, Yamagishi M, Yaku H, Tabata Y, Matsubara H, Oh H. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere‐derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J Am Coll Cardiol. 2008; 52:1858-1865 [DOI] [PubMed] [Google Scholar]
- 79.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004; 95:911-921 [DOI] [PubMed] [Google Scholar]
- 80.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere‐derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007; 115:896-908 [DOI] [PubMed] [Google Scholar]
- 81.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, Marban L, Marban E. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere‐derived cells. Circulation. 2012; 125:100-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carr CA, Stuckey DJ, Tan JJ, Tan SC, Gomes RS, Camelliti P, Messina E, Giacomello A, Ellison GM, Clarke K. Cardiosphere‐derived cells improve function in the infarcted rat heart for at least 16 weeks–an MRI study. PLoS One. 2011; 6:e25669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee ST, White AJ, Matsushita S, Malliaras K, Steenbergen C, Zhang Y, Li TS, Terrovitis J, Yee K, Simsir S, Makkar R, Marban E. Intramyocardial injection of autologous cardiospheres or cardiosphere‐derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post‐myocardial infarction. J Am Coll Cardiol. 2011; 57:455-465 [DOI] [PubMed] [Google Scholar]
- 84.Cheng K, Shen D, Sun B, Malliaras K, Smith R, Chowdhury S, Duong DT, Makkar R, Marban E. Irrelevance of c‐kit‐positive subpopulation to the therapeutic benefit of human cardiosphere‐derived cells. Circulation. 2012; 126:A13259 [Google Scholar]
- 85.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marban E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere‐derived cells transplanted into infarcted mice. Circ Res. 2010; 106:971-980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lauden L, Boukouaci W, Borlado LR, Lopez IP, Sepulveda P, Tamouza R, Charron D, Al‐Daccak R. Allogenicity of human cardiac stem/progenitor cells orchestrated by programmed death ligand 1. Circ Res. 2013; 112:451-464 [DOI] [PubMed] [Google Scholar]
- 87.Suncion VY, Schulman IH, Hare JM. Concise review: the role of clinical trials in deciphering mechanisms of action of cardiac cell‐based therapy. Stem Cells Transl Med. 2012; 1:29-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McCall FC, Telukuntla KS, Karantalis V, Suncion VY, Heldman AW, Mushtaq M, Williams AR, Hare JM. Myocardial infarction and intramyocardial injection models in swine. Nat Protoc. 2012; 7:1479-1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trachtenberg B, Velazquez DL, Williams AR, McNiece I, Fishman J, Nguyen K, Rouy D, Altman P, Schwarz R, Mendizabal A, Oskouei B, Byrnes J, Soto V, Tracy M, Zambrano JP, Heldman AW, Hare JM. Rationale and design of the transendocardial injection of autologous human cells (bone marrow or mesenchymal) in chronic ischemic left ventricular dysfunction and heart failure secondary to myocardial infarction (TAC‐HFT) trial: a randomized, double‐blind, placebo‐controlled study of safety and efficacy. Am Heart J. 2011; 161:487-493 [DOI] [PubMed] [Google Scholar]
- 90.Schuleri KH, Centola M, Choi SH, Evers KS, Dawoud F, George RT, Lima JA, Lardo AC. CT for evaluation of myocardial cell therapy in heart failure: a comparison with CMR imaging. JACC Cardiovasc Imaging. 2011; 4:1284-1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schuleri KH, Centola M, Evers KS, Zviman A, Evers R, Lima JA, Lardo AC. Cardiovascular magnetic resonance characterization of peri‐infarct zone remodeling following myocardial infarction. J Cardiovasc Magn Reson. 2012; 14:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim RJ, Shah DJ. Fundamental concepts in myocardial viability assessment revisited: when knowing how much is “alive” is not enough. Heart. 2004; 90:137-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, Kronenberg MW, Kostis JB, Kohn RM, Guillotte M. Prediction of mortality and morbidity with a 6‐minute walk test in patients with left ventricular dysfunction. SOLVD investigators. JAMA. 1993; 270:1702-1707 [PubMed] [Google Scholar]