Abstract
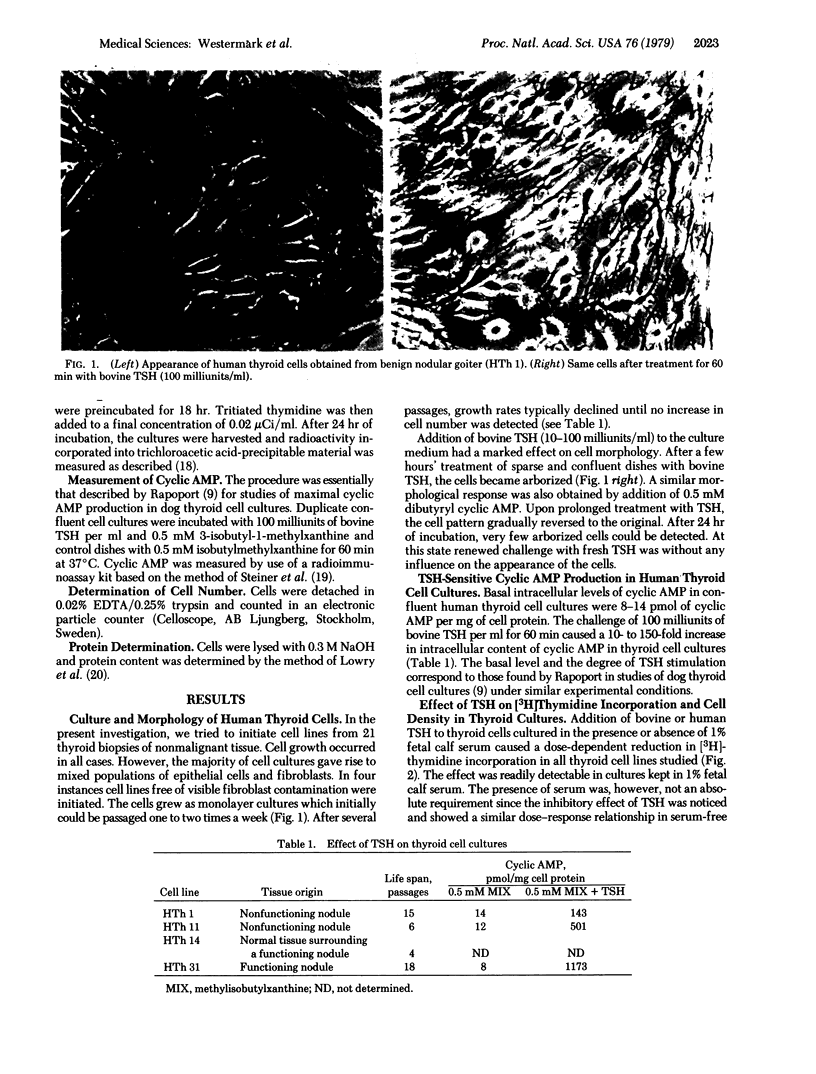
Thyroid cells, obtained from both normal human tissue and benign nodular goiter, were cultured and maintained in vitro in 4-18 passages. Cultures with confluent cells accumulated cyclic AMP (10-150 times the basal amount) upon addition of bovine thyrotropin (100 milliunits/ml), indicating that the cells in culture maintained a thyrotropin-sensitive adenylate cyclase system. Addition of high doses of thyrotropin also induced a characteristic and reversible change in the morphology of the cells.
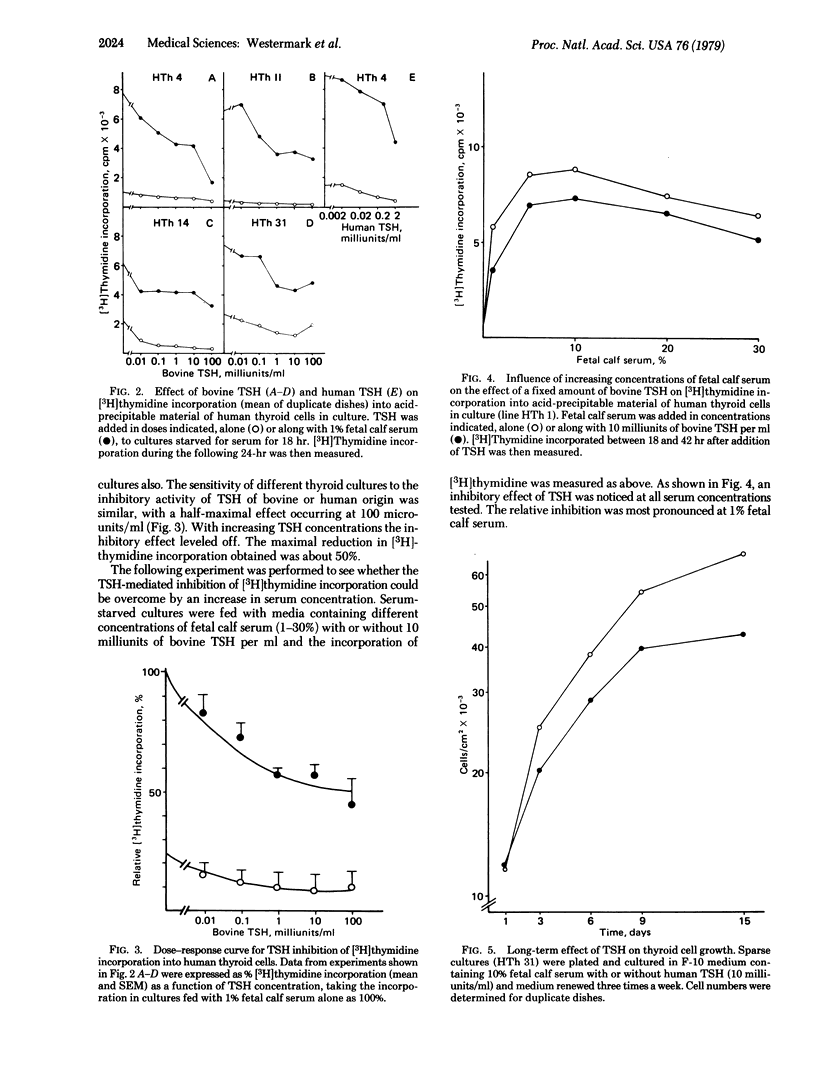
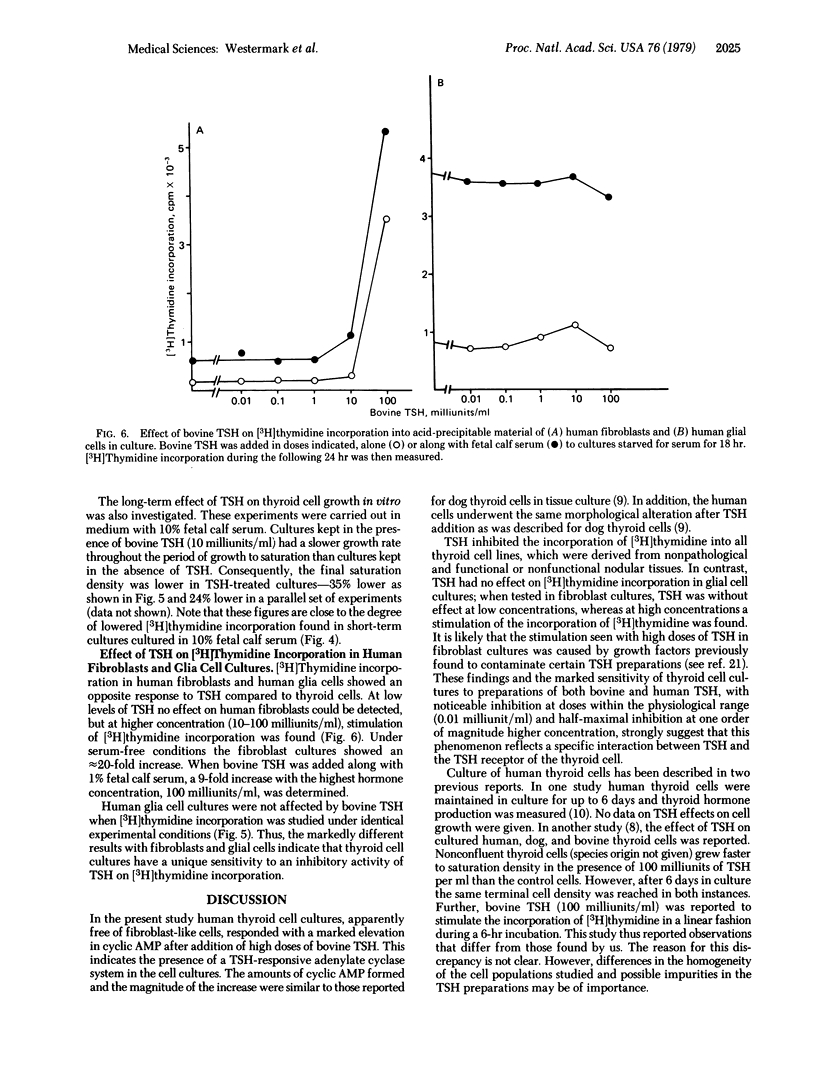
The effect of thyrotropin on cell growth was studied in short- and long-term experiments. Thyrotropin reduced [3H]thymidine incorporation in a dose-dependent fashion in all cultures of thyroid cells. The maximal inhibition over a 24-hr period was about 50%. The thyroid cells were notably sensitive, and the half-maximal effect occurred at about 100 milliunits of thyrotropin per ml. In contrast, the hormone had no effect on [3H]-thymidine incorporation into human glial cells. Low doses of thyrotropin also had no effect on human fibroblasts and, at high doses, a stimulation of [3H]thymidine incorporation was seen. Thyroid cell cultures grown in the presence of 10 milliunits of thyrotropin per ml for 7-14 days had a slower growth rate and 24-36% lower cell numbers at saturation density than control dishes, indicating that the hormone also had a long-term effect on cell proliferation. The data agree with in vitro studies by others of the effects of corticotropin and lutropin on target cells and suggest that in vivo the primary action of pituitary trophic hormones on endocrine tissues is not stimulation of growth.
Keywords: pituitary trophic hormones, goiter, cell differentiation
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartke A. The response of dwarf mice to murine thyroid-stimulating hormone. Gen Comp Endocrinol. 1968 Aug;11(1):246–247. doi: 10.1016/0016-6480(68)90124-x. [DOI] [PubMed] [Google Scholar]
- Bidey S. P., Marsden P., Anderson J., McKerron C. G., Berry H. In-vitro studies of normal human thyroid cells: responses to thyrotrophin and dibutyryl cyclic AMP. J Endocrinol. 1977 Jan;72(1):87–96. doi: 10.1677/joe.0.0720087. [DOI] [PubMed] [Google Scholar]
- Dickson J. A. Effects of thyrotrophic hormone on thyroid cells in filter-well culture. Endocrinology. 1966 Oct;79(4):721–731. doi: 10.1210/endo-79-4-721. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Fayet G., Pacheco H., Tixier R. Sur la reassociation in vitro des cellules isolees de thyrïde de porc et la biosynthése de la thyroglobuline. I. Conditions pour l'induction des reassociations cellulaires par la thyreostimuline. Bull Soc Chim Biol (Paris) 1970 Apr 17;52(3):299–306. [PubMed] [Google Scholar]
- GEDDA P. O. On the effect of thyrotropic hormone on thyroid in the guinea-pig. Acta Endocrinol Suppl (Copenh) 1960;35(Suppl 56):1–93. [PubMed] [Google Scholar]
- GYLLENSTEN L., JALLING B., TIDEN U. Relative erythrocyte content in the thyroid gland as measurement of the gland's activity. Acta Physiol Scand. 1959 Dec 12;47:328–332. [PubMed] [Google Scholar]
- Gospodarowicz D., Gospodarowicz F. The morphological transformation and inhibition of growth of bovine luteal cells in tissue culture induced by luteinizing hormone and dibutyryl cyclic AMP. Endocrinology. 1975 Feb;96(2):458–467. doi: 10.1210/endo-96-2-458. [DOI] [PubMed] [Google Scholar]
- Greig W. R., Smith J. F., Duguid W. P., Foster C. J., Orr J. S. Assessment of rat thyroid as a radiobiological model of the effects of x-irradiation on cell proliferation and DNA synthesis in vivo. Int J Radiat Biol Relat Stud Phys Chem Med. 1969;16(3):211–225. doi: 10.1080/09553006914551231. [DOI] [PubMed] [Google Scholar]
- HAM R. G. An improved nutrient solution for diploid Chinese hamster and human cell lines. Exp Cell Res. 1963 Feb;29:515–526. doi: 10.1016/s0014-4827(63)80014-2. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsby P. J., Gill G. N. Hormonal control of adrenocortical cell proliferation. Desensitization to ACTH and interaction between ACTH and fibroblast growth factor in bovine adrenocortical cell cultures. J Clin Invest. 1977 Aug;60(2):342–352. doi: 10.1172/JCI108782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERKOF P. R., LONG P. J., CHAIKOFF I. L. IN VITRO EFFECTS OF THYROTROPIC HORMONE. I. ON THE PATTERN OF ORGANIZATION OF MONOLAYER CULTURES OF ISOLATED SHEEP THYROID GLAND CELLS. Endocrinology. 1964 Feb;74:170–179. doi: 10.1210/endo-74-2-170. [DOI] [PubMed] [Google Scholar]
- Knopp J., Stolc V., Tong W. Evidence for the induction of iodide transport in bovine thyroid cells treated with thyroid-stimulating hormone or dibutyryl cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1970 Sep 10;245(17):4403–4408. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lissitzky S., Fayet G., Giraud A., Verrier B., Torresani J. Thyrotrophin-induced aggregation and reorganization into follicles of isolated porcine-thyroid cells. 1. Mechanism of action of thyrotrophin and metabolic properties. Eur J Biochem. 1971 Dec 22;24(1):88–99. doi: 10.1111/j.1432-1033.1971.tb19658.x. [DOI] [PubMed] [Google Scholar]
- Masui H., Garren L. D. Inhibition of replication in functional mouse adrenal tumor cells by adrenocorticotropic hormone mediated by adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3206–3210. doi: 10.1073/pnas.68.12.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASTAN I. Certain functions of isolated thyroid cells. Endocrinology. 1961 Jun;68:924–931. doi: 10.1210/endo-68-6-924. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- Philp J. R., Crooks J., Macgregor A. G., McIntosh J. A. The growth curve of the rat thyroid under a goitrogenic stimulus. Br J Cancer. 1969 Sep;23(3):515–523. doi: 10.1038/bjc.1969.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontén J., Macintyre E. H. Long term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand. 1968;74(4):465–486. doi: 10.1111/j.1699-0463.1968.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Pontén J., Saksela E. Two established in vitro cell lines from human mesenchymal tumours. Int J Cancer. 1967 Sep 15;2(5):434–447. doi: 10.1002/ijc.2910020505. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Suyama A. T. Inhibition of replication of normal adrenocortical cells in culture by adrenocorticotropin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):113–117. doi: 10.1073/pnas.72.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport B. Dog thyroid cells in monolayer tissue culture: adenosine 3', 5'-cyclic monophosphate response to thyrotropic hormone. Endocrinology. 1976 May;98(5):1189–1197. doi: 10.1210/endo-98-5-1189. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Kipnis D. M., Utiger R., Parker C. Radioimmunoassay for the measurement of adenosine 3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1969 Sep;64(1):367–373. doi: 10.1073/pnas.64.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONG W., KERKOF P., CHAIKOFF I. L. Iodine metabolism of dispersed thyroid cells obtained by trypsinization of sheep thyroid glands. Biochim Biophys Acta. 1962 Jun 18;60:1–19. doi: 10.1016/0006-3002(62)90365-7. [DOI] [PubMed] [Google Scholar]
- Weidman R. E., Gill G. N. Differential effects of ACTH or 8-Dr-cAMP on growth and replicationin a functional adrenal tumor cell line. J Cell Physiol. 1977 Jan;90(1):91–103. doi: 10.1002/jcp.1040900112. [DOI] [PubMed] [Google Scholar]
- Westermark B., Wasteson A., Uthne K. Initiation of DNA synthesis of stationary human glia-like cells by a polypeptide fraction from human plasma containing somatomedin activity. Exp Cell Res. 1975 Nov;96(1):58–62. doi: 10.1016/s0014-4827(75)80036-x. [DOI] [PubMed] [Google Scholar]
- Wilson B., Raghupathy E., Tonoue T., Tong W. TSH-like actions of dibutyryl-cAMP on isolated bovine thyroid cells. Endocrinology. 1968 Oct;83(4):877–884. doi: 10.1210/endo-83-4-877. [DOI] [PubMed] [Google Scholar]
- Winand R. J., Kohn L. D. Thyrotropin effects on thyroid cells in culture. Effects of trypsin on the thyrotropin receptor and on thyrotropin-mediated cyclic 3':5'-AMP changes. J Biol Chem. 1975 Aug 25;250(16):6534–6540. [PubMed] [Google Scholar]
- Wollman S. H., Breitman T. R. Changes in DNA and weight of thyroid glands during hyperplasia and involution. Endocrinology. 1970 Feb;86(2):322–327. doi: 10.1210/endo-86-2-322. [DOI] [PubMed] [Google Scholar]



