Abstract
Purpose.
Endothelial cells synthesize vasodilator nitric oxide (NO) and vasoconstrictor endothelin-1 (ET-1) from NO synthase (eNOS) and endothelin-converting enzyme-1 (ECE-1), respectively. Protein kinase C (PKC) and Rho kinase (ROCK) are major signaling molecules mediating vasoconstriction. Although endothelial cells express eNOS, ECE-1, endothelin B (ETB) receptors, PKC, and ROCK, their influences on ET-1–induced vasoconstriction remain elusive. We studied whether these endothelial signaling molecules modulate retinal arteriolar constriction to ET-1.
Methods.
Porcine retinal arterioles were isolated and pressurized for vasomotor study, under conditions with intact or denuded endothelium, using videomicroscopic techniques.
Results.
Retinal arterioles developed similar resting tone (≈45% of maximum diameter) with or without endothelium. Endothelial denudation attenuated vasoconstriction to ET-1 precursor, big ET-1, by almost equal to 50%, but did not affect vasoconstrictions to ET-1, ETB agonist sarafotoxin S6c, or PKC activator phorbol-12, 13-dibutyrate (PDBu). The ROCK inhibitor H-1152 caused vasodilation, and abolished vasoconstrictions to ET-1 and PDBu independent of endothelium. With L-type voltage-operated calcium channel (L-VOCC) blocker nifedipine, PDBu-induced vasoconstriction was abolished and converted to NO-mediated vasodilation in the presence of endothelium. The ET-1–induced vasoconstriction was unaffected by NO released from endothelium during flow elevation.
Conclusions.
Endothelial and smooth muscle ECE-1 contribute equally to synthesis of vasoactive ET-1 in retinal arterioles, with nominal role of endothelial ETB receptors in vasoconstriction to ET-1. The PKC activation leads to endothelium-dependent NO-mediated vasodilation when smooth muscle contraction is ablated by L-VOCC blockade. Endothelial cells and NO appear to have modest roles in modulating ROCK-dependent vasoconstriction, and are insufficient to counteract smooth muscle contractions to ET-1 and PKC activation.
Keywords: endothelins, endothelium, arterioles, PKC, retinal blood flow
Endothelin and protein kinase C (PKC) have been implicated in development of retinal disease, and have vasomotor roles in the retinal circulation. We report on the role of endothelium in vasomotor responses to endothelin system peptides and PKC activation in porcine retinal arterioles.
Introduction
The interplay of vascular endothelium and smooth muscle in the control of retinal arteriolar diameter is essential for retinal blood flow regulation.1 The endothelium produces vasoconstrictor and vasodilator substances, which lead to changes in arteriolar diameter via their effects on adjacent smooth muscle.1 An imbalance in the production or activity of these vasoactive factors may contribute to retinal disease via dysregulation of retinal blood flow. The 21 amino acid peptide endothelin-1 (ET-1) is synthesized by retinal arterioles to exert vasoconstriction, predominantly via endothelin type A (ETA) receptor activation.2 Elevated vitreous and/or plasma levels of ET-1 are associated with several important retinal diseases, including retinal vein occlusion,3 open angle glaucoma,4 and diabetic retinopathy,5,6 and ET-1 may contribute, in part, to these pathologies through its activity as a vasoconstrictor. On the other hand, ET-1 also can activate endothelial ET type B (ETB) receptors to exert vasodilation via released nitric oxide (NO).7–9 Although retinal arterioles express ETB receptors at endothelium and smooth muscle,2 the relative role of endothelial ETB receptors in influencing ET-1–induced vasoconstriction remains unclear. We also have demonstrated previously conversion of big ET-1, the 38 amino acid ET-1 precursor, to vasoactive ET-1 by endothelin converting enzyme-1 (ECE-1) in porcine retinal arterioles.2 Interestingly, ECE-1 is expressed not only in endothelial cells, but also in smooth muscle layers of these vessels.2 However, the relative contribution of endothelial versus smooth muscle ECE-1 for conversion of big ET-1 to vasoactive ET-1 in retinal arterioles is unknown.
Our recent study demonstrates in porcine retinal arterioles that ET-1–induced constriction is mediated by Rho kinase (ROCK), with expression of ROCK1 and ROCK2 isoforms in endothelial and smooth muscle layers.10 While a role for smooth muscle ROCK in vasoconstriction follows from its known activity as an inhibitor of myosin light chain phosphatase,11 endothelial ROCK also may contribute to vasoconstriction via an inhibitory effect upon NO production,12–15 resulting in imbalanced vasoconstrictor/vasodilator effects. Whether endothelial ROCK influences ET-1–induced retinal arteriolar constriction has not been addressed to our knowledge.
In a manner similar to ET-1, we also have shown that retinal arteriolar constriction to protein kinase C (PKC) activation by phorbol-12, 13-dibutyrate (PDBu) is mediated by ROCK signaling.10 However, it is unclear whether endothelial ROCK can influence PDBu-induced constriction of retinal arterioles. Moreover, in addition to vascular smooth muscle contraction, it has been shown in cultured endothelial cells that PKC activation can mediate increased production of vasodilator substances, such as NO16,17 and prostacyclin (PGI2).18 Because increased expression and/or activity of PKC has been implicated as a contributor to diabetic retinopathy19 and other retinal diseases,20 it is important to know whether endothelial PKC can modulate vasoconstriction of retinal arterioles.
In our study, we addressed the above issues by studying the role of endothelial and smooth muscle ECE-1 in vasomotor regulation, the contribution of endothelial ETB receptors to retinal arteriolar constriction to ET-1, and the roles of endothelial NO and ROCK in vasoconstrictions to ET-1 and PKC activation. An isolated vessel approach was used to obviate the potentially confounding influences on vasomotor activity from surrounding neuroglial tissue or hemodynamic changes that are inherent to in vivo preparations.
Methods
Animal Preparation
All animal procedures were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by the Scott and White Institutional Animal Care and Use Committee. Pigs of either sex (age range, 8–12 weeks; weight, 8–12 kg) purchased from Real Farms (San Antonio, TX) were sedated with Telazol (4.4 mg/kg, intramuscularly; TW Medical Veterinary Supply, Austin, TX), anesthetized with 2% to 5% isoflurane, and intubated. The procedure used for harvesting eyes has been described previously.2
Isolation, Denudation, and Cannulation of Microvessels
The techniques used for identification, isolation, cannulation, pressurization, and visualization of the retinal vasculature have been described previously.2,21 Isolated retinal arterioles (∼80 μm in situ) were cannulated with a pair of glass micropipettes and pressurized to 55 cmH2O intraluminal pressure.22 In a series of experiments involving intraluminal flow, the electrical resistances (measured by a BK Precision Universal LCR Meter, model 878; BK Precision Corporation, Yorba Linda, CA) of the two micropipettes used were matched (≤1% difference). Luminal flow was generated by adjusting the relative heights of the inflow and outflow reservoirs in opposite directions of the same magnitude, thereby producing a pressure gradient for flow across the length of the vessel without changing intraluminal pressure.21 Vasomotor activity of isolated vessels was recorded using videomicroscopic techniques23 throughout the experiments. Endothelial denudation was achieved by injecting air from one end of a vessel through the lumen for two minutes, as reported previously.24 After denudation, the opposite end of the vessel was cannulated and pressurized as described above. The denuded vessels that exhibited normal resting tone showed no vasodilation to endothelium-dependent vasodilator bradykinin (10 nM), and showed unaltered response to endothelium-independent vasodilator sodium nitroprusside (SNP, 1 μM) were accepted for data analysis.
Study of Vasomotor Function
Cannulated, pressurized arterioles were bathed in physiological saline solution (PSS) with 0.1% albumin (PSS-albumin; Affymetrix, Cleveland, OH) at a temperature of 36°C to 37°C to permit development of resting tone (stabilized within 60–90 minutes). To determine the role of endothelial and smooth muscle ECE-1 in the conversion of big ET-1 to ET-1, control and denuded arterioles with stable resting tone were exposed to big ET-1 (0.1 μM) for 20 minutes, and diameter changes were monitored. To address the contribution of NO, the retinal arteriolar response to accumulative increases in ET-1 concentration (1 × 10−13 M to 1 × 10−8 M) was assessed in the absence and presence of NOS inhibitor LG-nitro-L-arginine methyl ester25 (L-NAME, 10 μM). Vessels were exposed to L-NAME for at least 30 minutes before the addition of ET-1. The relative role of endothelial versus smooth muscle ETB receptors in retinal arterioles was determined by exposing vessels with and without intact endothelium to the selective ETB receptor agonist sarafotoxin S6c26 (10 nM; Tocris Bioscience, Ellisville, MO) and recording diameter changes after 20 minutes.
The role of endothelial versus smooth muscle ROCK in regulation of resting arteriolar diameter was assessed by exposing arterioles with and without endothelium to increasing concentrations of the nonselective ROCK inhibitor H-1152 (1 × 10−9 M to 1 × 10−6 M; EMD Chemicals, Gibbstown, NJ) and recording diameter changes for 5 minutes following addition of each concentration. In another cohort, the role of endothelial ROCK in ET-1–induced vasoconstriction was assessed by exposing vessels with and without endothelium to ET-1 (0.1 nM; BaChem, Bubendorf, Switzerland). After reaching a new stable diameter at 20 minutes after ET-1 administration, vessels then were treated with H-1152 (3 μM) and diameter changes were recorded for an additional 20 minutes.
To explore the role of endothelial ROCK in retinal arteriolar responses to PKC activation, retinal arterioles with and without endothelium were treated with PDBu (0.1 μM) for 20 minutes. After establishing stable constriction to PDBu, vessels were incubated with H-1152 (3 μM) for 20 minutes and diameter changes were recorded. To determine whether endothelial PKC activation linked to NOS or cyclooxygenase contributes to vasomotor regulation when smooth muscle contraction is compromised, retinal arterioles with and without endothelium were first incubated with the dihydropyridine L-type voltage-operated calcium channel (L-VOCC) blocker nifedipine (1 μM), or with nifedipine plus L-NAME (10 μM) or cyclooxygenase inhibitor indomethacin (10 μM) for 20 minutes, and then the vasomotor response to PDBu (0.1 μM) was assessed.
We have shown previously that retinal arterioles dilate to increased luminal flow via a mechanism related to NO release from the endothelium.21 The potential influence of endothelial NO on ET-1–induced vasoconstriction was explored in our study. The vessels with intact endothelium were subjected to luminal flow by generating a pressure gradient (60 cmH2O) across the cannulating micropipettes as described previously.21 After the vessel diameter stabilized following flow initiation (∼10 minutes), the constriction to ET-1 (0.1 nM) was assessed. In this set of studies, the experiments (ET-1 response assessed under conditions without and with luminal flow) were performed in the vessels from the same animal for paired comparison. At the end of each experiment described above, maximum vessel diameter was obtained by incubating vessels with a Ca2+-free PSS-albumin solution containing 0.3 mM SNP.
Chemicals
All chemical reagents were obtained from Sigma-Aldrich (St. Louis, MO) except as specifically stated. The ET-1, H-1152, big ET-1, L-NAME, and sarafotoxin S6c were dissolved initially in water. Nifedipine and PDBu were dissolved initially in ethanol and dimethyl sulfoxide, respectively. All subsequent dilutions of drugs for use in experiments were performed using PSS. The final concentrations of ethanol and dimethyl sulfoxide in the vessel bath were 0.01% and 0.001% by volume, respectively. These solvent concentrations had no significant effect on vessel viability or vasomotor activity (data not shown).
Data Analysis
Vessel diameters were normalized to resting diameter (observed before addition of agonist or inhibitor) to obtain percentage resting diameter. For the experiment assessing the effect of flow-mediated vasodilation on ET-1–induced constriction, the vessel diameter stabilized after 10 minutes of luminal flow stimulation was taken to be the resting diameter. The difference between the percentage resting diameter before addition of sarafotoxin S6c (see Fig. 3) or ET-1 (see Fig. 6) and the percentage resting diameter after 20 minutes of incubation with these peptides is defined as percentage change in resting diameter. As appropriate, Student's t-test and repeated or nonrepeated measures ANOVA with Tukey's multiple comparisons test were used to determine the level of significance of experimental interventions. Statistical analyses were done using Prism software (GraphPad, San Diego, CA). Data are reported as mean ± SEM, P < 0.05 was considered significant, and n represents number of vessels (1 per pig per treatment group).
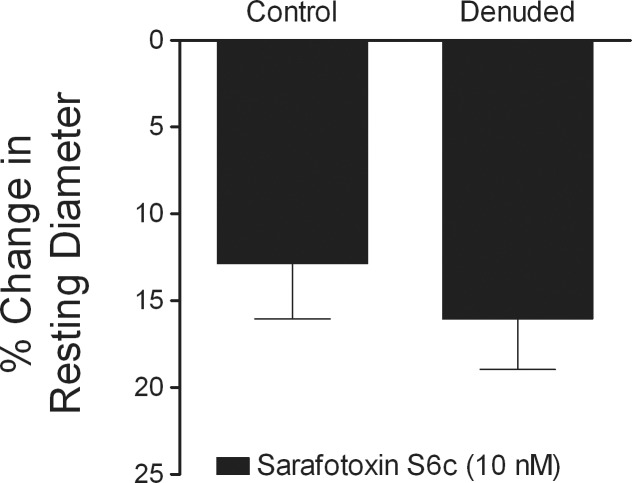
Figure 3.
Lack of functional vasomotor role for endothelial ETB receptors. Control (n = 7) and denuded (n = 7) vessels with resting tone were treated with sarafotoxin S6c (10 nM) for 20 minutes to produce vasoconstriction.
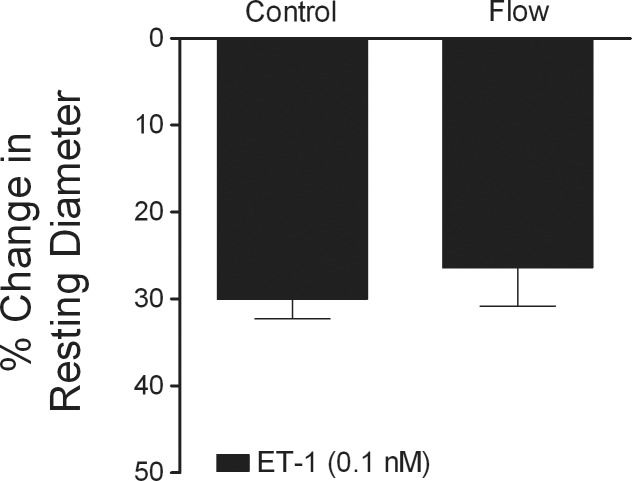
Figure 6.
Flow-induced production of vasodilator NO does not influence ET-1–induced constriction. Control retinal arterioles with resting tone (n = 5) were constricted with ET-1 (0.1 nM) for 20 minutes. Another group of vessels with resting tone was subjected to luminal flow stimulation (60 cmH2O pressure gradient) for 10 minutes (n = 5), and then treated with ET-1 (0.1 nM) for 20 minutes.
Results
Endothelial and Smooth Muscle ECE-1 in ET-1 Synthesis
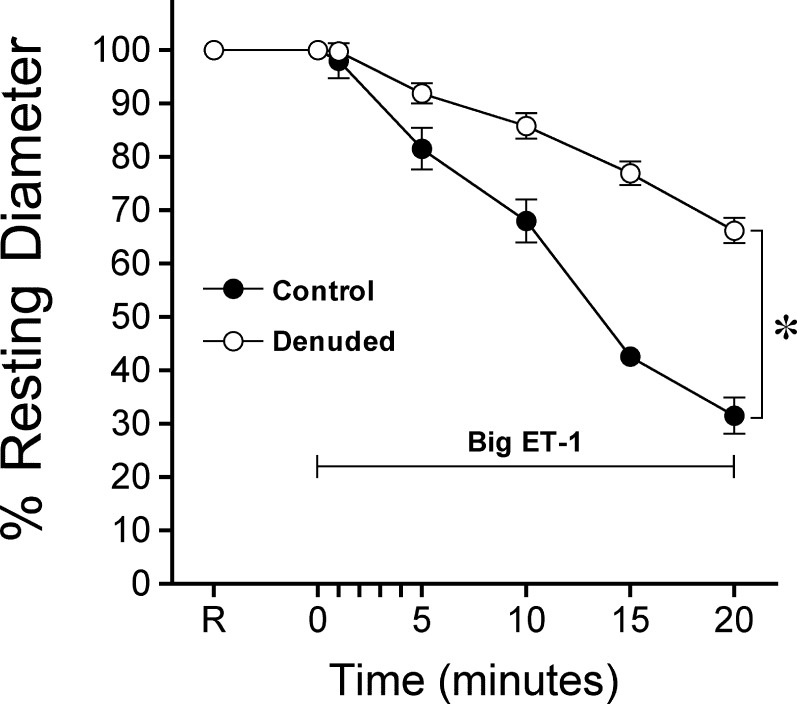
Isolated retinal arterioles developed stable tone (45 ± 1% of maximum diameter, n = 70), with resting and maximum diameter 41 ± 1 and 93 ± 1 μm, respectively. Endothelial removal affected neither resting tone (44 ± 2% of maximum diameter, n = 34), nor the resting (40 ± 1 μm) and maximum (90 ± 2 μm) diameters, but abolished dilation to the endothelium-dependent vasodilator bradykinin27,28 (10 nM; control, 74 ± 3% versus denuded, 0 ± 1% maximum dilation) without affecting the response to endothelium-independent vasodilator sodium nitroprusside (10 μM; control, 60 ± 7% versus denuded, 64 ± 5% maximum dilation). As shown in Figure 1, administration of big ET-1 (0.1 μM) caused a gradual constriction of retinal arterioles and this vasoconstriction was attenuated by almost equal to 50% in the denuded vessels.
Figure 1.
Endothelial ECE-1 involvement in constriction of retinal arterioles to big ET-1. Temporal course of vasoconstriction induced by big ET-1 (0.1 μM) was recorded in the presence (n = 7) or absence (n = 8) of endothelium. Control and denuded vessels with resting tone were treated with big ET-1 (0.1 μM) for 20 minutes. *P < 0.05 control versus denuded percentage resting diameters observed at 10, 15, and 20 minutes. R, resting diameter of vessels.
Vasomotor Influence of Endothelial ETB Receptors
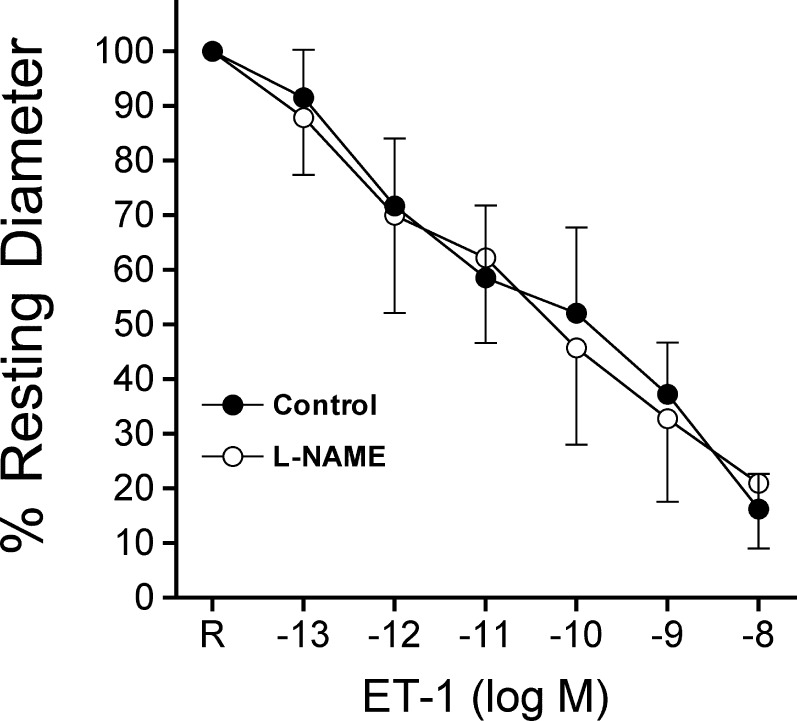
Vessels with intact endothelium constricted in a concentration-dependent manner to ET-1, and this response was not affected by L-NAME (10 μM, Fig. 2). The L-NAME treatment had no effect upon resting tone of retinal arterioles (control, 51 ± 3% of maximum diameter versus L-NAME, 46 ± 3% of maximum diameter, P = 0.34). Administration of ETB receptor agonist sarafotoxin S6c (10 nM) caused a small, but significant reduction of retinal arteriolar diameter, which was not affected by endothelial removal (Fig. 3).
Figure 2.
Lack of NO production in retinal arterioles in response to ET-1. Control (n = 4) and L-NAME pretreated (n = 4) vessels with intact endothelium and resting tone were exposed to increasing concentrations of ET-1 for 5 minutes at each concentration.
Influence of Endothelial ROCK on Vascular Response to ET-1
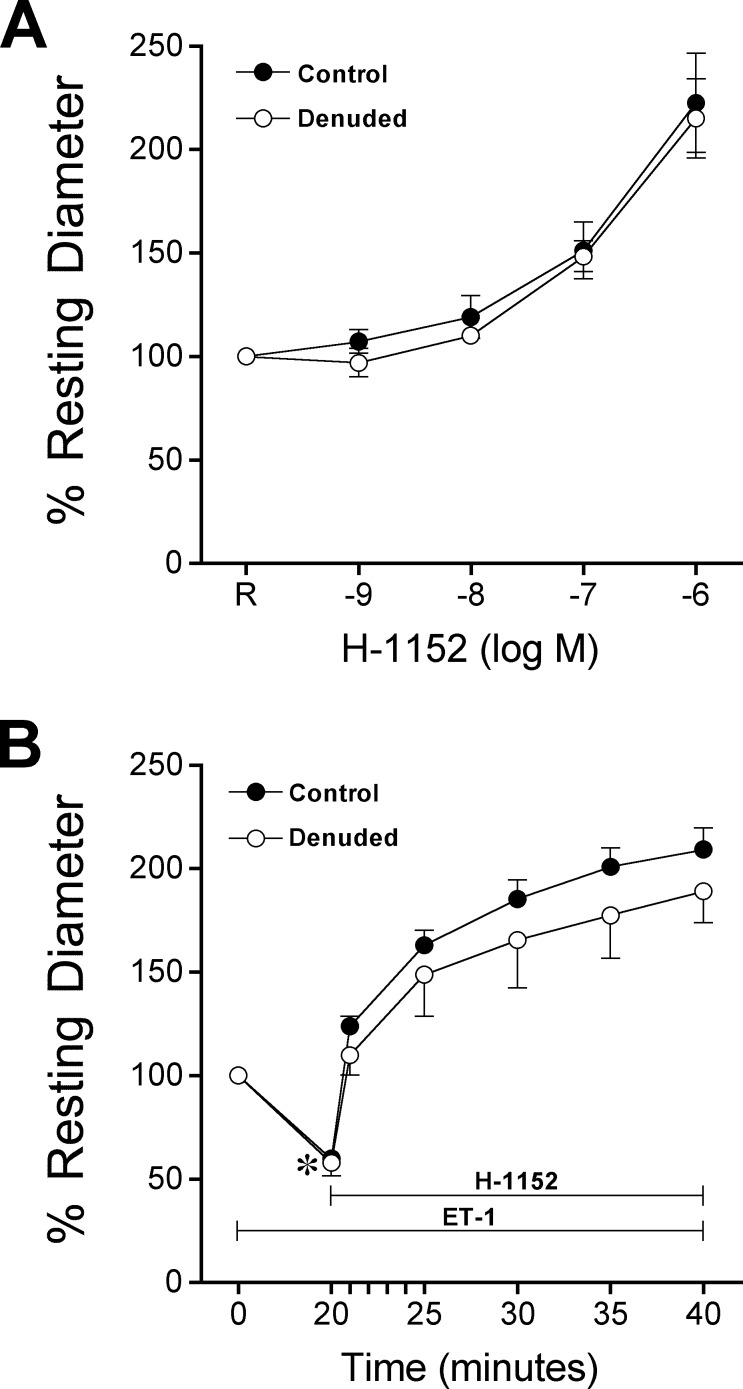
As shown in Figure 4A, retinal arterioles dilated (reached stable diameter in 5 minutes) to the ROCK inhibitor H-1152 in a concentration-dependent manner. This vasodilator response was not affected by endothelial removal (Fig. 4A). Administration of ET-1 (0.1 nM) to retinal arterioles caused a gradual vasoconstriction (i.e., ≈50% reduction in resting diameter) that stabilized within 20 minutes (Fig. 4B). This vasoconstriction was reversed by subsequent administration of H-1152 (3 μM) to the vessels (Fig. 4B). Endothelial removal did not affect vasoconstriction to ET-1 and the reversal effect of H-1152 (Fig. 4B).
Figure 4.
Smooth muscle ROCK maintains resting tone and mediates ET-1–induced constriction. (A) Control (n = 7) and denuded (n = 6) vessels with resting tone were treated with increasing concentrations of H-1152 for 5 minutes at each concentration. (B) Control (n = 7) and denuded (n = 7) vessels with resting tone were treated with ET-1 (0.1 nM) for 20 minutes to produce vasoconstriction, followed by administration of H-1152 (3 μM) for 20 minutes. *P < 0.05 versus resting diameter at 0 minutes.
Influence of Endothelial ROCK and PKC Activation on Vascular Response to PDBu
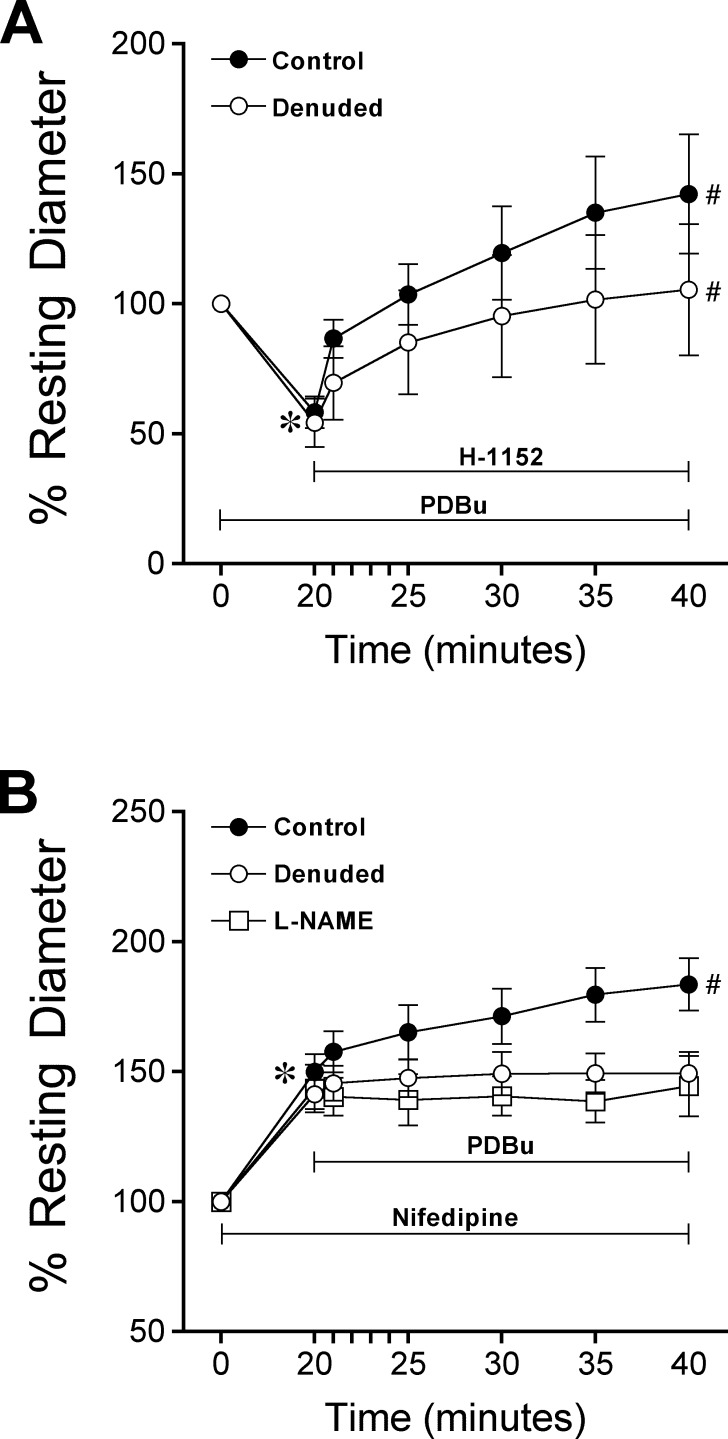
Exposure of retinal arterioles to the PKC activator PDBu (0.1 μM) caused significant vasoconstriction and reached a new stable diameter within 20 minutes (Fig. 5A). Subsequent treatment of the vessels with H-1152 caused reversal of PDBu-induced vasoconstriction (Fig. 5A). Endothelial denudation did not alter vasoconstriction to PDBu or the reversal effect of H-1152 (Fig. 5A). In another group, administration of nifedipine (1 μM) to retinal arterioles with or without intact endothelium for 20 minutes resulted in a similar degree of vasodilation (i.e., 50 ± 7% and 41 ± 6% above resting diameter in control and denuded vessels, respectively). Subsequent treatment of the vessels with PDBu (0.1 μM) for 20 minutes caused further dilation (by 34 ± 5% of resting diameter) in endothelium-intact vessels, but no change in diameter in denuded vessels (Fig. 5B). Moreover, this PDBu-induced endothelium-dependent vasodilation was abolished in the presence of L-NAME (Fig. 5B), but not indomethacin (dilation of control versus indomethacin-treated vessel at 40 minutes, 74 ± 8% vs. 73 ± 7% above resting diameter, P = 0.92, n = 3).
Figure 5.
Role of endothelium in PKC activation-induced vasomotor activity. (A) Control (n = 7) and denuded (n = 7) vessels with resting tone were treated with PDBu (0.1 μM) for 20 minutes, followed by incubation with H-1152 (3 μM) for 20 minutes. *P < 0.05 versus resting diameter at 0 minutes. #P < 0.05 versus diameter at time of H-1152 addition (20 minutes). (B) Pretreatment of control (n = 7) and denuded (n = 7) vessels with nifedipine (1 μM) for 20 minutes was followed by PDBu (0.1 μM) treatment for 20 minutes. In another set of experiments, control vessels were pretreated with nifedipine plus L-NAME (10 μM, n = 3) before administration of PDBu. *P < 0.05 versus resting diameter at 0 minutes. #P < 0.05, diameter at 40 minutes versus diameter at 20 minutes.
Influence of NO-Mediated Vasodilation on Vascular Response to ET-1
Exposure of retinal arterioles with intact endothelium to luminal flow (i.e., 60 cmH2O pressure gradient) resulted in vasodilation to 68 ± 2% of maximum diameter (data not shown), consistent with our previous results.21 Subsequent treatment of these vessels with ET-1 (0.1 nM) yielded vasoconstriction that was equivalent to that observed in vessels without intraluminal flow (Fig. 6).
Discussion
The regulation of vascular tone via vasodilator and vasoconstrictor influences is essential for maintenance of retinal blood flow, and perturbations of these vasomotor responses can contribute to retinal pathology.1 In the current study, we provided, to our knowledge, the first direct evidence for the relative contribution of the endothelium to vasomotor responses to the ET-1 system and PKC activation in isolated porcine retinal arterioles. We showed that endothelial and smooth muscle ECE-1 are involved in ET-1 production, but there is no role for endothelial ETB receptors in vasomotor control of retinal arterioles. The ET-1–induced retinal arteriolar constriction is mediated entirely through ROCK signaling in smooth muscle cells, and endothelial production of vasodilator NO does not influence vasoconstrictor activity elicited by ET-1. We also have shown the importance of the endothelium and NOS in exerting vasodilation to PKC activation when the responsible smooth muscle signaling, that is, L-VOCC activation, for contraction is disabled.
Our previous demonstration of endothelial and smooth muscle ECE-1 expression in porcine retinal arterioles2 suggests possible production of vasoactive ET-1 at both sites. Functional evidence in our study showing the almost equal to 50% reduction in constriction of denuded retinal arterioles to big ET-1 supports the equal importance of endothelial and smooth muscle layers contributing to the overall production of ET-1. In contrast with our findings, vasoconstriction to big ET-1 was shown to be endothelium-independent in porcine coronary arteries,29 and in rabbit basilar30 and saphenous31 arteries. Hence, an essentially equal functional role for endothelium and smooth muscle in processing exogenous big ET-1 to vasoactive ET-1 appears to be a unique feature of the retinal arterioles. Whether this is the case in other microvascular beds is unknown. Incubation of vascular endothelial cells with high glucose has been associated with increased ECE expression32 and ET-1 production.33 Moreover, since intravitreal treatment with pharmacologic blockade of ECE in vivo has been shown to improve retinal blood flow in early diabetes in rats,34 increased ECE activity/expression in diabetic retinal arterioles possibly could contribute to the observed flow deficiency.34,35 Although the direct evidence for an adverse role of local ECE-1 in the retinal arterioles during early diabetes remains unclear, our current findings on the equal contribution of endothelial and smooth muscle ECE-1 to ET-1 production should be considered.
Endothelin-1 has been suggested to stimulate vasorelaxation through endothelial NO release downstream of ETB receptor stimulation.7,8 In anesthetized dogs, when the NOS inhibitor NG-monomethyl-L-arginine (L-NMMA) and ET-1 were infused together intravenously, a greater increase in systemic vascular resistance was observed compared to that observed in the presence of ET-1 alone,36 suggesting that NO production, possibly via ETB receptor activation,37,38 can mitigate vasoconstrictor responses to ET-1. The potential role of NO in these previous observations is supported by a study using bioassay techniques in which administration of donor perfusate from freshly isolated endothelial cells treated with ET-1 enhances vasorelaxation of recipient arterial strips in a manner sensitive to NO scavenging.39 Moreover, selective ETB receptor stimulation causes retinal arteriolar vasoconstriction that is weaker than that in response to ET-1,2 possibly a result of endothelial ETB-induced production of NO.39 However, using an isolated vessel approach, we found in our study that inhibition of NOS had no effect on vasoconstriction to ET-1 (Fig. 2) and the vasoconstriction to ETB receptor activation was not altered by endothelial removal (Fig. 3). It appears that ET-1 does not lead to NO production to functionally counteract its vasoconstrictor activity in retinal arterioles. We previously showed that the smooth muscle-localized endothelin ETA receptors mediate retinal arteriolar constriction to ET-1 with nominal contribution from ETB, although there is ETB receptor expression in endothelial and smooth muscle cells.2 Interestingly, intravitreal injection of endothelin isoform, ET-3, has been shown to elicit an ETB-dependent NO-mediated increase in retinal blood flow in rats.40 However, we observed only modest concentration-dependent constriction of porcine retinal arterioles in response to ET-3 (n = 5, data not shown). The vasoconstriction (32 ± 6%) at the highest concentration of ET-3 (10 nM) was sensitive to ETB receptor antagonist BQ788. The species difference and/or the initiation of other indirect mechanisms by ET-3 in the above in vivo preparation in which the direct impact of this peptide on retinal arterioles was not verified might explain the observed discrepancy. Nonetheless, our data did not support a vasoactive role of endothelial ETB receptors in retinal arterioles, in contrast with their smooth muscle counterparts, which promote weak vasoconstriction (Fig. 3). This is in agreement with results from a study showing that ETB receptor blockade had no effect on ET-1–induced constriction of coronary arterioles from young rats.41 Taken together, these diverse findings may suggest heterogeneity among microvascular beds in whether ET-1 stimulation elicits endothelial ETB-mediated dilation, or that there may be activation of other signaling pathways for NO release secondary to ET-1 stimulation.
We have shown previously in retinal arterioles that ROCK signaling contributes to the maintenance of resting tone and ET-1–induced constriction.10 In the cell-culture system, activation of the RhoA/ROCK pathway appears to regulate negatively NO synthase (eNOS) activity in human umbilical vein endothelial cells13 and inhibit NO production in bovine aortic endothelial cells.42 Because endothelial and smooth muscle cells express ROCK isoforms in retinal arterioles,10 inhibition of ROCK is expected to promote smooth muscle relaxation by enhancing NO production from the endothelium in addition to the activation of myosin light chain phosphatase11 in smooth muscle cells. Indeed, ROCK inhibition caused retinal arteriolar dilation, but this response was not altered by endothelial denudation (Fig. 4A). These results suggest that tonic activation of ROCK in the smooth muscle is essential for maintenance of resting tone. However, ROCK appears to have little role in modulating eNOS activity, or alternatively, ROCK in endothelial cells may be inactive under resting conditions in terms of exerting eNOS regulation. Notably, endothelial ROCK activity seemingly becomes prominent under disease states,15,43 so its potential role in the development of retinal vascular disease deserves further evaluation.
In contrast with our findings presented in Figure 4B, the constrictor response to ET-1 was increased by endothelial denudation of porcine coronary,39 cat cerebral,44 and human internal mammary arteries.45 The discrepancy between those results and our findings may be due to differences in vessel preparation or vessel size. Nevertheless, the observed lack of a modulatory role of endothelium in the ET-1 constrictor response (Fig. 4B) is consistent with the absence of functional ETB receptors in the endothelium (Fig. 3). Our studies demonstrated that ET-1–induced constriction is mediated through ROCK signaling in smooth muscle alone, and the potential effects of endothelial ROCK on eNOS as discussed above appear to be insignificant in retinal arterioles upon ET-1 stimulation.
Our current data indicated that retinal arteriolar constriction resulting from PKC activation is independent of the presence of endothelium and is mediated through smooth muscle ROCK (Fig. 5A). We demonstrated previously that ET-1–induced constriction of retinal arterioles is independent of L-VOCC signaling, but activation of these channels is essential for PDBu-induced constriction of the same vessels.10 Interestingly, blockade of L-VOCC converted PDBu-induced constriction into dilation in a manner sensitive to endothelial removal (Fig. 5B). This result is consistent with several reports showing the activation of eNOS in association with increased femoral artery blood flow in rats overexpressing PKCα,16 and the augmentation of PGI218 or NO46 production by human umbilical vein endothelial cells in response to PKC activation. Endothelial PKC activation in intact porcine retinal arterioles appears to lead to production of NO, and its vasodilator action may be unmasked when the responsible signaling pathway, that is, L-VOCC activation, for smooth muscle contraction is disrupted (Fig. 5B). This may explain the observed greater reductions in overall mortality and cardiovascular events with L-VOCC inhibition in diabetic hypertensive patients compared to nondiabetic hypertensives,47 since diabetes has been associated with PKC activation.48 Notably, several studies have linked increased PKC activity to retinal blood flow abnormalities in diabetes.49–51 The results presented herein suggested that altered retinal vascular PKC expression/activity could have divergent vasomotor effects, depending on the site, that is, endothelium versus smooth muscle, of such changes.
Although our data did not support the involvement of NO in the ET-1–mediated response, there is a potential for endothelium-produced NO to influence ET-1–induced constriction upon stimulation. In the in vivo situation, the vascular endothelial cells are exposed continuously to shear stress, leading to a tonic production of NO from the endothelium.21,22,52 Under this condition, endothelial modulation of responses to ET-1 might be apparent, since endothelial NO production has been suggested to mitigate vasoconstriction to ET-1 in large conduit arteries.36,45 However, as shown in Figure 6, the presence of luminal flow had no effect on ET-1–induced vasoconstriction, suggesting that the released NO during shear stress stimulation21 does not prevail over the constrictor effect of ET-1. This is in agreement with our recent finding that treatment of retinal arterioles with exogenous NO causes a reduction in resting tone, but does not affect vasoconstriction to ET-1.10 It appears that the NO release upon shear stress stimulation is insufficient to counteract the constrictor action of ET-1 in the porcine retinal microvasculature.
In summary, our current findings demonstrated the equal importance of endothelial and smooth muscle ECE-1 in processing big ET-1 to vasoactive ET-1 in porcine retinal arterioles. Additionally, endothelial ETB receptors, endothelial ROCK, and eNOS do not contribute to vasomotor regulation of retinal arterioles in response to ET-1, but endothelial NO appears to modulate vasomotor function in response to PKC activation, especially when smooth muscle contraction is blunted. Interestingly, it was shown recently in a porcine model of type 1 diabetes that, while endothelium-dependent NO-mediated dilation of retinal arterioles is impaired, vasoconstriction to ET-1 is not altered.53 Since endothelial NO is not sufficient to modulate retinal arteriolar constriction to ET-1, the vascular endothelial dysfunction present in retinal pathologies, such as diabetes53,54 and glaucoma,55 may not necessarily result in increased retinal arteriolar constriction to ET-1 in vivo. Nonetheless, because increased ET-1 and PKC production/activity and endothelial dysfunction have been implicated in retinal disease, elucidation of the contribution of endothelium upon PKC/ET-1 system activation is important to our understanding how imbalances in vasomotor activity in the retinal vasculature may contribute to retinal disease. The role of ET-1 and PKC in retinal vascular pathophysiology deserves further investigation.
Acknowledgments
Supported by Retina Research Foundation (LK), Kruse Chair Endowment Fund (LK), and NIH EY018420 (TWH).
Disclosure: L.B. Potts, None; P.D. Bradley, None; W. Xu, None; L. Kuo, None; T.W. Hein, None
References
- 1. Haefliger IO, Flammer J, Bény JL, Lüscher TF. Endothelium-dependent vasoactive modulation in the ophthalmic circulation. Prog Retin Eye Res. 2001; 20: 209–225 [DOI] [PubMed] [Google Scholar]
- 2. Hein TW, Ren Y, Yuan Z, et al. Functional and molecular characterization of the endothelin system in retinal arterioles. Invest Ophthalmol Vis Sci. 2009; 50: 3329–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iannaccone A, Letizia C, Pazzaglia S, Vingolo EM, Clemente G, Pannarale MR. Plasma endothelin-1 concentrations in patients with retinal vein occlusions. Br J Ophthalmol. 1998; 82: 498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Emre M, Orgul S, Haufschild T, Shaw SG, Flammer J. Increased plasma endothelin-1 levels in patients with progressive open angle glaucoma. Br J Ophthalmol. 2005; 89: 60–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deng D, Evans T, Mukherjee K, Downey D, Chakrabarti S. Diabetes-induced vascular dysfunction in the retina: role of endothelins. Diabetologia. 1999; 42: 1228–1234 [DOI] [PubMed] [Google Scholar]
- 6. Ergul A. Endothelin-1 and diabetic complications: focus on the vasculature. Pharmacol Res. 2011; 63: 477–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gurbanov K, Rubinstein I, Hoffman A, Abassi Z, Better OS, Winaver J. Differential regulation of renal regional blood flow by endothelin-1. Am J Physiol Renal Physiol. 1996; 271: F1166–F1172 [DOI] [PubMed] [Google Scholar]
- 8. Schilling L, Feger GI, Ehrenreich H, Wahl M. Endothelin-1-induced contraction and relaxation of isolated rat basilar artery: effect of the endothelium. J Cardiovasc Pharmacol. 1995; 26: 197–199 [PubMed] [Google Scholar]
- 9. Liu S, Premont RT, Kontos CD, Huang J, Rockey DC. Endothelin-1 activates endothelial cell nitric-oxide synthase via heterotrimeric G-protein βγ subunit signaling to protein kinase B/Akt. J Biol Chem. 2003; 278: 49929–49935 [DOI] [PubMed] [Google Scholar]
- 10. Potts LB, Ren Y, Lu G, et al. Constriction of retinal arterioles to endothelin-1: requisite role of Rho kinase independent of protein kinase C and L-type calcium channels. Invest Ophthalmol Vis Sci. 2012; 53: 2904–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loirand G, Guérin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006; 98: 322–334 [DOI] [PubMed] [Google Scholar]
- 12. Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998; 273: 24266–24271 [DOI] [PubMed] [Google Scholar]
- 13. Ming XF, Viswambharan H, Barandier C, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002; 22: 8467–8477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Budzyn K, Marley PD, Sobey CG. Opposing roles of endothelial and smooth muscle phosphatidylinositol 3-kinase in vasoconstriction: effects of Rho-kinase and hypertension. J Pharmacol Exp Ther. 2005; 313: 1248–1253 [DOI] [PubMed] [Google Scholar]
- 15. Nohria A, Grunert ME, Rikitake Y, et al. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res. 2006; 99: 1426–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Partovian C, Zhuang Z, Moodie K, et al. PKCα activates eNOS and increases arterial blood flow in vivo. Circ Res. 2005; 97: 482–487 [DOI] [PubMed] [Google Scholar]
- 17. Li H, Oehrlein SA, Wallerath T, et al. Activation of protein kinase C α and/or ε enhances transcription of the human endothelial nitric oxide synthase gene. Mol Pharmacol. 1998; 53: 630–637 [DOI] [PubMed] [Google Scholar]
- 18. Garcia JG, Stasek J, Natarajan V, Patterson CE, Dominguez J. Role of protein kinase C in the regulation of prostaglandin synthesis in human endothelium. Am J Respir Cell Mol Biol. 1992; 6: 315–325 [DOI] [PubMed] [Google Scholar]
- 19. Frank RN. Potential new medical therapies for diabetic retinopathy: protein kinase C inhibitors. Am J Ophthalmol. 2002; 133: 693–698 [DOI] [PubMed] [Google Scholar]
- 20. Titchenell PM, Lin CM, Keil JM, Sundstrom JM, Smith CD, Antonetti DA. Novel atypical PKC inhibitors prevent vascular endothelial growth factor-induced blood-retinal barrier dysfunction. Biochem J. 2012; 446: 455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hein TW, Rosa RH Jr, Yuan Z, Roberts E, Kuo L. Divergent roles of nitric oxide and Rho kinase in vasomotor regulation of human retinal arterioles. Invest Ophthalmol Vis Sci. 2010; 51: 1583–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol. 1990; 259: H1063–H1070 [DOI] [PubMed] [Google Scholar]
- 23. Hein TW, Yuan Z, Rosa RH Jr, Kuo L. Requisite roles of A2A receptors, nitric oxide, and KATP channels in retinal arteriolar dilation in response to adenosine. Invest Ophthalmol Vis Sci. 2005; 46: 2113–2119 [DOI] [PubMed] [Google Scholar]
- 24. Hein TW, Xu W, Ren Y, Kuo L. Cellular signalling pathways mediating dilation of porcine pial arterioles to adenosine A2A receptor activation. Cardiovasc Res. 2013; 99: 156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pfeiffer S, Leopold E, Schmidt K, Brunner F, Mayer B. Inhibition of nitric oxide synthesis by NG-nitro-L-arginine methyl ester (L-NAME): requirement for bioactivation to the free acid, NG-nitro-L-arginine. Br J Pharmacol. 1996; 118: 1433–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams DL Jr, Jones KL, Pettibone DJ, Lis EV, Clineschmidt BV. Sarafotoxin S6c: an agonist which distinguishes between endothelin receptor subtypes. Biochem Biophys Res Commun. 1991; 175: 556–561 [DOI] [PubMed] [Google Scholar]
- 27. Nagaoka T, Hein TW, Yoshida A, Kuo L. Simvastatin elicits dilation of isolated porcine retinal arterioles: role of nitric oxide and mevalonate-Rho kinase pathways. Invest Ophthalmol Vis Sci. 2007; 48: 825–832 [DOI] [PubMed] [Google Scholar]
- 28. Jeppesen P, Aalkjaer C, Bek T. Bradykinin relaxation in small porcine retinal arterioles. Invest Ophthalmol Vis Sci. 2002; 43: 1891–1896 [PubMed] [Google Scholar]
- 29. Fukuroda T, Noguchi K, Tsuchida S, et al. Inhibition of biological actions of big endothelin-1 by phosphoramidon. Biochem Biophys Res Commun. 1990; 172: 390–395 [DOI] [PubMed] [Google Scholar]
- 30. Petersson J, Hanson GC, Lindberg BF, Högestätt ED. Contractile effect of big endothelin-1 and its conversion to endothelin-1 in rabbit cerebral arteries. Naunyn Schmiedebergs Arch Pharmacol. 1996; 354: 656–661 [DOI] [PubMed] [Google Scholar]
- 31. Ferlenga P, Morazzoni G, Da Ros B, et al. Characterization of big endothelin-1-induced contraction in rabbit saphenous artery. J Cardiovasc Pharmacol. 1995; 26: 78–80 [PubMed] [Google Scholar]
- 32. Keynan S, Khamaisi M, Dahan R, et al. Increased expression of endothelin-converting enzyme-1c isoform in response to high glucose levels in endothelial cells. J Vasc Res. 2004; 41: 131–140 [DOI] [PubMed] [Google Scholar]
- 33. Yamauchi T, Ohnaka K, Takayanagi R, Umeda F, Nawata H. Enhanced secretion of endothelin-1 by elevated glucose levels from cultured bovine aortic endothelial cells. FEBS Lett. 1990; 267: 16–18 [DOI] [PubMed] [Google Scholar]
- 34. Takagi C, Bursell SE, Lin YW, et al. Regulation of retinal hemodynamics in diabetic rats by increased expression and action of endothelin-1. Invest Ophthalmol Vis Sci. 1996; 37: 2504–2518 [PubMed] [Google Scholar]
- 35. Clermont AC, Bursell SE. Retinal blood flow in diabetes. Microcirculation. 2007; 14: 49–61 [DOI] [PubMed] [Google Scholar]
- 36. Lerman A, Sandok EK, Hildebrand FL Jr, Burnett JC Jr. Inhibition of endothelium-derived relaxing factor enhances endothelin-mediated vasoconstriction. Circulation. 1992; 85: 1894–1898 [DOI] [PubMed] [Google Scholar]
- 37. Ekelund U, Adner M, Edvinsson L, Mellander S. Effects of the combined ETA and ETB receptor antagonist PD145065 on arteries, arterioles, and veins in the cat hindlimb. J Cardiovasc Pharmacol. 1995; 26 (suppl 3): S211–S213 [PubMed] [Google Scholar]
- 38. Ohno T, Katori M, Majima M, Hayashi H, Saigenji K. Different responses of arterioles and venules in rat gastric mucosal microcirculation to endothelin-1 and endothelin-3. J Clin Gastroenterol. 1995; 21 (suppl 1): S56–S65 [PubMed] [Google Scholar]
- 39. Suzuki S, Kajikuri J, Suzuki A, Itoh T. Effects of endothelin-1 on endothelial cells in the porcine coronary artery. Circ Res. 1991; 69: 1361–1368 [DOI] [PubMed] [Google Scholar]
- 40. Mori F, King GL, Clermont AC, Bursell DK, Bursell SE. Endothelin-3 regulation of retinal hemodynamics in nondiabetic and diabetic rats. Invest Ophthalmol Vis Sci. 2000; 41: 3955–3962 [PubMed] [Google Scholar]
- 41. Shipley RD, Muller-Delp JM. Aging decreases vasoconstrictor responses of coronary resistance arterioles through endothelium-dependent mechanisms. Cardiovasc Res. 2005; 66: 374–383 [DOI] [PubMed] [Google Scholar]
- 42. Wolfrum S, Dendorfer A, Rikitake Y, et al. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004; 24: 1842–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cicek FA, Kandilci HB, Turan B. Role of ROCK upregulation in endothelial and smooth muscle vascular functions in diabetic rat aorta. Cardiovasc Diabetol. 2013; 12: 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kauser K, Rubanyi GM, Harder DR. Endothelium-dependent modulation of endothelin-induced vasoconstriction and membrane depolarization in cat cerebral arteries. J Pharmacol Exp Ther. 1990; 252: 93–97 [PubMed] [Google Scholar]
- 45. Lüscher TF, Yang Z, Tschudi M, et al. Interaction between endothelin-1 and endothelium-derived relaxing factor in human arteries and veins. Circ Res. 1990; 66: 1088–1094 [DOI] [PubMed] [Google Scholar]
- 46. da Silva CG, Specht A, Wegiel B, Ferran C, Kaczmarek E. Mechanism of purinergic activation of endothelial nitric oxide synthase in endothelial cells. Circulation. 2009; 119: 871–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tuomilehto J, Rastenyte D, Birkenhager WH, et al. Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. N Engl J Med. 1999; 340: 677–684 [DOI] [PubMed] [Google Scholar]
- 48. Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010; 106: 1319–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shiba T, Inoguchi T, Sportsman JR, Heath WF, Bursell S, King GL. Correlation of diacylglycerol level and protein kinase C activity in rat retina to retinal circulation. Am J Physiol. 1993; 265: E783–E793 [DOI] [PubMed] [Google Scholar]
- 50. Ishii H, Jirousek MR, Koya D, et al. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC β inhibitor. Science. 1996; 272: 728–731 [DOI] [PubMed] [Google Scholar]
- 51. Bursell SE, Takagi C, Clermont AC, et al. Specific retinal diacylglycerol and protein kinase C beta isoform modulation mimics abnormal retinal hemodynamics in diabetic rats. Invest Ophthalmol Vis Sci. 1997; 38: 2711–2720 [PubMed] [Google Scholar]
- 52. Kuo L, Chilian WM, Davis MJ. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol. 1991; 261: H1706–H1715 [DOI] [PubMed] [Google Scholar]
- 53. Hein TW, Potts LB, Xu W, Yuen JZ, Kuo L. Temporal development of retinal arteriolar endothelial dysfunction in porcine type 1 diabetes. Invest Ophthalmol Vis Sci. 2012; 53: 7943–7949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nguyen TT, Kawasaki R, Wang JJ, et al. Flicker light-induced retinal vasodilation in diabetes and diabetic retinopathy. Diabetes Care. 2009; 32: 2075–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Resch H, Garhofer G, Fuchsjäger-Mayrl G, Hommer A, Schmetterer L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009; 87: 4–12 [DOI] [PubMed] [Google Scholar]