Abstract
Patient: Female, 60
Final Diagnosis: Recurrent incisional hernia
Symptoms: —
Medication: —
Clinical Procedure: Limited ileo-cecal resection
Specialty: Surgery
Objective:
Diagnostic/therapeutic accidents
Background:
Iatrogenic entero-atmospheric fistula is devastating and its management is extremely difficult because it is often associated with fluid and electrolyte disturbances, nutritional problems, and life-threatening sepsis.
Case Report:
A 60-year-old woman underwent laparoscopic repair of a recurrent incisional hernia that was complicated by iatrogenic cecal injury necessitating a limited ileocecal resection and onlay prosthetic mesh repair of the hernia. Postoperatively, sloughing of the overlying skin led to mesh exposure. An attempted rotational flap coverage was complicated by small bowel injury, which was recognized and repaired. However, an entero-atmospheric fistula developed after the removal of contaminated mesh. The fistula was initially treated by vacuum-assisted closure dressing and later was converted to a ‘stoma’. Six months later, the small bowel segment bearing the fistula was excised and bowel continuity was restored.
Conclusions:
In selected cases, the conversion of entero-atmospheric fistula to a ‘stoma’ allows the patient to be discharged home early and maintain good nutritional status while awaiting the definitive surgical intervention.
Keywords: laparoscopy, incisional hernia repair, colon injury, entero-atmospheric fistula, stoma
Background
The sudden appearance of an entero-atmospheric fistula (EAF) after any abdominal surgery is devastating for both patient and surgeon. It is often associated with fluid and electrolyte disturbances, as well as nutritional deficiency and life-threatening sepsis [1,2]. Hence, its treatment is challenging and is associated with high morbidity and mortality [2].
The initial management is conservative and, although often futile, it should be tried before definitive treatment is contemplated [2]. Generally speaking, proximal high-output fistulae rarely close without surgery, while distal low-output fistulae may close spontaneously with conservative therapy, provided there is no distal obstruction and no foreign body is present [3,4]. In EAF, spontaneous healing is hampered by lack of vascularized tissue coverage over the exposed bowel and by continuous efflux of irritating enteric contents [3]. Several methods have been tried to overcome these problems and to facilitate fistula management [4–6].
We report a case of EAF that developed after attempted laparoscopic repair of a recurrent incisional hernia. It was managed by converting the fistula to a ‘stoma’. This case highlights some challenging management issues that could be of benefit to surgeons regularly performing laparoscopic incisional hernia repairs. It also introduces a feasible temporary solution to this daunting complication.
Case Report
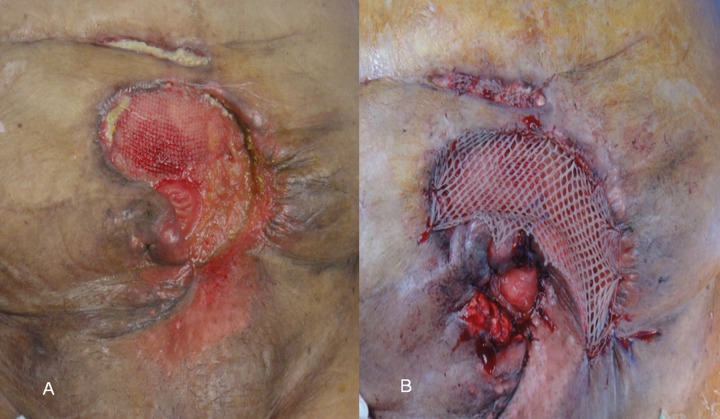
A 60-year-old woman, known to have asthma, hypertension, and diabetes, was admitted for laparoscopic repair of recurrent incisional hernia (post-caesarian section) following 4 previous open repairs. Clinically, she was obese (BMI 45 kg/m2) and the abdomen showed multiple scars of previous surgeries, with a medium-size incisional hernia in the right lower abdomen, which was easily reducible. After attention to the co-morbidities, she underwent a laparoscopic repair. Dense adhesions were encountered and upon mobilization of hernia contents, the cecum was inadvertently injured in multiple places. We converted to an open procedure and a limited ileo-cecal resection was performed. As no absorbable meshes were available, the defect was reluctantly repaired using Proceed mesh (Ethicon Inc, Johnson and Johnson Company, USA). On the fifth postoperative day, a small area (3×5 cm) of skin necrosis sloughed, exposing the underlying mesh. This was managed initially with frequent dressing, but later a rotational flap was used to cover the exposed mesh. During mobilization of the flap, the small bowel was inadvertently injured, but it was repaired in 2 layers using 3/0 polydioxanone (PDS) sutures. Four days later, small bowel contents were noted in the subcutaneous drain, but there were no signs of peritonism. The contaminated mesh was removed and the fistula was intubated using a T-tube. The bowel was temporarily covered with Permacol biological mesh (Covidien, Cedex, France), and the wound was dressed. Later, the T-tube was removed and a vacuum-assisted closure (VAC) machine was used. Because the wound around the fistula was granulating well after the disintegration of the biological mesh (Figure 1A), the EAF was converted to a ‘stoma’ by the application of split-skin skin graft over the granulating area and suturing the fistula edges to skin (Figure 1B). A stoma bag was then applied and oral fluids and later soft and solid diets were gradually introduced. Initially, the ‘stoma’ output was liquid, causing distressing leakage and soiling, but after the introduction of solid diet and Peptamen (Nestlé, Vevey, Switzerland) per mouth, contents started to solidify. She was given stoma care education and discharged home after the ‘stoma’ output was 3–4 soft bowel movements/day. The total hospital stay was 75 days.
Figure 1.
The fistula after disintegration of the biological mesh and exposing healthy granulation tissues prior to skin grafting and ‘stoma’ formation (A). A split skin graft was used to cover the granulated area and the elevated inferior lip of skin was rotated as a flap (B). The edges of the fistula were sutured to the skin graft and flap to form a ‘stoma’ to which a stoma bag can be applied.
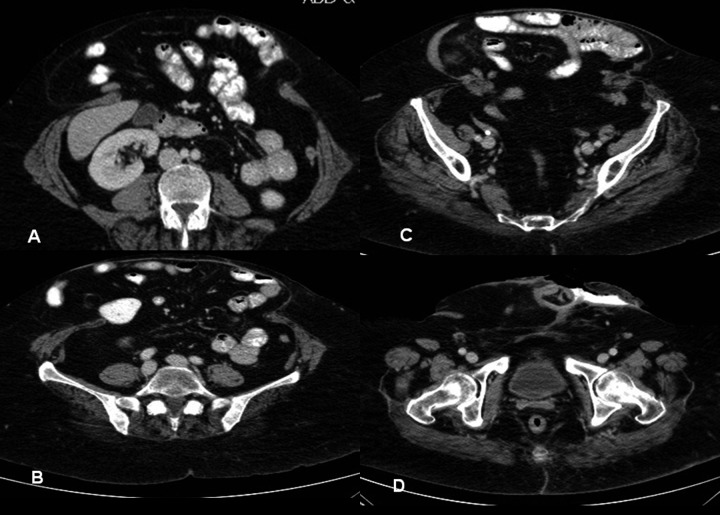
She was readmitted 6 months later for ‘stoma’ closure. Preoperative nutritional status was adequate and CT evaluation showed a hernia containing large and small bowels (Figure 2). The abdomen revealed skin excoriation at the inferior aspect of the ‘stoma’ (Figure 3). At surgery, the abdomen was entered through a fresh upper mid-line incision. All bowel loops were dissected free from the hernia sac after division of adhesions with extreme caution to avoid creating enterotomies. The fistula-bearing small bowel segment (Figure 4) was then resected, and bowel continuity was restored by a stapled side-to-side anastomosis. The abdominal wall defect was closed using underlay biological Permacol mesh (Covidien, Cedex, France). She was electively ventilated for 24 hours and was discharged home 14 days later. At 9-month follow-up, she was noted to have a recurrent hernia, but was advised to stay on conservative treatment because she was asymptomatic, her hernia was reducible, and the likelihood of recurrence was high.
Figure 2.
Abdominal CT scan showing several cuts of the abdominal hernia prior to the definitive corrective surgery. The large hernia had a wide-neck and contained small and large bowels (A–C). The fistula (‘stoma’) opening site is demonstrated clearly in (D).
Figure 3.
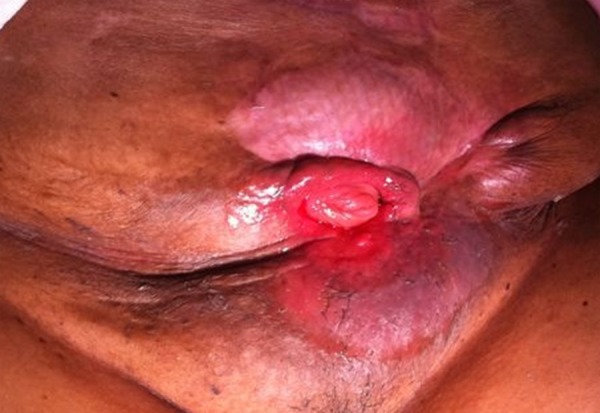
The ‘stoma’ as it looked some 6 months after the initial injury and prior to the final definitive procedure.
Figure 4.
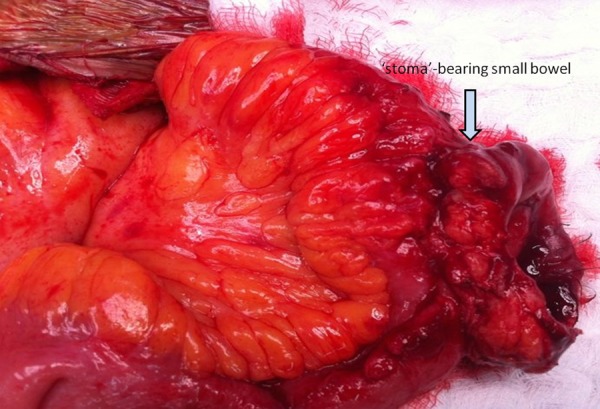
Operative view showing the ‘stoma’-bearing small bowel after releasing hernia contents, division of all adhesions, and adequate fistula mobilization. This ‘stoma’-bearing segment was then resected and a stapled side-to-side anastomosis was fashioned.
Discussion
EAF is defined as presence of an enteric fistula in the setting of an open abdomen. It is a serious complication that occurs after trauma and any major abdominal procedure [1,2]. Its management is complex and leads to increased cost. Moreover, the morbidity and mortality remain high despite modern advances in medical care [1,2]. Patients who survive the initial metabolic insults may require future surgical intervention to close the fistula. This is often challenging and technically demanding, and is poorly described in the literature [1].
The initial management is nil per mouth, total parenteral nutrition, control of sepsis, and containment of fistula output [2,4]. An attempt at non-surgical management should be tried before definitive treatment is contemplated. This may allow spontaneous closure, thus avoiding the risks of a major reoperative procedure [2]. The conservative approach was tried in this case, despite fistula failure to show signs of healing. Early surgical intervention was deferred because the abdomen was considered ‘hostile’. Resection of the non-healing fistula constitutes the final definitive operation. This requires careful planning and meticulous execution of the resection to maximize the chances of successful resolution [2].
In EAF, spontaneous healing is hampered by lack of vascularized tissue coverage over the exposed bowel. Moreover, continuous efflux of irritating enteric contents and chronic exposure of the viscera contribute to increased protein loss, septic infection, and high mortality [3]. Several methods have been described to avoid visceral exposure, such as use of catheters to intubate the fistula and early mobilization of skin and subcutaneous flaps [4]; both methods were tried in this case. Control of the efflux is achieved by surgical exteriorization and proximal diversion. This may be difficult to perform due to mesenteric shortening as a result of soft tissue and bowel edema [4]. Alternatively, a “floating stoma” may be created wherein the edges of the fistula are sutured to a plastic silo over which a stoma appliance is placed [5,6]. However, coverage of the fistula with well-vascularized soft tissue represents the most effective strategy for control and eventual healing [7]. In this case, we employed an approach that entailed coverage of the exposed but granulating the area with a split-skin graft and suturing the fistula edges to the skin, creating a ‘stoma’, albeit in a non-conventional position (Figure 1B). This meant converting the EAF to a ‘stoma’ that can be cared for in the usual manner, allowing the patient to be discharged home and obviating the need to stay in hospital while waiting for the definitive surgical procedure. This has contributed immensely to reducing the hospital length of stay and financial costs, and also improved the patient’s psychological morale and wellbeing. This method was made possible by the fact that the fistula was located in the distal ileum, as indicated by the CT scan (Figure 2D) and by the solidification of the fistula affluent after the trial of Peptamen (Nestlé S.A., Vevey, Switzerland) oral consumption. This approach would have been difficult to employ if the fistula had been located in the proximal jejunum.
Other essential surgical strategies are temporary coverage of the open abdomen with an absorbable mesh, skin grafting of the exposed area, and the selective use of vacuum-assisted wound closure [8,9]. Unfortunately, there were no absorbable meshes available at the time of ileocecal resection, and attempted use of a number of biological meshes was hampered by their poor quality due to manufacturer error. However, VAC dressing was used in our patient with beneficial effect in controlling the efflux and minimizing dressing changes. That proved to be rewarding for both patient and nurses. However, during its use, extreme caution was exercised by applying intermittent mode, low suction pressure to avoid creating more fistulae [9].
It may be argued that the fistula happened due to an ileo-colic anastomotic stricture that has caused back pressure on the site of enterotomy repair. However, this was not the case, as it was not evident at the time of the definitive surgical procedure.
Although some authors have advocated early surgical intervention for fistula closure [8], others advocated against re-operating earlier than 3 months from the date of insult or trauma [2]. The delayed option will allow those fistulas that are likely to close an opportunity to do so, while at the same time decreasing the risks of multiple enterotomies and difficult dissection in the immediate postoperative period. We opted here for the delayed surgical intervention, which proved to be beneficial in achieving an eventual successful outcome. Regardless of which strategy is adopted, it is important to ensure bowel patency distal to the fistula prior to surgical fistula takedown, because the presence of untreated distal bowel obstruction will preclude a favorable outcome of this procedure [2].
We report this case to highlight some challenging issues in the management of iatrogenic EAF and to introduce a feasible temporary solution by converting the fistula to a manageable ‘stoma’ in selected cases with a distal small bowel fistula.
Conclusions
We believe that in selected patients with distal iatrogenic EAF, the conversion of the fistula into ‘stoma’ is feasible and effective in temporarily controlling the fistula. This is cost-effective because it is associated with reduced hospital stay and allows the patient to be discharged home early. It also allows the patient to maintain good nutritional status while awaiting the definitive surgical intervention. The delayed surgical intervention is advocated and it is recommended to wait at least 3–6 months from the date of the last operation before attempting the fistula/’stoma’ closure.
Footnotes
Conflict of interests
None to declare.
References:
- 1.Sriussadaporn S, Sriussadaporn S, Kritayakirana K, Pak-art R. Operative management of small bowel fistulae associated with open abdomen. Asian J Surg. 2006;29(1):1–7. doi: 10.1016/S1015-9584(09)60284-0. [DOI] [PubMed] [Google Scholar]
- 2.Evenson AR, Fischer JE. Current management of enterocutaneous fistula. J Gastrointest Surg. 2006;10:455–64. doi: 10.1016/j.gassur.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Girard S, Sideman M, Spain DA. A novel approach to the problem of intestinal fistulization arising in patients managed with open peritoneal cavities. Am J Surg. 2002;184:166–67. doi: 10.1016/s0002-9610(02)00916-9. [DOI] [PubMed] [Google Scholar]
- 4.Ramsay PT, Mejia VA. Management of enteroatmospheric fistulae in the open abdomen. Am Surg. 2010;76(6):637–39. [PubMed] [Google Scholar]
- 5.Subramaniam MH, Liscum KR, Hirshberg A. The floating stoma: a new technique for controlling exposed fistulae in abdominal trauma. J Trauma. 2002;53(2):386–88. doi: 10.1097/00005373-200208000-00037. [DOI] [PubMed] [Google Scholar]
- 6.Cipolla J, Baillie DR, Steinberg SM, et al. Negative pressure wound therapy: Unusual and Innovative Applications. OPUS Scientist. 2008;2:15–29. [Google Scholar]
- 7.Kearney R, Payne W, Rosemurgy A. Extra-abdominal closure of enterocutaneous fistula. Am Surg. 1997;63:406–9. [PubMed] [Google Scholar]
- 8.Marinis A, Gkiokas G, Anastasopoulos G, et al. Surgical techniques for the enteroatmospheric fistulae. Surgical Infections. 2009;10(1):47–52. doi: 10.1089/sur.2008.044. [DOI] [PubMed] [Google Scholar]
- 9.Woodfield JC, Barry PR, Bissett IP, McKee M. Experience with the use of vacuum dressings in the management of acute enterocutaneous fistulas. ANZ Surg. 2006;76:1085–87. doi: 10.1111/j.1445-2197.2006.03956.x. [DOI] [PubMed] [Google Scholar]