Abstract
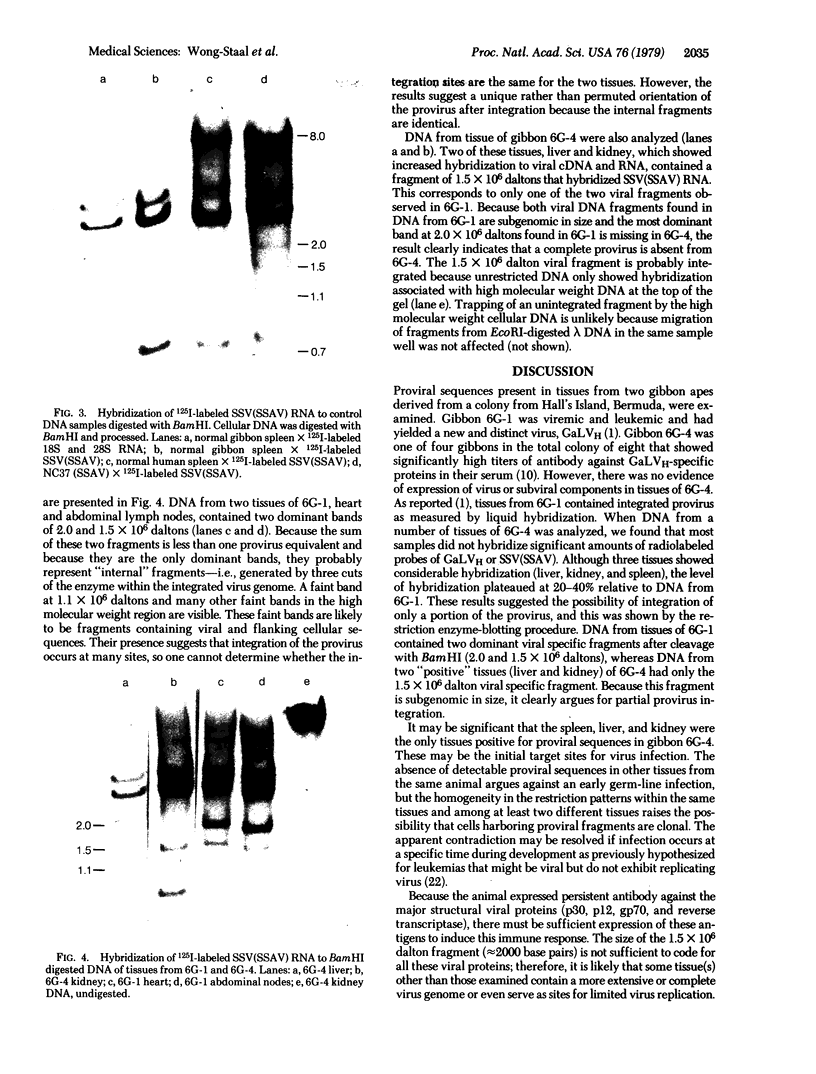
Integrated viral DNA sequences were detected in tissues from two gibbon apes, a leukemic gibbon (6G-1) from whose leukocytes a distinct strain of gibbon ape leukemia virus (GaLVH) was isolated, and gibbon 6G-4, a contact of 6G-1 from the same colony that had uremia and cachexia of unknown origin. Although 6G-4 had no detectable neoplasia or viral proteins, its serum contained persistent antibody against GaLV antigens. Whereas DNA from most of the tissues of 6G-1 contained GaLV provirus, DNA from only three tissues (kidney, spleen, and liver) from 6G-4 showed detectable viral sequences, and the extent of hybridization in each case was lower than with 6G-1. After cleavage with BamHI, two virus-specific DNA fragments were detected in tissues of 6G-1. Only one of these fragments was detected in the positive tissues of 6G-4. The results indicate that: (i) 6G-4 was exposed to and infected by GaLV; (ii) early target sites for infection of gibbon by GaLV may be limited to a few tissues; and (iii) infection can be contained by integration of only partial provirus in a few tissues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aulakh G. S., Gallo R. C. Rauscher-leukemia-virus-related sequences in human DNA: presence in some tissues of some patients with hemotopoietic neoplasias and absence in DNA from other tissues. Proc Natl Acad Sci U S A. 1977 Jan;74(1):353–357. doi: 10.1073/pnas.74.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M. A very large repeating unit of mouse DNA containing the 18S, 28S and 5.8S rRNA genes. Cell. 1977 Aug;11(4):795–805. doi: 10.1016/0092-8674(77)90292-6. [DOI] [PubMed] [Google Scholar]
- Current concepts of leukemia and lymphoma: etiology, pathogenesis, and therapy. Ann Intern Med. 1976 Sep;85(3):351–366. doi: 10.7326/0003-4819-85-3-351. [DOI] [PubMed] [Google Scholar]
- Essex M., Cotter S. M., Sliski A. H., Hardy W. D., Jr, Stephenson J. R., Aaronson S. A., Jarrett O. Horizontal transmission of feline leukemia virus under natural conditions in a feline leukemia cluster household. Int J Cancer. 1977 Jan;19(1):90–96. doi: 10.1002/ijc.2910190113. [DOI] [PubMed] [Google Scholar]
- Essex M. Horizontally and vertically transmitted oncornaviruses of cats. Adv Cancer Res. 1975;21:175–248. doi: 10.1016/s0065-230x(08)60973-2. [DOI] [PubMed] [Google Scholar]
- Gallagher R. E., Schrecker A. W., Walter C. A., Gallo R. C. Oncornavirus lytic activity in the serum of gibbon apes. J Natl Cancer Inst. 1978 Mar;60(3):677–682. doi: 10.1093/jnci/60.3.677. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Gallagher R. E., Wong-Staal F., Aoki T., Markham P. D., Schetters H., Ruscetti F., Valerio M., Walling M. J., O'Keeffe R. T. Isolation and tissue distribution of type-C virus and viral components from a gibbon ape (Hylobates lar) with lymphocytic leukemia. Virology. 1978 Feb;84(2):359–373. doi: 10.1016/0042-6822(78)90255-6. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Kawakami T. G., Buckley P. M. Antigenic studies on gibbon type-C viruses. Transplant Proc. 1974 Jun;6(2):193–196. [PubMed] [Google Scholar]
- Kawakami T. G., Huff S. D., Buckley P. M., Dungworth D. L., Synder S. P., Gilden R. V. C-type virus associated with gibbon lymphosarcoma. Nat New Biol. 1972 Feb 9;235(58):170–171. doi: 10.1038/newbio235170a0. [DOI] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakower J. M., Tronick S. R., Gallagher R. E., Gallo R. C., Aaronson S. A. Antigenic characterization of a new gibbon ape leukemia virus isolate: seroepidemiologic assessment of an outbreak of gibbon leukemia. Int J Cancer. 1978 Dec;22(6):715–720. doi: 10.1002/ijc.2910220613. [DOI] [PubMed] [Google Scholar]
- Levin R., Ruscetti S. K., Parks W. P., Scolnick E. M. Expression of feline type-C virus in normal and tumor tissues of the domestic cat. Int J Cancer. 1976 Nov 15;18(5):661–671. doi: 10.1002/ijc.2910180515. [DOI] [PubMed] [Google Scholar]
- Olsen R. G., Hoover E. A., Schaller J. P., Mathes L. E., Wolff L. H. Abrogation of resistance to feline oncornavirus disease by immunization with killed feline leukemia virus. Cancer Res. 1977 Jul;37(7 Pt 1):2082–2085. [PubMed] [Google Scholar]
- Prensky W. The radioiodination of RNA and DNA to high specific activities. Methods Cell Biol. 1976;13:121–152. doi: 10.1016/s0091-679x(08)61800-2. [DOI] [PubMed] [Google Scholar]
- Reitz M. S., Miller N. R., Wong-Staal F., Gallagher R. E., Gallo R. C., Gillespie D. H. Primate type-C virus nucleic acid sequences (woolly monkey and baboon types) in tissues from a patient with acute myelogenous leukemia and in viruses isolated from cultured cells of the same patient. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2113–2117. doi: 10.1073/pnas.73.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. P., Dungworth D. L., Kawakami T. G., Callaway E., Lau D. T. Lymphosarcomas in two gibbons (Hylobates lar) with associated C-type virus. J Natl Cancer Inst. 1973 Jul;51(1):89–94. doi: 10.1093/jnci/51.1.89. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Svoboda J., Popovic M., Sainerová H., Mach O., Shoyab M., Baluda M. A. Incomplete viral genome in a non-virogenic mouse tumour cell line (RVP3) transformed by Prague strain of avian sarcoma virus. Int J Cancer. 1977 Jun 15;19(6):851–858. doi: 10.1002/ijc.2910190617. [DOI] [PubMed] [Google Scholar]
- Theilen G. H., Gould D., Fowler M., Dungworth D. L. C-type virus in tumor tissue of a woolly monkey (Lagothrix spp.) with fibrosarcoma. J Natl Cancer Inst. 1971 Oct;47(4):881–889. [PubMed] [Google Scholar]
- Todaro G. J., Lieber M. M., Benveniste R. E., Sherr C. J. Infectious primate type C viruses: Three isolates belonging to a new subgroup from the brains of normal gibbons. Virology. 1975 Oct;67(2):335–343. doi: 10.1016/0042-6822(75)90435-3. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Isolation of a sequence-specific endonuclease (BamI) from Bacillus amyloliquefaciens H. J Mol Biol. 1975 Sep 5;97(1):123–125. doi: 10.1016/s0022-2836(75)80028-3. [DOI] [PubMed] [Google Scholar]
- Wolfe L. G., Deinhardt F., Theilen G. H., Rabin H., Kawakami T., Bustad L. K. Induction of tumors in marmoset monkeys by simian sarcoma virus, type 1 (Lagothrix): a preliminary report. J Natl Cancer Inst. 1971 Nov;47(5):1115–1120. [PubMed] [Google Scholar]
- Wong-Staal F., Gillespie D., Gallo R. C. Proviral sequences of baboon endogenous type C RNA virus in DNA of human leukaemic tissues. Nature. 1976 Jul 15;262(5565):190–195. doi: 10.1038/262190a0. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Reitz M. S., Paran M., Gallo R. C. Mechanism of stimulation of murine type-C RNA tumor virus production by glucocorticoids: post-transcriptional effects. J Virol. 1974 Oct;14(4):802–812. doi: 10.1128/jvi.14.4.802-812.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]