Abstract
Globozoospermia is a rare but severe teratozoospermia, characterized by ejaculates consisting completely of round-headed spermatozoa that lack an acrosome or, in partial globozoospermia, containing a variable proportion (20.0–90.0%) of acrosomeless spermatozoa. Men that are affected with total globozoospermia are infertile, and even the application of intracytoplasmic sperm injection (ICSI) has met with disappointingly low success rates.
In humans, several case reports of globozoospermia have demonstrated that two or more siblings were affected in each family, which suggested a genetic component to this disease. Currently, three genes are known to be associated with total globozoospermia in humans, SPATA16 , PICK1 and DPY19L2 genes. Mutations in SPATA16 and PICK1 are rare causes of globozoospermia, found in only one patient each. Several studies have suggested that DPY19L2 mutations are the major cause of globozoospermia in patients from different ethnic origins and different geographic regions. The most common DPY19L2 mutation is the 200 kb deletion arising from a nonallelic homologous recombination (NAHR) between the flanking low copy repeats (LCRs). Here we describe the presence of a homozygous deletion of the DPY19L2 gene in two infertile Macedonian patients with 100.0% round headed spermatozoa, thus suggesting that this deletion represents a major cause of globozoospermia among Macedonian men.
Keywords: Globozoospermia , DPY19L2 gene , Intra cytoplasmic sperm injection (ICSI) , Nonallelic homologous recombination (NAHR) , Low copy repeats (LCRs) , Deletion(s) , Male infertility
INTRODUCTION
Globozoospermia is a rare but severe form of teratozoospermia, accounting for less than 0.1% of male infertility [ 1 ] . It is characterized by ejaculates consisting completely of round-headed spermatozoa that lack an acrosome (total globozoospermia) or, in partial globozoospermia, containing a variable proportion (20.0–90.0%) of acrosomeless spermatozoa.
The genetic component to globozoospermia was suggested many years ago by several case reports which demonstrated that two or more siblings were affected in each family [ 2 – 5 ] . Furthermore, genetic studies in mice have provided direct evidence that disruption of several genes, including GOPC (Golgi-associated PDZ and coiled-coil motif containing protein), HRB (HIV-1 Rev binding protein) and CSNK2A2 (casein kinase 2, α prime polypeptide) results in a globozoospermia phenotype with decreased fertility. However, there has not been a clear link between homozygous mutations in these genes and globozoospermia in humans [ 6 ] .
Currently, three genes are known to be associated with total globozoospermia in humans. In 2007, a genome-wide study of six brothers from a consanguineous Ashkenazi Jewish family suggested that a homozygous mutation (c.848G>A or R283Q) in SPATA16 (spermatogenesis-associated 16) was associated with male infertility in human globozoospermia [ 7 ] .
Later, using the candidate gene screening strategy, a homozygous missense mutation (G198A) in exon 13 of the PICK1 (protein interacting with C kinase 1) gene was identified in a Chinese family. The family member affected by this homozygous missense mutation showed a complete lack of acrosome [ 8 ] . However, no mutations in these genes were detected in other men with globozoospermia, thus suggesting that SPATA16 and PICK1 genes are not the main loci associated with this condition [ 9 ] .
In 2011, a large homozygous deletion, encompassing ∼200 kb, including the entire DPY19L2 (protein dpy-19 homolog 2) gene was identified in four (out of 21 screened) unrelated patients [ 10 ] . At the same time, a study of 20 patients with globozoospermia originating mainly from Tunisia identified the DPY19L2 deletion, at a much higher rate (75.0%), thus suggesting that deletions involving this gene might be a major cause of globozoospermia [ 11 ] . This was confirmed when a larger cohort including 64 globozoospermic patients was screened. This study showed that the DPY19L2 gene was mutated in 66.7% (36 out of 64) globozoospermic patients [ 12 ] . In addition to the deletion, several point mutations were also identified. Out of 36 patients with the mutated gene, 69.4% were homozygotes, 19.4% were compound heterozygotes for both this deletion and a point mutation and 11.1% showed a homozygous point mutation. Molecular analysis of the DPY19L2 gene among Chinese globozoospermic patients revealed that a genetic defect was present in nine (60.0%) of the 15 unrelated patients [ 13 ] . Four patients were homozygous for the deletion and five were homozygous for a point mutation. This study confirmed that DPY19L2 mutations are the major cause of globozoospermia in patients from different ethnic origins and different geographic regions.
MATERIALS AND METHODS
Among more than 200 infertile men, two patients with 100.0% of globozoocephalic spermatozoa with no acrosome in their semen were recruited for genetic study at the Macedonian Academy of Sciences and Arts, Research Center for Genetic Engineering and Biotechnology (RCGEB) “Georgi D. Efremov”, Skopje, Republic of Macedonia laboratories. Patient 1 was a 30-year-old man with two unsuccessful intra cytoplasmic sperm injection (ICSI) attempts, while patient 2 was a 35-year-old man who has previously experienced three unsuccessful ICSI attempts. Both patients were of Macedonian ethnic origin, living in Tetovo, a town in the western part of the Republic of Macedonia. Although they originated from the same town, they were not related and no consanguinity was reported in their families.
The search for mutations in the SPATA16 and PICK1 genes was performed on genomic DNA by sequencing of all exons and exon/intron boundaries using BigDye™ Terminator Cycle Sequencing Ready Reaction Kit on an ABI PRISM™ 3100 Genetic Analyzer (Life Technologies Corporation, Carlsbad, CA, USA). The oligonucleotide primers used for polymerase chain reaction (PCR) and sequencing were designed by Primer 3 software [ 14 ] and synthesized by Integrated DNA Technologies, Coralville, IA, USA.
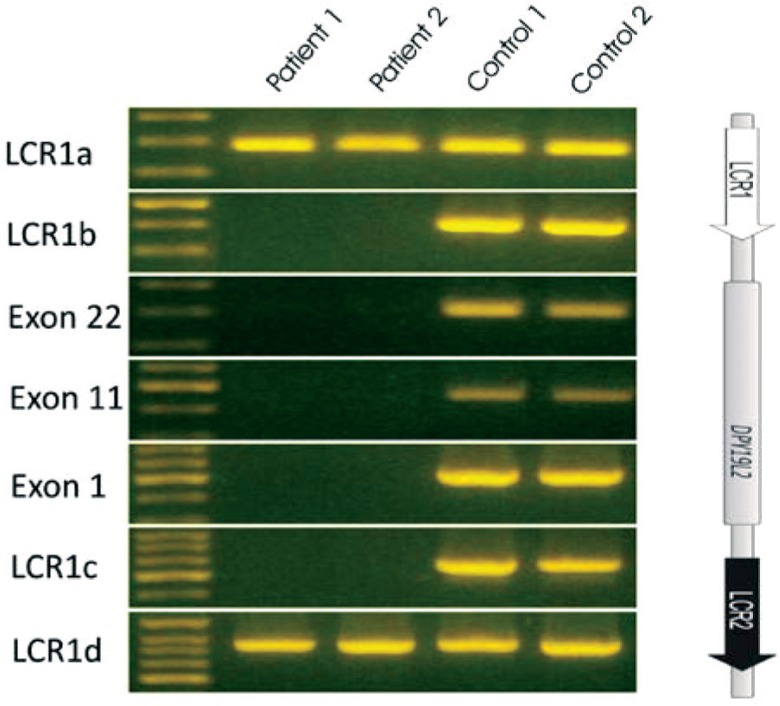
The search for copy number variants (CNVs) was performed by oligonucleotide array comparative genomic hybridization (aCGH) using the 180K Agilent Human Genome CGH Microarrays and Genomic Workbench software (Agilent Technologies, Santa Clara, CA, USA). The deletion at 12q14.2, including the entire DPY19L2 gene, was confirmed with classical PCR using seven loci, of which three were intragenic, located on exons 1, 11, and 22 of the DPY19L2 gene, while the other four were located in the low copy repeat (LCR) regions. The primers in the centromeric LCR1 region, named LCRa and LCRb, were localized approximately 25 and 9 kb 3’ of DPY19L2, respectively, and the primers in the telomeric LCR2 region, named LCRc and LCRd, were localized approximately 62 and 77 kb 5’ of DPY19L2, respectively [ 11 ] . For further confirmation of the deletion we performed a gap-PCR, using primers flanking the DPY19L2 deletion [ 10 ] . This PCR generates a fragment of 1700 bp only in the patients carrying the DPY19L2 deletion.
RESULTS
We have sequenced the SPATA16 and PICK1 genes in the first globozoospermic patient (patient 1). However, we found no mutations that might be responsible for the infertility in this patient. Only two polymorphisms in the SPATA16 gene were identified: rs 115897458 or codon 18 (C A T>C G T) (His→Arg) and rs508508 or codon 225 (A C T>A G T) (Thr→Ser).
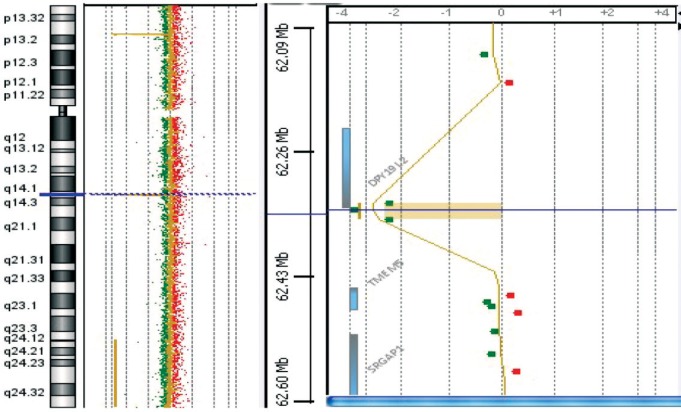
Then, aCGH analysis was carried out in patient 1 and showed the presence of a homozygous deletion including the DPY19L2 gene ( Figure 1 ). Because of the low number of probes in that region, classical PCR was performed for narrowing down the breakpoints and confirming the deletion. Patient 2 was studied by PCR analysis only. Both patients showed the same pattern of deletion breakpoints with PCR amplification only of outer loci of LCR region and no amplification of the inner loci LCR regions and the three exons in the DPLY19L2 gene (exons 1, 11 and 22), ( Figure 2 ), with the maximum size of the deleted region being approximately 210 kb. The gap-PCR analysis generated a PCR fragment of approximately 1700 bp in both patients with globozoospermia, thus confirming the presence of a DPLY19L2 gene deletion.
Figure 1.
Oligonucleotide array CGH of patient 1 showing the homozygous deletion of three probes in the telomeric region of DPLY19L2 gene.
Figure 1.
Polymerase chain reaction analysis of the DPY19L2 gene in two patients and controls showing that the deletion breakpoints are localized extragenically within the LCR regions.
DISCUSSION
Here we present the genetic analysis of two patients with 100.0% round-headed spermatozoa. A homozygous deletion of the DPY19L2 gene was detected in both patients, thus suggesting that this deletion represents a major cause of globozoospermia among Macedonian men.
The mechanism underlying the recurrent DPY19L2 gene deletion is a nonallelic homologous recombination (NAHR) between the two highly homologous 28 kb LCRs present on each side of DPY19L2 [ 10 ] . Thus far, nine breakpoint zones have been identified in patients from different regions [ 12 , 13 ] . The fact that the same breakpoints are shared by patients from different regions and ethnic origin, and that patients from the same country have different breakpoints, excludes the founder effect and strongly suggests that the deletion results from recurrent events linked to the specific architectural feature of this region.
Intracytoplasmic sperm injection is the only treatment for patients with globozoospermia. However, fertilization rates after ICSI in these patients are severely reduced [ 7 , 15 ] . Fertilization failures in the patients with globozoospermia have been attributed to a deficiency in oocyte activation capacity [ 16 ] . A recent study of a large cohort of globozoospermic patients has shown that the fertilization rates after ICSI with assisted oocyte activation (AOA) are restored to normal when compared with conventional ICSI in globozoospermic patients with and without a mutations in the DPY19L2 gene [ 17 ] . Thus, it was proposed that the first-line therapeutic approach for complete globozoospermia should include ICSI with AOA regardless of the DPY19L2 status. Although at present the molecular diagnosis does not influence the choice of treatment in patients with globozoospermia, it is very important for adequate genetic counseling of couples with this rare form of male infertility.
Acknowledgments
This study was supported by project CRP/ MAC09-01 from the ICGIB, Trieste (to DPK).
REFERENCES
- 1. Perrin A , Coat C , Nguyen MH , Talagas M , Morel F , Amice J , et al. Molecular cytogenetic and genetic aspects of globozoospermia: a review. Andrologia. 2013 ; 45 ( 1 ): 1 – 9 . doi: 10.1111/j.1439-0272.2012.01308.x. [DOI] [PubMed] [Google Scholar]
- 2. Florke-Gerloff S , Topfer-Petersen E , Muller-Esterl W , Mansouri A , Schatz R , Schirren C , et al. Biochemical and genetic investigation of roundheaded spermatozoa in infertile men including two brothers and their father. Andrologia. 1984 ; 16 ( 3 ): 187 – 202 . doi: 10.1111/j.1439-0272.1984.tb00262.x. [DOI] [PubMed] [Google Scholar]
- 3. Carrell DT , Emery BR , Liu L . Characterization of aneuploidy rates, protamine levels, ultrastructure, and functional ability of round-headed sperm from two siblings and implications for intracytoplasmic sperm injection. Fertil Steril. 1999 ; 71 ( 3 ): 511 – 516 . doi: 10.1016/s0015-0282(98)00498-1. [DOI] [PubMed] [Google Scholar]
- 4. Kilani Z , Ismail R , Ghunaim S , Mohamed H , Hughes D , Brewis I , et al. Evaluation and treatment of familial globozoospermia in five brothers. Fertil Steril. 2004 ; 82 ( 5 ): 1436 – 1439 . doi: 10.1016/j.fertnstert.2004.03.064. [DOI] [PubMed] [Google Scholar]
- 5. Dirican EK , Isik A , Vicdan K , Sozen E , Suludere Z . Clinical pregnancies and livebirths achieved by intracytoplasmic injection of round headed acrosomeless spermatozoa with and without oocyte activation in familial globozoospermia: case report. Asian J Androl. 2008 ; 10 ( 2 ): 332 – 336 . doi: 10.1111/j.1745-7262.2008.00248.x. [DOI] [PubMed] [Google Scholar]
- 6. Christensen GL , Ivanov IP , Atkins JF , Campbell B , Carrell DT . Identification of polymorphisms in the Hrb, GOPC, and Csnk2a2 genes in two men with globozoospermia. J Androl. 2006 ; 27 ( 1 ): 11 – 15 . doi: 10.2164/jandrol.05087. [DOI] [PubMed] [Google Scholar]
- 7. Dam AH , Koscinski I , Kremer JA , Moutou C , Jaeger AS , Oudakker AR , et al. Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am J Hum Genet. 2007 ; 81 ( 4 ): 813 – 820 . doi: 10.1086/521314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu G , Shi QW , Lu GX . A newly discovered mutation in PICK1 in a human with globozoospermia. Asian J Androl. 2010 ; 12 ( 4 ): 556 – 560 . doi: 10.1038/aja.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dam AH , Feenstra I , Westphal JR , Ramos L , van Golde RJ , Kremer JA . Globozoospermia revisited. Hum Reprod Update. 2007 ; 13 ( 1 ): 63 – 75 . doi: 10.1093/humupd/dml047. [DOI] [PubMed] [Google Scholar]
- 10. Koscinski I , Elinati E , Fossard C , Redin C , Muller J , Velez de la Calle J , et al. DPY19L2 deletion as a major cause of globozoospermia. Am J Hum Genet. 2011 ; 88 ( 3 ): 344 – 350 . doi: 10.1016/j.ajhg.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harbuz R , Zouari R , Pierre V , Ben Khelifa M , Kharouf M , Coutton C , et al. A recurrent deletion of DPY19L2 causes infertility in man by blocking sperm head elongation and acrosome formation. Am J Hum Genet. 2011 ; 88 ( 3 ): 351 – 361 . doi: 10.1016/j.ajhg.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elinati E , Kuentz P , Redin C , Jaber S , Vanden Meerschaut F , Makarian J , et al. Globozoospermia is mainly due to DPY19L2 deletion via nonallelic homologous recombination involving two recombination hotspots. Hum Mol Genet. 2012 ; 21 ( 16 ): 3695 – 3702 . doi: 10.1093/hmg/dds200. [DOI] [PubMed] [Google Scholar]
- 13. Zhu F , Gong F , Lin G , Lu G . DPY19L2 gene mutations are a major cause of globozoospermia: identification of three novel point mutations. Mol Hum Reprod. 2013 ; 19 ( 6 ): 395 – 404 . doi: 10.1093/molehr/gat018. [DOI] [PubMed] [Google Scholar]
- 14. Koressaar T , Remm M . Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007 ; 23 ( 10 ): 1289 – 1291 . doi: 10.1093/bioinformatics/btm091. [DOI] [PubMed] [Google Scholar]
- 15. Sahu B , Ozturk O , Serhal P . Successful pregnancy in globozoospermia with severe oligoasthenospermia after ICSI. J Obstet Gynaecol. 2010 ; 30 ( 8 ): 869 – 870 . doi: 10.3109/01443615.2010.515321. [DOI] [PubMed] [Google Scholar]
- 16. Rybouchkin A , Dozortsev D , Pelinck MJ , De Sutter P , Dhont M . Analysis of the oocyte activating capacity and chromosomal complement of round-headed human spermatozoa by their injection into mouse oocytes. Hum Reprod. 1996 ; 11 ( 10 ): 2170 – 2175 . doi: 10.1093/oxfordjournals.humrep.a019071. [DOI] [PubMed] [Google Scholar]
- 17. Kuentz P , Vanden Meerschaut F , Elinati E , Nasr-Esfahani MH , Gurgan T , Iqbal N , et al. Assisted oocyte activation overcomes fertilization failure in globozoospermic patients regardless of the DPY19L2 status. Hum Reprod. 2013 ; 28 ( 4 ): 1054 – 1061 . doi: 10.1093/humrep/det005. [DOI] [PubMed] [Google Scholar]