Abstract
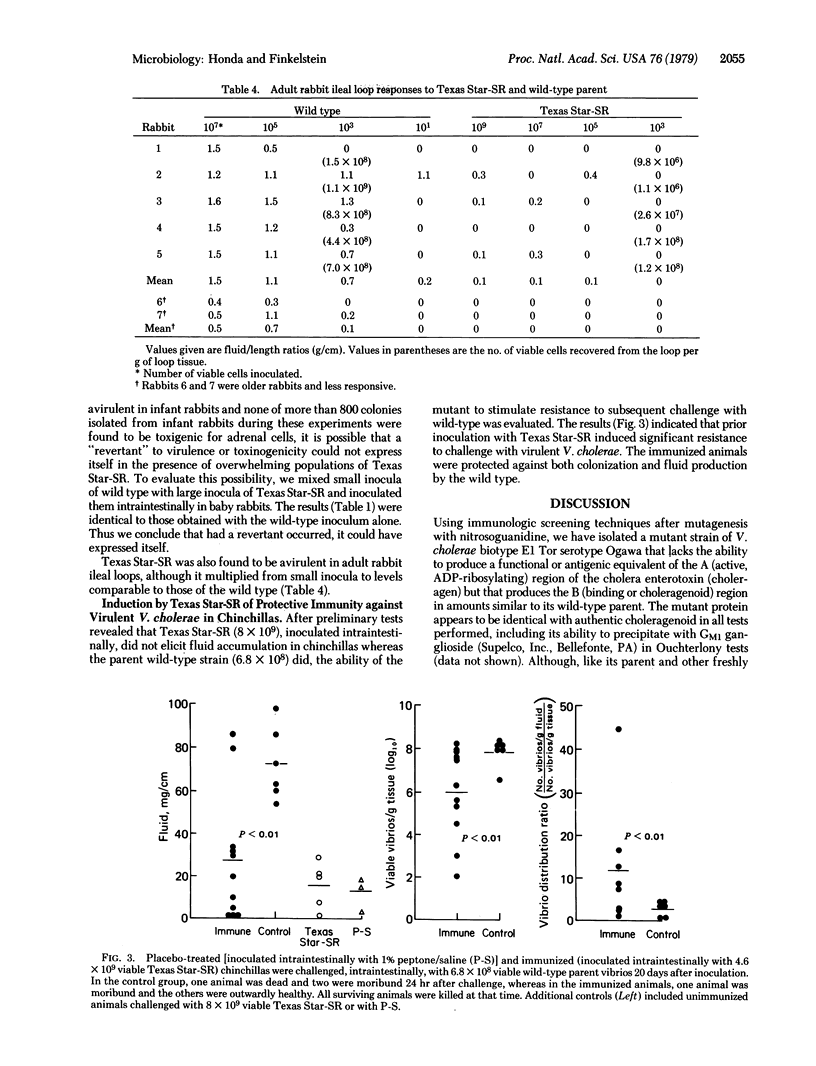
After mutagenesis with nitrosoguanidine and selection by immuno-halo techniques, an avirulent mutant, designated Texas Star-SR, which produces no detectable A (active; ADP-ribosylating) region of the cholera enterotoxin (choleragen) but produces the B region (choleragenoid) in amounts similar to the hypertoxinogenic wild-type parent Vibrio cholerae (biotype E1 Tor serotype Ogawa), has been isolated. The mutant retains the colonizing ability, motility, prototrophy, and serologic characteristics of the parent. In relevant intestinal experimental models, it has been shown to be avirulent and to induce protection against challenge with virulent cholera vibrios. The mutant appears to be suitable for further evaluation in volunteers as a candidate living enteric vaccine against cholera and related enterotoxic enteropathies.
Full text
PDF
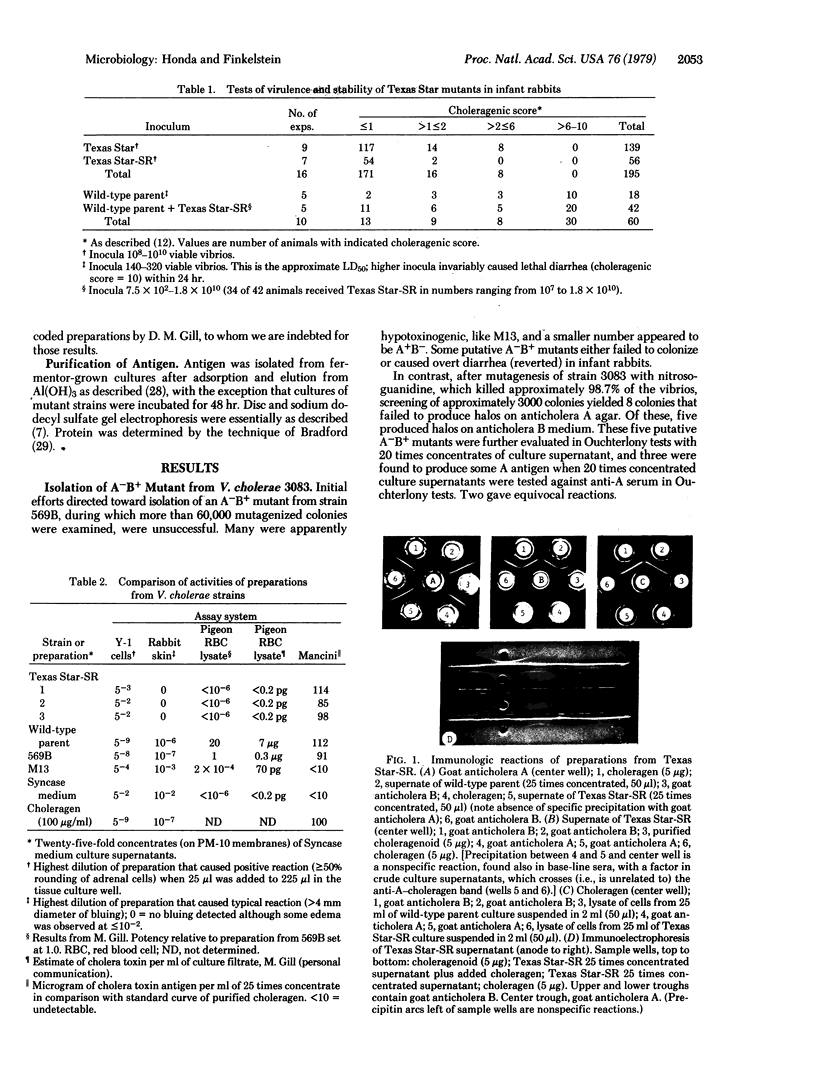
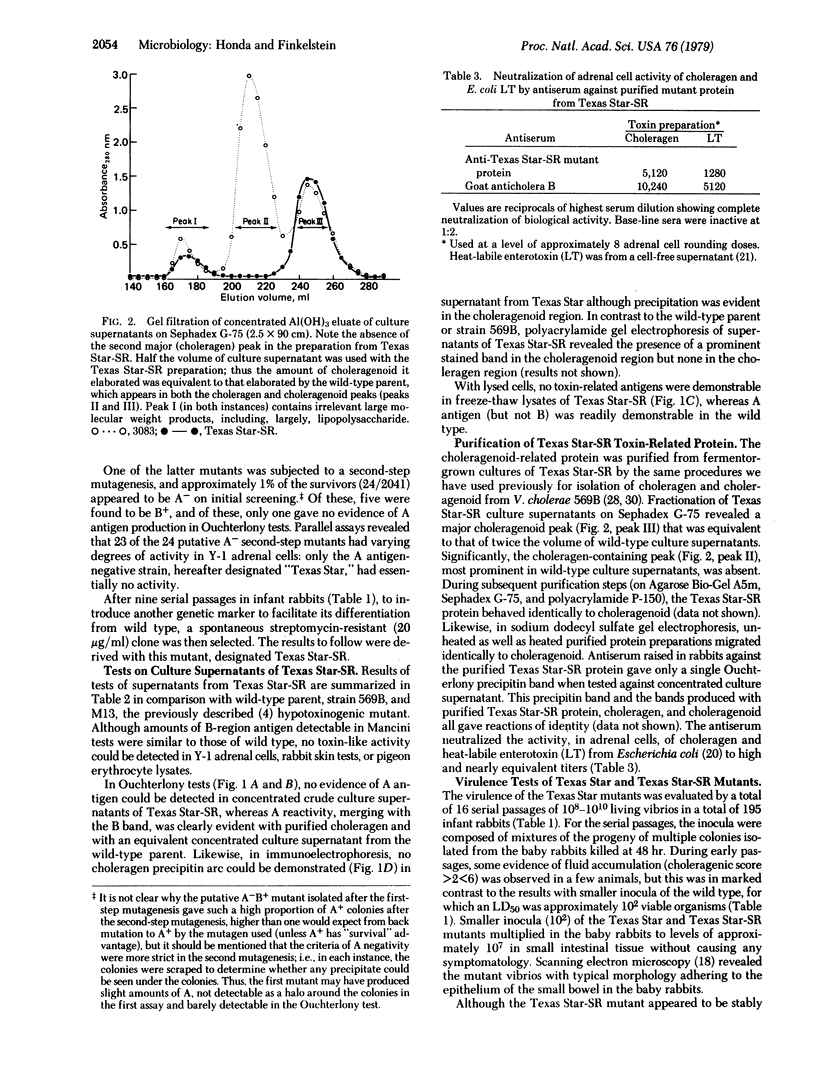

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baselski V. S., Medina R. A., Parker C. D. Survival and multiplication of Vibrio cholerae in the upper bowel of infant mice. Infect Immun. 1978 Nov;22(2):435–440. doi: 10.1128/iai.22.2.435-440.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachman U., Goss S. J., Pickett M. J. Experimental cholera in the chinchilla. J Infect Dis. 1974 Apr;129(4):376–384. doi: 10.1093/infdis/129.4.376. [DOI] [PubMed] [Google Scholar]
- Blachman U., Graboff S. R., Haag G. E., Gottfeld E., Pickett M. J. Experimental Cholera in Chinchillas: the Immune Response in Serum and Intestinal Secretions to Vibrio cholerae and Cholera Toxin. Infect Immun. 1974 Nov;10(5):1098–1104. doi: 10.1128/iai.10.5.1098-1104.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Craig J. P., Pierce N. F., Hornick R. B. Response of man to infection with Vibrio cholerae. II. Protection from illness afforded by previous disease and vaccine. J Infect Dis. 1974 Oct;130(4):325–333. doi: 10.1093/infdis/130.4.325. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Immunological cross-reactivity between a heat-labile enterotoxin(s) of Escherichia coli and subunits of Vibrio cholerae enterotoxin. Infect Immun. 1978 Sep;21(3):1036–1039. doi: 10.1128/iai.21.3.1036-1039.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman M., Neoh S. H., LaRue M. K., Delaney R. Dissociation and recombination of the subunits of the cholera enterotoxin (choleragen). J Immunol. 1974 Jul;113(1):145–150. [PubMed] [Google Scholar]
- Finkelstein R. A., Fujita K., LoSpalluto J. J. Procholeragenoid: an aggregated intermediate in the formation of choleragenoid. J Immunol. 1971 Oct;107(4):1043–1051. [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A. Monospecific equine antiserum against cholera exo-enterotoxin. Infect Immun. 1970 Dec;2(6):691–697. doi: 10.1128/iai.2.6.691-697.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Sobocinski P. Z., Atthasampunna P., Charunmethee P. Pathogenesis of experimental cholera: identification of choleragen (procholeragen A) by disc immunoelectrophoresis and its differentiation from cholera mucinase. J Immunol. 1966 Jul;97(1):25–33. [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- Freter R. Parameters affecting the association of vibrios with the intestinal surface in experimental cholera. Infect Immun. 1972 Aug;6(2):134–141. doi: 10.1128/iai.6.2.134-141.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., King C. A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975 Aug 25;250(16):6424–6432. [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K., Vasil M. L., Finkelstein R. A. Studies on toxinogenesis in Vibrio cholerae. III. Characterization of nontoxinogenic mutants in vitro and in experimental animals. J Clin Invest. 1975 Mar;55(3):551–560. doi: 10.1172/JCI107962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai G. J., Burrows W. The titration of cholera toxin and antitoxin in the rabbit ileal loop. J Infect Dis. 1966 Dec;116(5):606–614. doi: 10.1093/infdis/116.5.606. [DOI] [PubMed] [Google Scholar]
- Klapper D. G., Finkelstein R. A., Capra J. D. Subunit structure and N-terminal amino acid sequence of the three chains of cholera enterotoxin. Immunochemistry. 1976 Jul;13(7):605–611. doi: 10.1016/0019-2791(76)90173-7. [DOI] [PubMed] [Google Scholar]
- Knoop F. C. The interaction of cholera toxin subunit A with cultured adrenal cells. Can J Microbiol. 1978 Aug;24(8):915–921. doi: 10.1139/m78-153. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Affinity filters, a new approach to the isolation of tox mutants of Vibrio cholerae. Proc Natl Acad Sci U S A. 1978 Feb;75(2):941–945. doi: 10.1073/pnas.75.2.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. T., Clements J. D., Finkelstein R. A. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect Immun. 1976 Aug;14(2):527–547. doi: 10.1128/iai.14.2.527-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch F. E., Jr, Murphy J. R., Graf L. H., Field M. Isolation of nontoxinogenic mutants of Vibrio cholerae in a colorimetric assay for cholera toxin using the S49 mouse lymphosarcoma cell line. J Infect Dis. 1978 Jun;137(6):747–755. doi: 10.1093/infdis/137.6.747. [DOI] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J. Synergistic protective effect in rabbits of immunization with Vibrio cholerae lipopolysaccharide and toxin/toxoid. Infect Immun. 1976 Mar;13(3):735–740. doi: 10.1128/iai.13.3.735-740.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Holmes R. K., Finkelstein R. A. Studies on toxinogenesis in Vibrio cholerae. II. An vitro test for enterotoxin production. Infect Immun. 1974 Jan;9(1):195–197. doi: 10.1128/iai.9.1.195-197.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward W. E., Gilman R. H., Hornick R. B., Libonati J. P., Cash R. A. Efficacy of a live oral cholera vaccine in human volunteers. Dev Biol Stand. 1976;33:108–112. [PubMed] [Google Scholar]