Abstract
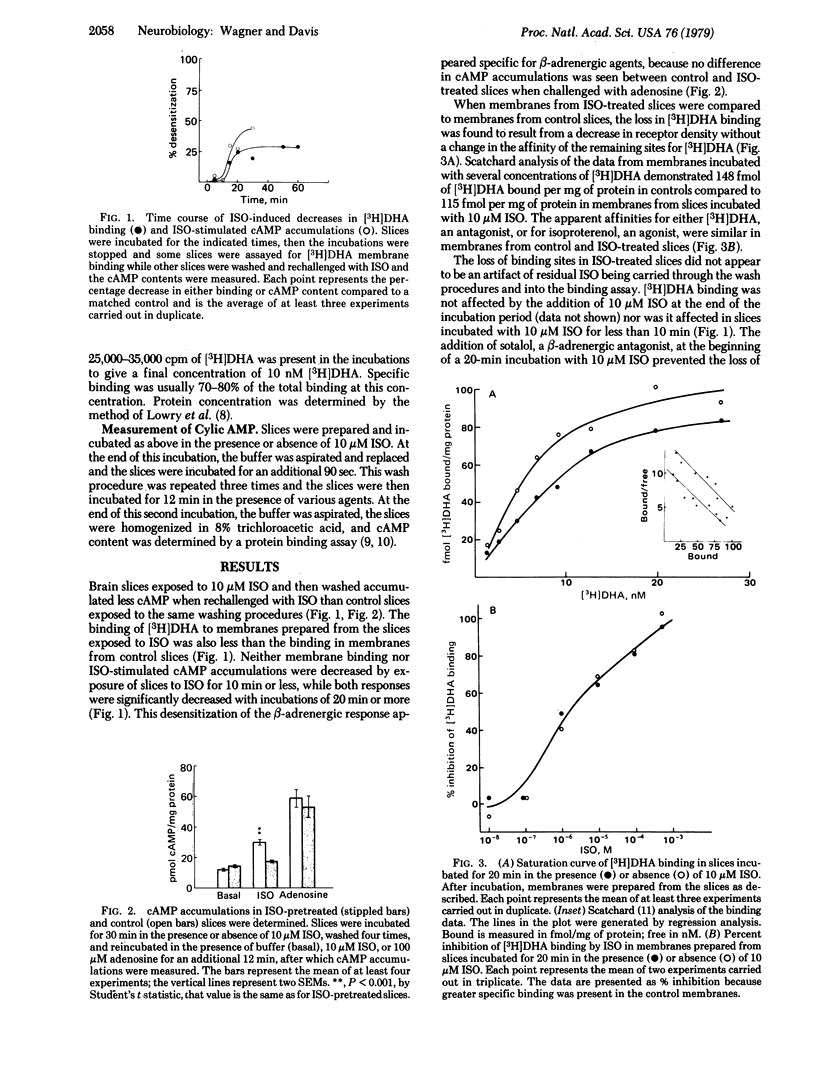
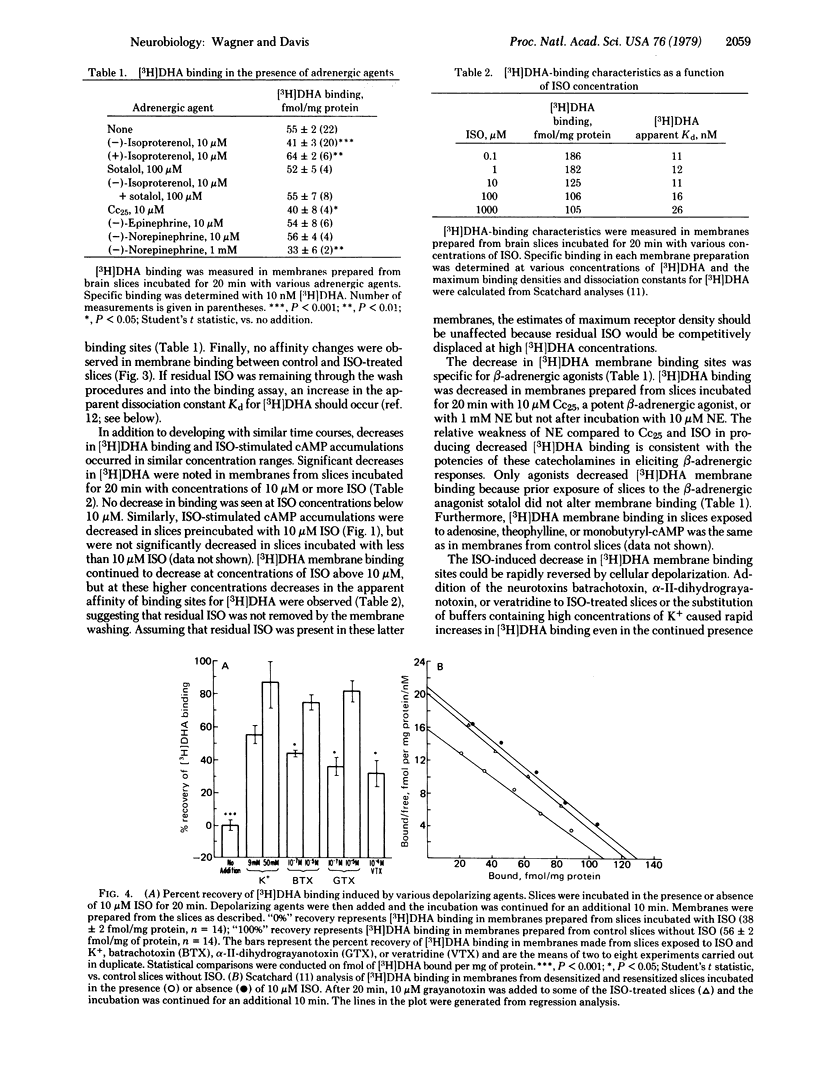
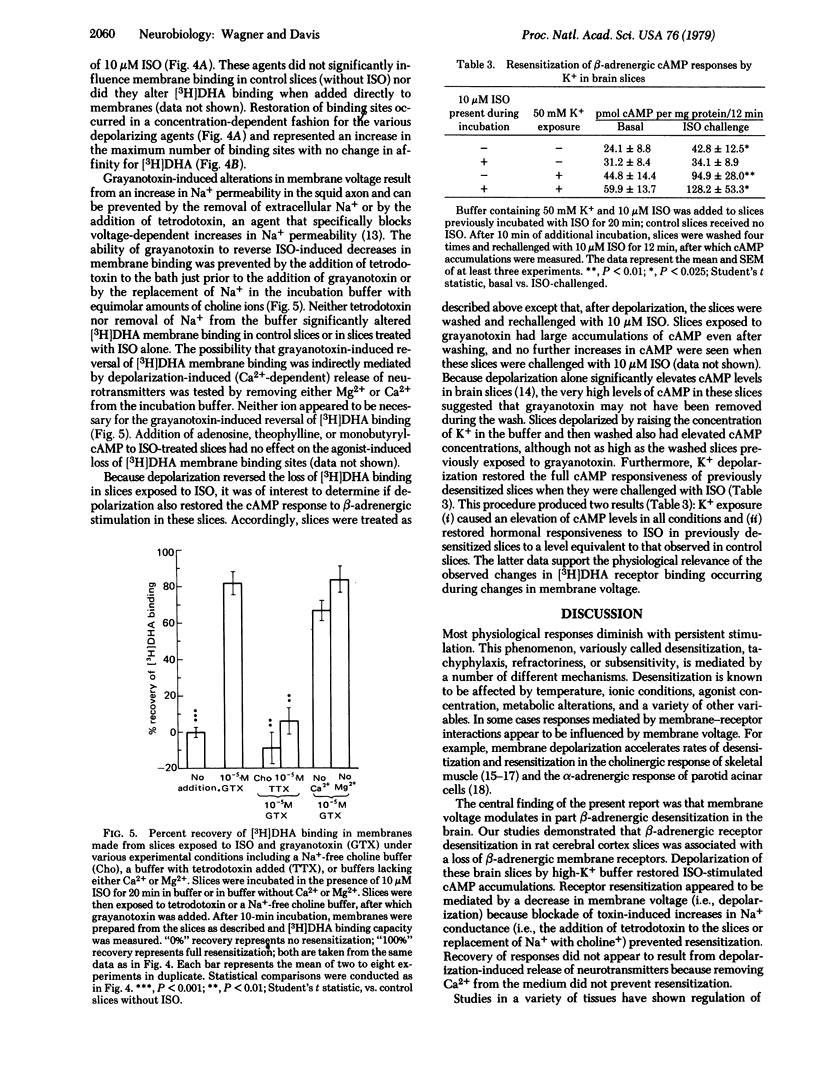
Rat cerebral cortex slices exposed to (-)-isoproterenol and then washed accumulated significantly less cyclic AMP when rechallenged with isoproterenol than did control slices. The isoproterenol-induced desensitization was associated with a concurrent reduction in [3H]dihydroalprenolol membrane binding but with no change in the affinity of [3H]dihydroalprenolol or isoproterenol for the binding sites. beta-Adrenergic receptor desensitization was rapidly reversed by slice depolarization with high-[K+] buffers, batrachotoxin, grayanotoxin, or veratridine, even in the continued presence of isoproterenol. Restoration of binding by grayanotoxin was prevented by tetrodotoxin or by removing Na+ from the buffer. These data demonstrate partial participation of the membrane receptor in beta-adrenergic desensitization of rat brain slices and suggest that brain beta-adrenergic receptors, like cholinergic receptors in skeletal muscle and alpha-adrenergic receptors in rat parotid, are regulated in part by membrane voltage.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. W., Davis J. N., Lefkowitz R. J. Direct identification and characterisation of beta-adrenergic receptors in rat brain. Nature. 1975 Dec 4;258(5534):437–440. doi: 10.1038/258437a0. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Huang Y. C., Breckenridge B. M., Wolff D. J. Identification of a calcium-binding protein as a calcium-dependent regulator of brain adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Jan;72(1):64–68. doi: 10.1073/pnas.72.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund D. B., Snyder S. H. Beta adrenergic receptor binding in membrane preparations from mammalian brain. Mol Pharmacol. 1976 Jul;12(4):568–580. [PubMed] [Google Scholar]
- Catterall W. A., Ray R., Morrow C. S. Membrane potential dependent binding of scorpion toxin to action potential Na+ ionophore. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2682–2686. doi: 10.1073/pnas.73.8.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Hoyler E., Davis J. N. Failure to find beta-adrenergic receptor binding in guinea pig cerebral cortex with [3H]dihydroalprenolol. Brain Res. 1977 May 27;127(2):313–316. doi: 10.1016/0006-8993(77)90547-9. [DOI] [PubMed] [Google Scholar]
- Davis J. N., Lefkowitz R. J. Beta-adrenergic receptor binding: synaptic localization in rat brain. Brain Res. 1976 Aug 20;113(1):214–218. doi: 10.1016/0006-8993(76)90023-8. [DOI] [PubMed] [Google Scholar]
- DeVellis J., Brooker G. Reversal of catecholamine refractoriness by inhibitors of RNA and protein synthesis. Science. 1974 Dec 27;186(4170):1221–1223. doi: 10.1126/science.186.4170.1221. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRASTYAN E., LISSAK K., MADARASZ I., DONHOFFER H. Hippocampal electrical activity during the development of conditioned reflexes. Electroencephalogr Clin Neurophysiol. 1959 Aug;11(3):409–430. doi: 10.1016/0013-4694(59)90040-9. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnegy M. E., Uzunov P., Costa Regulation of dopamine stimulation of striatal adenylate cyclase by an endogenous Ca++ -binding protein. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3887–3890. doi: 10.1073/pnas.73.11.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi S., Rall T. W., McIlwain H. The effect of electrical stimulation upon the accumulation of adenosine 3',5'-phosphate in isolated cerebral tissue. J Neurochem. 1969 Apr;16(4):485–491. doi: 10.1111/j.1471-4159.1969.tb06847.x. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Rall T. W. The influence of chemical agents on the accumulation of adenosine 3',5'-Phosphate in slices of rabbit cerebellum. Mol Pharmacol. 1968 Jul;4(4):367–378. [PubMed] [Google Scholar]
- Kalisker A., Rutledge C. O., Perkins J. P. Effect of nerve degeneration by 6-hydroxydopamine on catecholamine-stimulated adenosine 3',5'-monophosphate formation in rat cerebral cortex. Mol Pharmacol. 1973 Sep;9(5):619–629. [PubMed] [Google Scholar]
- Kordas M. The effect of membrane polarization on the time course of the end-plate current in frog sartorius muscle. J Physiol. 1969 Oct;204(2):493–502. doi: 10.1113/jphysiol.1969.sp008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lauzon G. J., Kulshrestha S., Starr L., Bär H. P. On the accumulation of adenosine 3':5'-monophosphate in Ehrlich cells and adenylate cyclase desensitization in response to epinephrine. J Cyclic Nucleotide Res. 1976;2(2):99–114. [PubMed] [Google Scholar]
- Levin S. V., Rozental' D. L., Gol'fand K. A., Komissarchik Ia Iu. Izmenenie sorbtsii anionnogo krasitelia--biriuzovogo priamogo svetoprochnogo--membranami aksona kraba pri vozbuzhdenii. Tsitologiia. 1968 Mar;10(3):312–321. [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocil F. Dependence of acetylcholine desensitization on the membrane potential of frog muscle fibre and on the ionic changes in the medium. J Physiol. 1970 Oct;210(3):507–518. doi: 10.1113/jphysiol.1970.sp009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickey J. V., Tate R., Mullikin D., Lefkowitz R. J. Regulation of adenylate cyclase-coupled beta adrenergic receptor binding sites by beta adrenergic catecholamines in vitro. Mol Pharmacol. 1976 May;12(3):409–419. [PubMed] [Google Scholar]
- Nahorski S. R. Altered responsiveness of cerebral beta adrenoceptors assessed by adenosine cyclic 3',5'-monophosphate formation and (3H)propranolol binding. Mol Pharmacol. 1977 Jul;13(4):679–689. [PubMed] [Google Scholar]
- Nahorski S. R. Association of high affinity stereospecific binding of 3H-propranolol to cerebral membranes with beta adrenoceptors. Nature. 1976 Feb 12;259(5543):488–489. doi: 10.1038/259488a0. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Resolution of some components of adenylate cyclase necessary for catalytic activity. J Biol Chem. 1977 Oct 25;252(20):6966–6969. [PubMed] [Google Scholar]
- Schultz J., Daly J. W. Acummulation of cyclic adenosine 3', 5'-monophosphate in cerebral cortical slices from rat and mouse: stimulatory effect of alpha- and beta-adrenergic agents and adenosine. J Neurochem. 1973 Nov;21(5):1319–1326. doi: 10.1111/j.1471-4159.1973.tb07585.x. [DOI] [PubMed] [Google Scholar]
- Schultz J., Daly J. W. Cyclic adenosine 3',5'-monophosphate in guinea pig cerebral cortical slices. I. Formation of cyclic adenosine 3',5'-monophosphate from endogenous adenosine triphosphate and from radioactive adenosine triphosphate formed during a prior incubation with radioactive adenine. J Biol Chem. 1973 Feb 10;248(3):843–852. [PubMed] [Google Scholar]
- Seyama I., Narahashi T. Increase in sodium permeability of squid axon membranes by -dihydrograyanotoxin II. J Pharmacol Exp Ther. 1973 Feb;184(2):299–307. [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Sporn J. R., Molinoff P. B. beta-Adrenergic receptors in rat brain. J Cyclic Nucleotide Res. 1976;2(3):149–161. [PubMed] [Google Scholar]
- Strittmatter W. J., Davis J. N., Lefkowitz R. J. alpha-Adrenergic receptors in rat parotid cells. II. Desensitization of receptor binding sites and potassium release. J Biol Chem. 1977 Aug 10;252(15):5478–5482. [PubMed] [Google Scholar]
- Su Y. F., Harden T. K., Perkins J. P. Isoproterenol-induced desensitization of adenylate cyclase in human astrocytoma cells. Relation of loss of hormonal responsiveness and decrement in beta-adrenergic receptors. J Biol Chem. 1979 Jan 10;254(1):38–41. [PubMed] [Google Scholar]
- Williams L. T., Lefkowitz R. J. Slowly reversible binding of catecholamine to a nucleotide-sensitive state of the beta-adrenergic receptor. J Biol Chem. 1977 Oct 25;252(20):7207–7213. [PubMed] [Google Scholar]



