Abstract
The design and engineering of innovative biopolymer-based biomaterials for a variety of biomedical applications should be based on the understanding of the relationship between their nanoscale structure and mechanical properties. Down the road, such understanding could be fundamental to tune the properties of engineered tissues, extracellular matrices for cell delivery and proliferation/differentiation, etc. In this tutorial review, we attempt to show in what way biomaterial structural data can help to understand the bulk material properties. We begin with some background on common types of biopolymers used in biomaterials research, discuss some typical mechanical testing techniques and then review how others in the field of biomaterials have utilized small-angle scattering for material characterization. Detailed examples are then used to show the full range of possible characterization techniques available for biopolymer-based biomaterials. Future developments in the area of material characterization by small-angle scattering will undoubtedly facilitate the use of structural data to control the kinetics of assembly and final properties of prospective biomaterials.
Biopolymers in Biomaterials Research
The new emphasis in the field of tissue engineering has been on the design and engineering of biocompatible and/or biodegradable materials that aid in the regeneration of tissues (1). One of the most prevalent approaches involves employing biopolymer-based biomaterials as a support system for the delivery of cells and/or growth factors to damaged tissues (2,3). This undertaking requires that the biomaterial provides sufficient mechanical support during tissue rebuilding.
In addition to providing mechanical support, it is important for the biomaterial to support and sustain an environment that enables appropriate cellular growth, adhesion and differentiation. An appropriate cell environment is the key to eliciting new tissue development (4). As supports for cell growth, synthetic biodegradable polymers have been widely used (5). However, in order to stimulate or modulate new tissue development, synthetic materials must be supplemented with bioactive molecules like BMP (bone morphogenetic protein) and FGF-2 (fibroblast growth factor 2) to name a few (6). In contrast, naturally derived polymers have a built-in ability to stimulate tissue growth. Several of these biopolymers (e.g. chondroitin sulfate, heparan sulfate, collagen, etc.) are components of or mimic the extracellular matrix (ECM), thus they ordinarily interact with cells. For example, since the ECM is important in regulating elements of cell adhesion, division and differentiation, biopolymers are a good starting point for trying to reproduce native properties of tissues.
This review focuses on biomaterials assembled from natural polysaccharides and engineered peptides. Natural biomaterials are assembled mostly from polysaccharides (e.g., glycogen, chitosan) and polypeptides (e.g. elastin, collagen), while most of the soft tissues include the combinations of the above (e.g., proteoglycans). From a material engineering standpoint, the advantage of polysaccharides is structural diversity and commercial availability of numerous naturally occurring polysaccharides while the advantage of peptides is their programmability and ease of synthesis. Together, they offer ample engineering opportunities and challenges.
Polysaccharides
Alginate is one of the most commonly used biopolymers. It is used in a variety of medical applications including cell encapsulation and delivery because it is commercially available, gels under mild conditions and has relatively low cytotoxicity. Alginates are naturally derived polysaccharide block copolymers composed of regions of β-D-mannuronic acid monomers (M-blocks), regions of α-L-guluronic acid (G-blocks) (7). The percentage and distribution of each M or G monomer depends on the species of seaweed used for alginate isolation (8). Alginate gels are formed when divalent cations such as calcium and magnesium interact with negatively charged G-blocks to form ionic bridges between different polymer chains. The gelation of alginate is usually triggered by the addition of divalent metal cations (e.g., Ca2+, Zn2+, Mg2+) resulting in V-shaped chelate-like complexing of two adjacent sugar rings and general shrinkage of the whole polysaccharide chain. As a result, the mechanical properties and mesh size of alginate gels can be easily controlled by varying the M to G ratio, the molecular weight of the alginate and the type of divalent cation selected for cross-linking (9).
Cellulose is an inexpensive, readily available biopolymer in part because it is the most abundant organic polymer in the world (10). Cellulose can be easily modified with acetate, methyl or hydroxypropyl groups to name a few, which allows for the crystallization of cellulose derivatives with targeted narrow distributions of molecular weights and makes it desirable as a biomaterial (11). It is a linear polysaccharide composed of D-glucose units linked by glycosidic bonds where every other glucose residue is rotated about 180° (12,13). One such modified cellulose, hydroxypropylcellulose (HPC), is a neutral and water-soluble cellulose ether with a unique combination of properties including thermoplasticity, water solubility and surface activity (14). The advantages of cellulose ethers are that they are biocompatible and hence can be used for many pharmaceutical purposes (15).
Chitosan has been investigated for many different tissue engineering applications because it is structurally similar to GAGs (glycosaminoglycans) found in the extracellular matrix. Chitosan can also be degraded by enzymes, giving it desirable biodegradable properties (16). It is a linear polysaccharide made of D-glucosamine and N-acetyl-D-glucosamine units derived from chitin. Chitin is a natural polysaccharide found particularly in the exoskeleton of crustaceans and insects (17). The degree of N-deacetylation usually varies from 75% to 90% (8). Positively charged chitosan is soluble in dilute acids which protonate the free amino groups.
Chondroitin sulfate is one of the most abundant GAGs, found in cartilage, synovial fluid, bone, and heart valves (18). Chondroitin sulfate is a component of proteoglycans, covalently linked to a protein core (19). It is made of repeating disaccharide units of D-glucuronic acid and N-acetyl galatosamine, which are sulfated at either 4- or 6-positions (20). The benefit of using GAGs as biomaterials is having a material that can bind to and modulate growth factors and cytokines and is also involved in cell adhesion, proliferation and differentiation (21). Moreover, GAGs degrade to non-toxic oligosaccharides. These characteristics together with their defined physical and chemical characteristics make them very interesting biopolymers.
Also known as animal starch, glycogen is a neutral biopolymer made exclusively of D-glucose units. As a biodegradable material, glycogen is excellent since it degrades to simple glucose monomers. Glycogen is a polymer of α(1→4) glycosidic bonds, linked with α(1→6)-linked branches (22). Glycogen is a neutral polysaccharide with a molecular weight ranging from 400 kDa to millions of Da, depending on the amount of branching and polymerization (23). In the body, glycogen is polymerized by the protein glycogenin, usually stored in the liver and muscle tissue as an energy depot.
The following table (Table 1) gives a brief overview of some common polysaccharides utilized in our work and how they have been utilized by others in the field of tissue engineering.
Table 1.
Polysaccharide properties and their common uses in tissue engineering.
| Polysaccharide | Source | Charge | Shape | M.W range (kDa) | Tissue Engineering Applicability |
|---|---|---|---|---|---|
| Alginate | algae | negative | linear | 350-450 | bone,24 cartilage,25 blood vessels,26 cardiac tissue27 |
| Cellulose | plants | neutral | linear | 80 | Cartilage28-30 |
| Chitosan | crustacean shell | positive | linear | 100-1000 | bone,31 cartilage,31,32 nerve tissue31 |
| Chondroitin | cartilage | negative | linear | 20 | Cartilage33 |
| Glycogen | liver & muscles | neutral | branched | 400-several thousand | no known application |
Peptides
Peptide biopolymers are attracting interest as biomaterials due to their biodegradable, programmable and bioresorbable nature. Unlike synthetic polymers, peptides can be made with precise control over sequence, chain length and stereochemistry. Peptide-based networks with viscoelastic properties have been designed for various biomedical applications (34).
Peptide hydrogels created by Zhang et al. are based on an alternating sequence of hydrophobic and charged residues, (e.g. Arg-Ala-Asp-Ala) (35-38). These ionic self-complementary peptides self-assemble to form β-sheets in physiological media and tangle to create hydrogels. These hydrogels have served as scaffolds for human endothelial cells (38). They have also been shown to support liver cell differentiation into hepatocyte spheroids (37) and neural cell proliferation and differentiation (35, 36).
Schneider et al. created hydrogels with peptides termed ‘β-hairpin peptides’ (39-41). The β-hairpin peptides self-assemble in charge screening conditions, folding to hairpin and forming β-sheets. These β-sheets also interact and stack into fibers which in turn form a greatly entangled hydrogel. The β-hairpin peptide gels have been used to successfully encapsulate mesenchymal stem cells and hepatocytes (40). They have also been shown to support fibroblast growth, attachment and migration in vitro (39-41).
Stupp et al. created peptide amphiphiles (PAs) which self-assemble into biomaterials (42-46). It was shown that these materials could encapsulate cells, which were viable for several weeks and continued to proliferate (42). PAs were also modified with an RGD sequence and then used to deliver therapeutic bone marrow mononuclear cells in vivo (43). It was found that the oliopeptide material contributed significant support to the cells following injection. The RGD PAs were also used as a coating to improve bladder cell attachment to a poly(glycolic) acid scaffold in vitro (44). By incorporating the neural cell adhesion sequence, IKVAV, into their PAs, the Stupp group was also able to create peptide materials that enhance selective differentiation of neural progenitor cells into neurons in vitro (45) and improve motor and sensory axon regeneration in vivo (46).
Yu et al. designed a set of mutually complementary, self-repulsive peptide modules to gain better control and tunability of the assembling process (47). These peptide modules assemble into a hydrogel upon mixing oppositely charged peptide modules. As opposed to the above mentioned self-assembling peptide systems, such separation of positively and negatively charged peptide amino acids into different peptide modules has several advantages. For example, it provides high flexibility to control pH, temperature, concentration, and ionic strength of peptide solutions which is very limited in self-assembling systems. One of the co-assembled hydrogels was capable of rapid recoveries from repeated shear-induced breakdowns, a property desirable for designing injectable biomaterials (47). The compatibility of these hydrogels with entrapped biomolecules was also confirmed using high-resolution, 1H-15N heteronuclear NMR spectroscopy (48). Sticky-ends in the peptide fibers did not provide any advantages over blunt-ends in terms of material properties, suggesting that peptides might be aligned perpendicular to the fiber axis (49). Using pulsed-field gradient NMR techniques, it was found that the retardation of the diffusion of small molecules inside this type of hydrogels increases linearly with their logP values of partition coefficients which are the measure of their hydrophobicity (50). These hydrogels can be used to culture human mesenchymal stem cells and negative charges can improve the biocompatibility of hydrogels assembled from D-peptides (51).
Peptide-Polysaccharide Composite Materials
Since the ECM contains both polypeptides and polysaccharides, groups have developed composite materials from peptides and polysaccharides in an effort to make better ECM mimetics. According to Yamada et al., addition of a laminin active peptide into agarose gels resulted in a matrix that could support both the 2D and 3D culture of a variety of cells (52). This group also observed improvements in cell behavior when peptides were incorporated in chitosan and alginate gels (53). Conjugation of heparin, a linear glycosaminoglycan, to peptides such as collagen, gelatin and fibrin has been highly attractive since these conjugations sequester growth factors and prevent their bulk release (54-58). The Guan group has designed saccharide-peptide hydrogels that can be used to tailor cell responses by varying the cross-linking time and thereby, varying the mechanical modulus (59). Stupp et al. combined high molecular weight hyaluronic acid with their peptide amphiphiles to create composite sacs that could maintain human mesenchymal stem cell viability for up to four weeks (60). By combining charged polysaccharides with charged peptide modules, we found that peptides improved the stiffness of pre-assembled polysaccharide networks and polysaccharides improved the resistance of peptide-based hydrogels to strain break (61). Structural analysis of these composite peptide-polysaccharide networks showed strong interactions between the charged polysaccharides and peptides.
Mechanical Testing in Biomaterials Research
When creating new biomaterials for implantation, the primary goal is to restore function or integrity to the tissue or biological component. For example, in an arthritic joint, the goal of an implant is to restore the smooth surface at the ends of the bones. The mechanical requirements on an implant of this type would be quite demanding. For such reasons, in addition to biocompatibility testing, biomaterials created for this purpose would need to undergo mechanical testing which could mimic conditions that the implant would encounter in the body. Mechanical testing gives researchers an insight into the longevity and success of a material in different mechanical environments.
Compression-Tensile Testing
The measurement of the mechanical behavior of a sample under compression and tension can be performed to provide basic biomaterial mechanical data that is critical for material design and performance assessment. The requirements for compression and tensile strength values and the methods for testing these properties are specified in various standards (i.e., ASTM, American Society for Testing and Materials) for a wide variety of biomaterials.
The compression test is a method for determining the behavior of biomaterials under a compressive load. Compression tests are performed by loading the sample between two plates, and then applying a force to the sample by moving the plates together. During the test, the sample is compressed and deformation versus the applied load is recorded. The compression test is used to determine yield point, yield strength, and compressive strength.
The tensile test is a method for determining behavior of biomaterials under tensile loading. The tests are conducted by fixing the sample into the test instrument and then applying a force to the sample by separating the testing machine crossheads. The crosshead speed can be varied to control the rate of strain in the test sample. Data from the tests are used to determine yield strength and elastic modulus. Measurement of the sample dimensions after testing also provides elongation values to characterize material ductility. In compression and tensile testing, the applied force is perpendicular to the surface of the material being tested. For reliable measurements, such force should be low enough to ensure linear dependence between the applied stress and the strain of the tested biomaterial.
Oscillatory Rheometry
Another aspect of the mechanical properties of soft materials is how they respond to shear forces, which are parallel to the surface of the material being tested. This is accomplished using an oscillatory rheometer. An oscillatory rheometer is a device that can be used to measure the viscoelastic properties of soft biomaterials. The basic principle of an oscillatory rheometer is to make cyclic shear deformations in the sample and then measure the resulting stress responses. The tests are conducted by placing the sample between two plates. While the bottom plate remains stationary, a motor rotates the top plate, which imposes a time-dependent strain on the sample. Simultaneously, the time-dependent stress is quantified by measuring the torque that the sample imposes on the top plate. Common measurements include time-sweep, which measures time evolution of the oscillation frequency-dependent linear viscoelastic moduli, G′(ω) (elastic modulus) and G″(ω) (viscous modulus), frequency-sweep, which shows material dependence on angular frequency of the applied stress and strain-sweep, which measures biomaterial strain yield values (γyield).
Rheometry is a common tool in biopolymer-based hydrogel mechanical characterization because rheometers are relatively inexpensive and are small enough to fit on a bench top. Additionally, rheometry theory and measurements have been well described (62,63). Rheometry is the most prevalent method for the mechanical characterization of peptide-based hydrogels. A thorough review of this subject has been made by Yan and Pochan (64). Rheometry is also a common method for the mechanical characterization of polysaccharide hydrogels. Due to the prevalence of polysaccharides in food preparation, as thickeners and texturizers for example, rheometry of polysaccharide gels has also been reviewed in detail elsewhere (65-67). With growing interest in utilizing polysaccharide-based hydrogels as biomaterials, many new polysaccharide materials and polysaccharide blends have been characterized by rheometry. This area of study is quite robust and out of the scope of this review.
Compression-Tensile Testing vs. Oscillatory Rheometry
Both compression-tensile testers and rheometers are excellent choices for biomaterial mechanical testing. Rheometers are a better choice for testing very soft materials since these materials can be difficult to work with in a compression-tensile tester. Rheometry also allows one to monitor material stiffness changes over time, e.g., kinetics of the gelation, which could not be as easily done using a compression-tensile tester. However, when working with stronger materials, compression-tensile testers provide a wider range of mechanical tests. Because rheometers measure shear moduli and compression-tensile testers measure Young's moduli, these two instruments can also complement each other, giving a better overall picture of biomaterial properties. In biological tissues, the Young's modulus E and the shear modulus G are related by a scaling factor of 3 as E = 3G (68).
Small Angle Scattering in Biomaterials Research
Structural characterization is an important tool in understanding mechanical properties of biomaterials. To understand how structural features of materials translate to their bulk mechanical characteristics is of paramount importance for targeted design of novel biomaterials with preset properties. There are many characterization techniques available, including electron microscopy, atomic force microscopy, X-ray diffraction, small-angle scattering and polarized light microscopy. Utilizing these techniques in combination with mechanical characterization techniques can provide a wealth of information about novel biomaterials. However, the majority of the above listed techniques require special procedures for sample preparation (heating, freezing, drying, vitrification, etc.) which in case of biomaterials may alter the structure significantly. For example, using scanning electron microscopy (SEM), it was found that increasing material crystallinity and crosslinking of polyethylene increased the size of the material microstructure, which increased both wear and fatigue resistance (69). Even in this case of a rather robust polymer like polyethylene, one cannot totally exclude that fixation, drying and metal coating of the sample for SEM has not interfered with the material structure. Using differential scanning calorimetry (DSC) and X-ray diffraction, a relationship between increased triple-helix content in gelatin and its increased mechanical properties was found (70). The structure of cross-linked collagen was also monitored with SEM, which showed that under an applied load collagen morphology could straighten and result in a stiffer material (71). Similarly, in both above mentioned studies, collagen samples were heated, dried and/or metal coated before the analysis. This is one of the reasons why small-angle scattering is a technique which is becoming more commonly utilized in sensitive biomaterials characterization. It is unique from other techniques because samples can be analyzed in situ without special preparations such as freezing, drying or dying, which as has been mentioned above can often alter the sample structure.
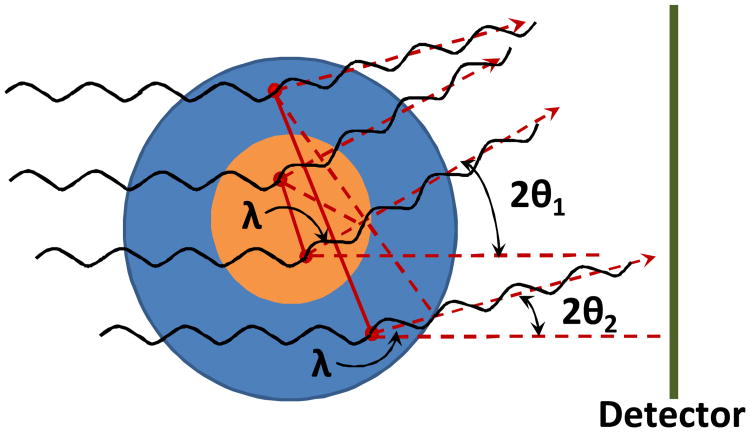
Small-angle scattering (SAS, angle ∼0-2°) has become a powerful tool for elucidating the structure of biomaterials. SAS is a scattering method based on the small deflection of collimated radiation away from the straight path after it interacts with structures that are larger than the wavelength of the radiation (72). The “angle” in SAS refers to the angle of radiation deflection (Figure 1). SAS techniques can give information about the shape and size of structures in a sample. SAS is used more often in biomaterials research than techniques like wide-angle scattering (WAS, angle ∼2-90°) because WAS tells about the amount of crystallinity and the higher organized structures within a sample. Often, biomaterials are fairly amorphous so WAS is not very informative. Small-angle X-ray scattering (SAXS) and small-angle neutron scattering (SANS) are two SAS techniques, each with their own advantages and disadvantages. Both techniques have been used extensively to characterize biomaterials and are described in more detail in this review.
Fig. 1.
Pictorial presentation of small-angle scattering in the case of the particles of different size. 2θ1 and 2θ2 are the angles between incident and scattered waves from two particles, λ is a wavelength of incident radiation and is a path difference between two waves scattered from two arbitrary points within a particle. Since the scattering is elastic, the wavelength of the scattered radiation is unchanged and also equals to λ. Red dashed arrows show the direction of the scattered radiation to the detector.
Structural information from SAXS and SANS
One might argue that it was the appearance of the seminal book of Glatter and Kratky (73) that indeed ignited the interest, first, in the structural biology community and later, in the biomaterials community to use SAS as a key to get much needed structural information. This book literally paved way to understanding how to extract important structural data from scattering profiles, and relate them to various properties of (bio)macromolecules and/or their assemblies. The concepts of the radius of gyration (Rg) and maximum particle dimension (Dmax) have been since successfully employed to characterize the dimensional features of the scatterers. Pair-wise distance distribution functions (P(r)) derived from the scattering profiles were indicative of the shapes of the scattering species as well as the most probable set of distances in their molecules. Rapid onset of software development significantly simplified the data processing and expanded the user community significantly. ATSAS (74), NCNR SANS from NIST (75), IRENA & NIKA (76) suites became the indispensable instruments to study structure-property relationships in chemistry, biology, and materials science. Unfortunately, a real fly in the ointment for biomaterial applications is that in most cases the reliable conclusions from SAXS/SANS data could be made for monodisperse systems, i.e., formed by essentially identical particles. Biomaterials, on the contrary, are characterized by a significant amount of polydispersity. However, they are often assembled from the particles with the same morphology, e.g., fibers. Therefore, when characterizing biomaterials, more important parameters would be radius of gyration of the fiber cross-section (Rc), reflecting the size of the fiber cross-section; correlation length (Lc), reflecting the mesh size of the network (77); persistence length of the fiber (Lp) (78) which is also closely related to the mechanical properties of biomaterial—MacKintosh theorem (79); mass-fractal dimension (df), reflecting the morphology of the ‘building brick’ of the network (80), to name a few. An important achievement was the possibility to model the 2D shape of a fiber cross-section (81) which makes it possible not only to visualize the shape of the cross-section, but also to estimate its area and mass per unit length of the fiber. More recently, a novel approach, QUAFIT, has been suggested to analyze polydisperse mixtures of aggregates formed from the same initial monomer. The program fits the experimental scattering data on the assumption that any given aggregate is a result of optimum assembly of monomers according to a given point-symmetry group (82). QUAFIT has been successfully applied to reconstruct the low resolution shapes of the decameric hemocyanine aggregates and to study the kinetics of their assembly and dissociation (83).
There is a plethora of reviews devoted to SAXS/SANS application to study the structure-property relationships in a variety of materials. Fratzl (84) discusses SAXS/SANS techniques in material science to unravel the structure of alloys, ceramic and crystalline materials including very inhomogeneous systems. Jacques and Trewhella (85) discuss mainly the applications of SANS in structural biology. However, this review is extremely useful for researchers in other fields of science, too, since it provides step by step guidance helping to organize the experiment, to analyze the data, and to avoid most common mistakes and to resolve the most frequent problems. Of great interest is the review by Putnam (86) where the authors discuss not only the ways to use SAXS in the analysis of solution structures of biomacromolecules, but touch on the problem of comparison of the low resolution structure in solution with high resolution crystal structure. The complex issue of biomacromolecular assembly in solution is also considered. Of particular importance is the review by Guilbaud and Saiani (87) specifically devoted to the peptide-based self-assembled materials and hydrogels. Here, the authors discuss the radius of gyration concept and its connection to bulk material properties, special attention is also paid to the analysis of mass-fractal dimensions and their link to the morphology of assembled materials.
Below, we will discuss each technique in more detail and consider their advantages and weaknesses from an experimental viewpoint.
Small-Angle X-ray Scattering (SAXS)
SAXS is a widely used technique in the biomaterials community. It is especially used to determine basic structural characteristics such as material correlation length and fiber mass fractal dimension. Gamani et al. used SAXS to characterize cross-linked hylaronan. Using a scattering profile, I(Q) vs. Q, they determined the polymer junction zone for each gel type (88). SAXS was also used to study how the chemical compositions and concentrations of calcium-alginate gels affected their structures. It was found that lateral association of chain segments, which dictates junction zone lengths, depends on a number of material factors elucidated by SAXS (89) Shinohara et al. used SAXS to investigate the structure of slide-ring gels in various solvent types. They were able to see a “pulley effect”, a unique property of their gels, by simply examining the shape of the raw scattering data (90). Tada et al. used SAXS to study the change in structure of curdlan gels over time (91). SAXS was also used to examine changes to hydroxyapatite size in bone after extreme heating (92). Choudhary et al. found that hydrophobic modification of alginate hydrogels resulted in different SAXS scattering profiles. These profiles were evaluated using fractal analysis and they found that there were different regions of more loosely or tightly packed networks which they attribute to regions of hydrophilic or hydrophobic interactions (93).
Small-Angle Neutron Scattering (SANS)
SANS is another scattering technique used in biomaterials characterization. Since there is a large difference between neutron scattering lengths of hydrogen and deuterium, changing the ratio of H2O/D2O in the solvent (contrast variation/matching) often allows to exclude one or more components in the multi-component systems and to elucidate their structural organization.
Markarian et al. used SANS contrast matching to study single chain dimensions of novel materials made from sulfonated poly(styrenesulfonate) and poly(diallyldimethylammonium chloride) at different ionic strengths (94). Others use SANS to examine molecular distribution and conformation. Wilson et al. used both SANS and ultra small-angle neutron scattering (USANS) to measure silica nanoparticle dispersion in dental nanocomposites (95). Luk et al. characterized the distribution of water in semicrystalline and amorphous polymers (96). Feuz et al. determined the conformations of poly(L-lysine)-graft-poly(ethylene glycol) molecular brushes (97). Still others used SANS in conjunction with rheometry to measure the structural response of fibrin gel to shear deformation in so-called SANS rheology experiments which will be addressed in more detail below (98).
SAXS vs. SANS
Due to an often high flux of the X-ray beam from the synchrotron, modern 2D detectors in a fixed position that allow the capture of a wide range of Q-values, data acquisition of a SAXS profile even for the low scatterers could be completed within seconds (99). This makes time-dependent structural studies possible and allows one to monitor the kinetics of biomaterial assembly. For SANS, this task is much more challenging. First, high-flux neutrons are required, and those might be obtained from spallation sources or high-flux reactors (100). Second, to cover the widest possible range of Q-values the plane of detector in SANS is positioned at different distances from the sample in order to vary the captured angle 2θ. All such movements of the detector plane require significant time, and this makes kinetics data collection for the full Q-range almost impossible. However, in case of high scatterers, slow reaction, and narrow Q-range, high-flux SANS still allows one to monitor the kinetics of large assembly formation. For example, micelle to vesicle transition for lecithin in the presence of bile salt has been successfully monitored by SANS, however, with > 60 s time resolution at a fixed detector plane position within a Q-range from only 0.006 to 0.06 Å-1 (101). In general, kinetic studies are much less feasible with SANS, especially if a nuclear reactor is used as a source of cold neutrons and the sample contains low scatterers, a common feature of most biomaterials, such as hydrogels, which usually contain less than 1% w/w of material in water. In this case, data collection takes much longer time, usually from 1 to 2 hours. Smaller sample size and sample preparation in H2O are other practical advantages of SAXS, while due to high neutron capture cross-section of H atom, SANS samples must be preapred in D2O. Additional advantage of SAXS is its higher monochromicity (both from a synchrotron and X-ray tube), while SANS data usually have certain contribution from neutron wavelength smearing. However, SANS is much less harmful for most biomaterials, while doing SAXS, the user should always be aware of possible sample degradation under the action of X-rays (radiolysis, solvated electron, etc.). Moreover, SANS opens wider possibilities to elucidate different structural features of multicomponent hydrogels. Using the technique of contrast variation, which involves the varying ratio of D2O/H2O in the sample, one could “mask” the scattering of each component separately (102). Technically, SANS also allows one to detect the scattering at lower angles as compared to SAXS, thus providing the observation of larger scattering particles. The maximum scattering size is defined as d = 2π/Q, where Q = 4πsinθ/λ and 2θ is a scattering angle, thus the smaller angles correspond to bigger sizes (usually > 1000 Å for SANS and ∼ 600–700 Å for SAXS, see also Figure 1).
SAXS and SANS Rheology
Undoubtedly, the structure-property relationships in biomaterials would be much better understood if one would be able to monitor the response of material to shear force and simultaneously observe the structural changes evoked by such force at the nanoscale level. Attempts to create such an attractive and comprehensive research technique have been implemented in SAXS/SANS rheology. However, the present state of these techniques does not allow one to perform the full sequence of oscillatory rheometry experiments mentioned above. For example, it is very difficult, if at all possible, to measure the frequency spectrum (frequency-sweep experiments) due to the significantly different time scale of the SANS measurement and the duration of the shear force application (at low frequencies the time could be ∼1000 s, while at high frequencies it would drop to ∼0.06 s). Yield-sweep experiments are also not straightforward, since the material could break much faster than the existing SANS setup would be able to detect it. Thus, at present, the most reliable data in SAXS/SANS rheology could be obtained only in the experiments where the structural changes of material are monitored under shear flow or oscillatory shear stress (time-sweep experiments (103).
For example, a SAXS shear-cell was used to demonstrate the dynamics of molecular orientation of liquid crystalline polymers to reveal the anisotropy and estimate the orientation angle under different shear-strain (104), particle orientation and assembly and decay of particle networks has also been shown in biopolymer/clay dispersions (105). SAXS and WAXS also demonstrate the increase in biopolymer/clay particle orientation with the growth in shear rate, relaxation effects after the flow cessation were also observed (106). An original shear cell has been designed to monitor the structure of fibrin gel under variable strain—strain-hardening effects were observed which strongly correlated with structural transitions in fibrin fibrous networks (98). A more sophisticated SANS rheology setup based on a commercial rheometer with titanium concentric cylinder geometry has been used to study sol-gel transitions in organogels, and this setup has allowed one to capture a significant Q-range (0.002-0.2 Å-1) and to monitor in situ the formation and dissolution of fibers (107). Still, despite the above mentioned limitations SAXS/SANS rheology provides an interesting insight into the structural changes in biomaterials under stress, and its constraints very likely will be overcome in the future.
We next summarize our work as two case studies to illustrate how mechanical studies in conjunction with SAS studies help to elucidate the properties of engineered materials. The first case study involves peptide-based hydrogels. The second case involves peptide-polysaccharide composite materials.
Case Study 1: Using SAS to Understand the Mechanical Advantage of Homochirality in Peptide Hydrogels
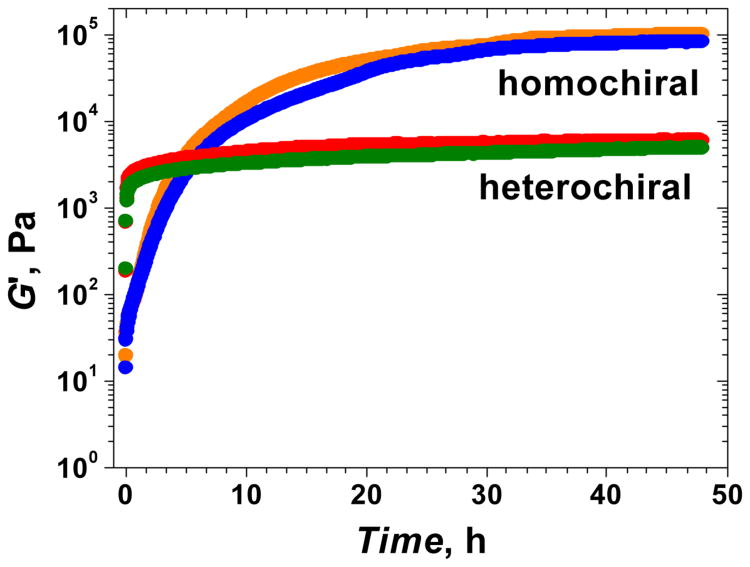
The term chirality is used to describe the structural property of an object that is non-superimposable on its mirror image. Interestingly, biomacromolecules are homochiral, where proteins contain exclusively L-amino acids while nucleic acids and most polysaccharides contain exclusively D-sugars. As a result, biochemical interactions within the body are chirality-dependent (108). Recently, our lab found a mechanical advantage for biomacromolecule homochirality (109). We found that oliopeptide hydrogels containing either all L-peptides or all D-peptides were mechanically stiffer than hydrogels containing a mix of L- and D-peptides (Figure 2). The peptides used for each gel type are listed in Table 2 below. Using both SAXS and SANS together, we were able to explain the mechanical advantage of homochirality in peptide hydrogels.
Fig. 2.
Dynamic rheological characterization of heterochiral and homochiral peptide pairs. Homochiral pairs lead to higher G′ values at 48 h of gelation. Blue: (L+L−); Orange: (D+D−); Green: (L+D−); Red: (D+L−). Reprinted with permission from ref. 109. Copyright 2012 American Chemical Society.
Table 2. Name, sequence, charge and M. W. of peptide modulesa.
| Name | L-peptide module | D-peptide moduleb | Name | chargec | M.W. (Da) |
|---|---|---|---|---|---|
| L+ | Ac-LK-LW-(LK-LA)3-LK-LW-LK-am | Ac-DK-DW-(DK-DA)3-DK-DW-DK-am | D+ | +6 | 1,413 |
| L- | Ac-LE-LW-(LE-LA)3-LE-LW-LE-am | Ac-DE-DW-(DE-DA)3-DE-DW-DE-am | D- | -6 | 1,419 |
A: alanine; E: glutamic acid; K: lysine; W: tryptophan; Ac-: acetylation; -am: amidation.
The two positive modules, L+ and D+, form a mirror image pair. So do the negative modules, L− and D−.
Gelation is induced by pairing oppositely charged peptide modules. (L+L−) and (D+D−) are two homochiral gels that are mirror images of each other; (L+D−) and (D+L−) are two heterochiral gels that are mirror images of each other.
The combined usage of SAXS and SANS techniques to characterize hydrogel structures has significant advantages. Due to the high flux of an X-ray beam from a synchrotron, we were able to reduce the data collection time down to 0.2 s, which facilitated the use of SAXS to monitor the gelation process in real time. For SANS, in comparison, the data collection time is about 1–2 h, making it unsuited for monitoring the gelation process. On the other hand, because of the different wavelength parameters (0.689 Å for SAXS vs. 6 Å and 8 Å for SANS) and detector setup, the SANS instrument allows collection of much lower Qmin values as compared to the SAXS (0.001 Å−1 for SANS vs. 0.007 Å −1 for SAXS). Lower Q values provide the possibility to reliably observe molecular assemblies of much greater size (up to ∼2000 Å for SANS vs. up to ∼500 Å for SAXS).
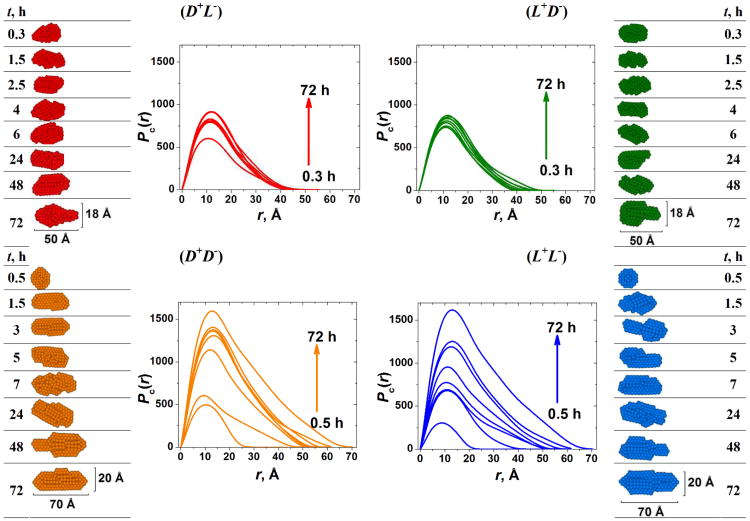
Using SAXS, we found that kinetics for fiber growth in homochiral gels began more slowly than for heterochiral gels but eventually the final dimensional characteristics of the homochiral fibers outgrew those of the heterochiral fibers (Figure 3).
Fig. 3.
SAXS monitoring of the gelation process. Left and right columns show the time evolution of the 2D average cross-section of the peptide fibers. Red: (D+L−); Green: (L+D−); Orange: (D+D−); Blue: (L+L−). Reprinted with permission from ref. 109. Copyright 2012 American Chemical Society.
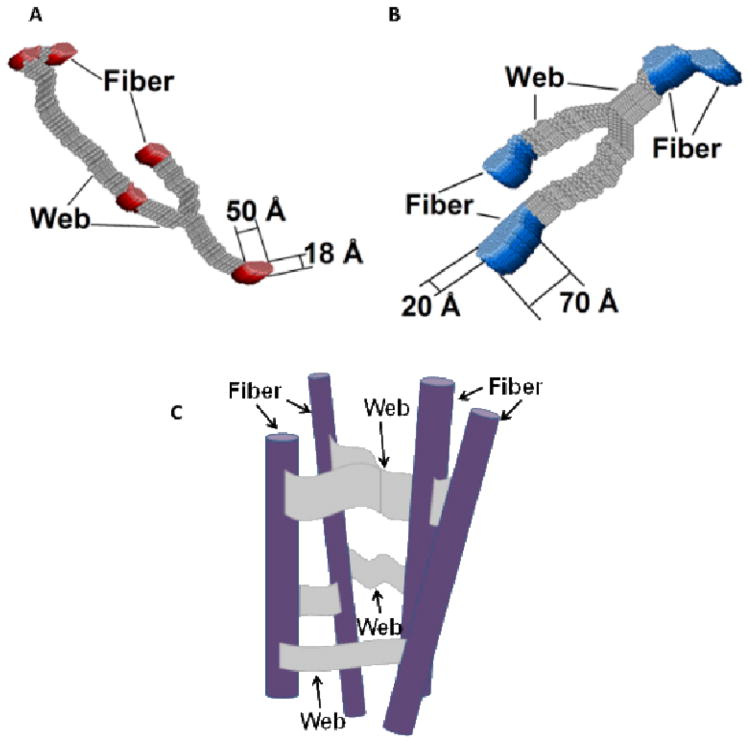
Using SANS, we looked at the structural features on a larger scale which showed that homochiral gels contain thicker fibers that are connected to each other by thicker webs than the fibers in heterochiral gels (Figure 4). Taken together, SAXS and SANS analysis explains that homochiral gels contain thicker, more well connected fibers than heterochiral gels, which is ultimately why they have a mechanical advantage.
Fig. 4.
Pictorial presentation of the 3D slice of the hydrogels under study showing the cross sections of the individual fibers interconnected with flat, “lappet-like” webs shown in gray. Reconstruction from the 2D cross-shape restored from SANS data with the low Qmin values (∼0.003–0.005 Å−1): (A) for the heterochiral hydrogel pair (L+D−) and (D+L−). and (B) for the homochiral hydrogel pair: (L+L−) and (D+D−). (C) shows a pictorial cartoon illustrating the schematic fibrous network organization. Reprinted with permission from ref. 109. Reprinted with permission from ref. 109. Copyright 2012 American Chemical Society.
Case Study 2: Using SAS to Understand the Mechanical Properties of polysaccharide-peptide composite materials
After successful mechanical improvement of a novel polysaccharide-based material (110) we wanted to incorporate the newly created polysaccharide network into a peptide-based hydrogel (61). In doing so, the resulting material would be more like natural ECM, containing both polysaccharides and polypeptides. In addition, we hoped to create a material with hybrid mechanical properties, one with the stiffness of our peptide hydrogels and the resistance to strain deformation provided by the polysaccharides. What resulted was, in fact, a composite material with good strain deformation resistance and relatively good stiffness values as well (Figure 5). From the mechanical data, it seemed clear that an interaction between polysaccharides and peptides had occurred, but it was unclear how the system had changed structurally in order to give these mechanical results. This is where SAS became important once again.
Fig. 5.
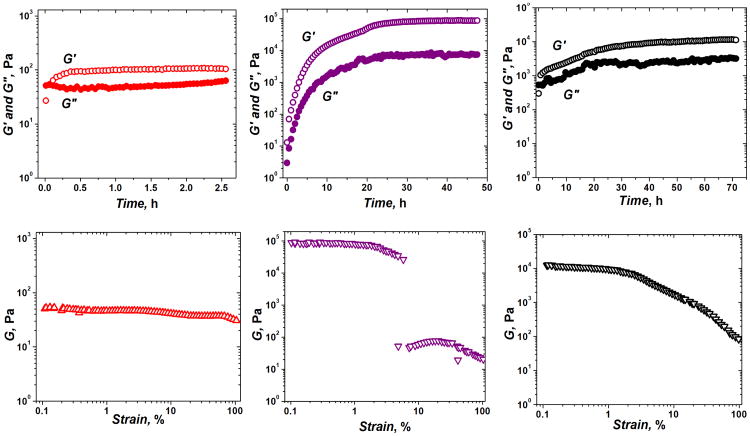
Dynamic oscillatory rheological characterization of peptide, polysaccharide and composite materials. The graphs in red are the time (top) and strain (bottom) sweep for the chitosan+alginate (CA) network. The graphs in purple are the time (top) and strain (bottom) sweep for the peptide (P) hydrogel. The graphs in black are the time (top) and strain (bottom) sweep for the chitosan+alginate+peptide (CAP) network. G = (G′+G″)½. Reprinted with permission from ref. 61. Copyright 2011 Wiley Periodicals, Inc.
Using SANS, we were able to understand how the fibers within each material had changed upon interaction. In Figure 6 below, the huge difference in fiber size between pure peptides and pure polysaccharides is quite evident. Upon interaction with peptides, polysaccharide network fibers appear to be broken down, resulting in much smaller hybrid peptide-polysaccharide fibers. This significant change in fiber structure helps explain the significant change in mechanical values upon peptide-polysaccharide mixing.
Fig. 6.
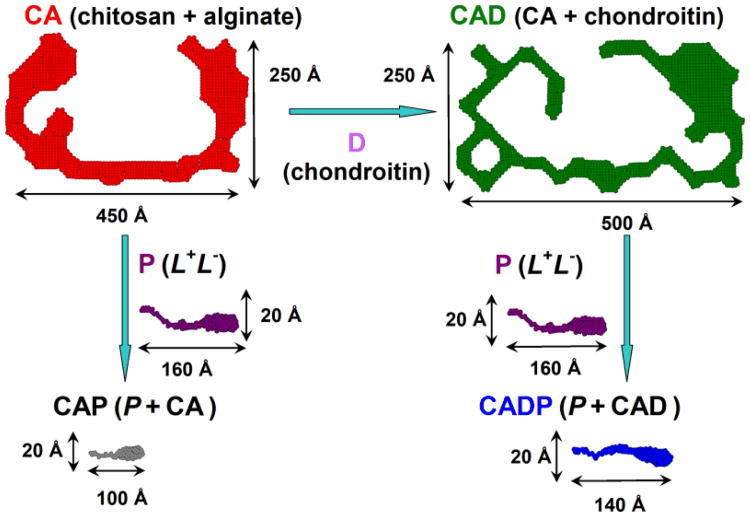
Pictorial description of the 2D shapes of a fiber cross-section in polysaccharide and composite peptide-polysaccharide networks. The addition of chondroitin D to CA (chitosan + alginate, red) leads to much bigger and thicker fiber CAD (CA + chondroitin, green); while the addition of the peptides P (L+ + L−, purple) to the above polysaccharide networks with the formation of CAP (CA + P, black) or CADP (CAD + P, blue) completely disrupts the structures of polysaccharides and results in the fibers with the cross-section very similar to the pure peptide network P. Reprinted with permission from ref. 61. Copyright 2011 Wiley Periodicals, Inc.
In order to understand why the strain behavior of the composite gel was more similar to the polysaccharide gels, we examined the correlation lengths of the three fiber networks. In contrast to the fiber characterization described above, correlation length (Lc) is a property of the entire fiber network, describing the average distance between fibers within the network and as such, reflects the mesh size of the network. The Lc values for CA, P and CAP were 150, 60 and 98Å, respectively. Not surprisingly, the hybrid network CAP has a correlation length in between CA and P networks. A larger space between the fibers seems to provide a greater flexibility, resistance to deformation, and lower susceptibility to break. Using a variety of characterization techniques available with SANS, we were able to get a better understanding of the structure-property relationship of our hybrid peptide-polysaccharide networks.
Conclusions
In this review, we demonstrate how structural information at the nanoscale level could be used as an instrument for understanding the mechanical properties of biopolymer-based biomaterials. In the case of hydrogel fibrous networks, at the individual fiber level, small-angle scattering techniques (SAXS and SANS) reveal information about the average cross-sectional dimensions of the fiber, its elementary building blocks from mass-fractal dimensions and its persistence length. Clearly, thicker fiber cross-sections, more compact elementary building blocks and longer persistence length of the fiber will translate into a mechanically stiffer biomaterial. The first case study shows that component chirality may be used to tune biomaterial mechanical properties, since thicker and more strongly connected homochiral fibers ultimately have a mechanical advantage over the heterochiral ones. However, the latter assemble a network much faster.
At the level of network organization, both SANS and SAXS provide the possibility to trace not only the interconnection of the fibers, but also the mesh size of the network (based on the correlation length). In general, greater mesh size translates into a weaker biomaterial. On the other hand, one might expect the networks with greater mesh size would be more flexible and less prone to breaking. Indeed, as the second case study shows, the engineering of peptide-based biomaterials by the addition of less stiff but more elastic polysaccharide networks increases the mesh size of the network. This translates into a hybrid biomaterial which is stiffer than polysaccharide networks alone and has significantly improved strain-resistant properties of otherwise brittle pure peptide networks. Biopolymer-based biomaterials provide a range of mechanical properties that can be tuned using various combinations of structurally different components. A greater understanding of the mechanical-structural relationship will continue to aid in the development of biopolymer-based biomaterials with the appropriate environments for cell delivery and growth.
Acknowledgments
Our work on biomaterials has been supported by the NIH under grant EB004416. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357. Beamtime was awarded through the program of General User Proposals to GUP-24524. SANS experiments at NIST NCNR were based upon activities supported in part by the National Science Foundation under Agreement No. DMR-0944772. The writing of this manuscript was supported in part by EB012003.
References
- 1.Vacanti JP, Langer R. The Lancet. 1999;354:32–34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 2.Kim BS, Mooney DJ. Trends Biotechnol. 1998;16:224–230. doi: 10.1016/s0167-7799(98)01191-3. [DOI] [PubMed] [Google Scholar]
- 3.Yoshimoto H, Shin YM, Terai H, Vacanti JP. Biomaterials. 2003;24:2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 4.Heath CA. Trends Biotechnol. 2000;18:17–19. doi: 10.1016/s0167-7799(99)01396-7. [DOI] [PubMed] [Google Scholar]
- 5.Gunatillake P, Mayadunne R, Adhikari R. Biotechnol Annu Rev. 2006;12:301–347. doi: 10.1016/S1387-2656(06)12009-8. [DOI] [PubMed] [Google Scholar]
- 6.Boontheekul T, Mooney DJ. Curr Opin Biotechnol. 2003;14:559–565. doi: 10.1016/j.copbio.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Stevens MM, Qanadilo HF, Langer R, Shastri VP. Biomaterials. 2004;25:887–894. doi: 10.1016/j.biomaterials.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 8.George M, Abraham TE. J Control Release. 2006;114:1–14. doi: 10.1016/j.jconrel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Drury JL, Dennis RG, Mooney DJ. Biomaterials. 2004;25:3187–3199. doi: 10.1016/j.biomaterials.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Brown RM. J Polym Sci A, Polym Chem. 2004;42:487–495. [Google Scholar]
- 11.Nishinari K, Takahashi R. Curr Opin Colloid Interface Sci. 2003;8:396–400. [Google Scholar]
- 12.Saxena IM, Brown RM. Ann Bot. 2005;96:9–21. doi: 10.1093/aob/mci155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown RM, Saxena IM, Kudlicka K. Trends Plant Sci. 1996;1:149–156. [Google Scholar]
- 14.Kadajji VG, Betageri GV. Polymers. 2011;3:1972–2009. [Google Scholar]
- 15.Clasen C, Kulicke WM. Prog Polym Sci. 2001;26:1839–1919. [Google Scholar]
- 16.Zhang H, Neau SH. Biomaterials. 2001;22:1653–1658. doi: 10.1016/s0142-9612(00)00326-4. [DOI] [PubMed] [Google Scholar]
- 17.Malafaya PB, Silva GA, Reis RL. Adv Drug Deliv Rev. 2007;59:207–233. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Wang JY, Roehrl MH. Proc Natl Acad Sci USA. 2002;99:14362–14367. doi: 10.1073/pnas.222536599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pieper JS, Oosterhof A, Dijkstra PJ, Veerkamp JH, van Kuppevelt TH. Biomaterials. 1999;20:847–858. doi: 10.1016/s0142-9612(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee CT, Kung PH, Lee YD. Carbohydr Polym. 2005;61:348–354. [Google Scholar]
- 21.Lippiello L. Osteoarthr Cartil. 2003;11:335–342. doi: 10.1016/s1063-4584(03)00026-8. [DOI] [PubMed] [Google Scholar]
- 22.Berg JM, Tymoczko JL, Stryer L. Biochemistry. 7th. W. H. Freeman and Company; New York: 2012. Glycogen Metabolism; p. 338. [Google Scholar]
- 23.Hers HG, Hue L. Annu Rev Biochem. 1983;52:617–653. doi: 10.1146/annurev.bi.52.070183.003153. [DOI] [PubMed] [Google Scholar]
- 24.Loebsack A, Greene K, Wyatt S, Culberson C, Austin C, Beiler R, Roland W, Eiselt P, Rowley J, Burg K, Mooney D, Holder W, Halberstadt C. J Biomed Mater Res. 2001;57:575–581. doi: 10.1002/1097-4636(20011215)57:4<575::aid-jbm1204>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Li ZS, Ramay HR, Hauch KD, Xiao DM, Zhang MQ. Biomaterials. 2005;26:3919–3928. doi: 10.1016/j.biomaterials.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 26.Freeman I, Cohen S. Biomaterials. 2009;30:2122–2131. doi: 10.1016/j.biomaterials.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 27.Sapir Y, Kryukov O, Cohen S. Biomaterials. 2011;32:1838–1847. doi: 10.1016/j.biomaterials.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Muller FA, Muller L, Hofmann L, Greil P, Wenzel MM, Staudenmaier R. Biomaterials. 2006;27:3955–3963. doi: 10.1016/j.biomaterials.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 29.Svensson A, Nicklasson E, Harrah T, Panilaitis B, Kaplan DL, Brittberg M, Gatenholm P. Biomaterials. 2005;26:419–431. doi: 10.1016/j.biomaterials.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 30.Pulkkinen H, Tiitu V, Lammentausta E. Biomed Mater Eng. 2006;16:S29–S35. [PubMed] [Google Scholar]
- 31.Guerra GD, Barbani N, Gagliardi M, Rosellini E, Cristallini C. Int J Carb Chem. 2011;2011:1–9. [Google Scholar]
- 32.Suh JK, Matthew HW. Biomaterials. 2000;24:2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 33.Wang DA, Varghese S, Sharma B, Strehin I, Farmanian S, Gorham J, Fairbrother DH, Cascio B, Elisseeff JH. Nature Mat. 2007;6:385–392. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kopecek J, Yang J. Acta Biomater. 2009;5:805–816. doi: 10.1016/j.actbio.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmes TC, de Lacalle S, Su X, Liu G, Rich A, Zhang S. Proc Natl Acad Sci USA. 2000;97:6728–6733. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semino CE, Kasahara J, Hayashi Y, Zhang S. Tissue Eng. 2004;10:643–655. doi: 10.1089/107632704323061997. [DOI] [PubMed] [Google Scholar]
- 37.Semino CE, Merok JR, Crane GG, Panagiotakos G, Zhang S. Differentiation. 2003;71:262–270. doi: 10.1046/j.1432-0436.2003.7104503.x. [DOI] [PubMed] [Google Scholar]
- 38.Genove E, Shen C, Zhang S, Semino CE. Biomaterials. 2005;26:3341–3351. doi: 10.1016/j.biomaterials.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Haines LA, Rajagopal K, Ozbas B, Salick DA, Pochan DJ, Schneider JP. J Am Chem Soc. 2005;127:17025–17029. doi: 10.1021/ja054719o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, Pilarz M, Lamm MS, Pochan DJ, Schneider JP. Proc Natl Acad Sci USA. 2007;104:7791–7796. doi: 10.1073/pnas.0701980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kretsinger JK, Haines LA, Ozbas B, Pochan DJ, Schneider JP. Biomaterials. 2005;26:5177–5186. doi: 10.1016/j.biomaterials.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 42.Beniash E, Hartgerink JD, Storrie H, Stendahl JC, Stupp SI. Acta Biomater. 2005;1:387–397. doi: 10.1016/j.actbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Webber MJ, Tongers J, Renault MA, Roncalli JG, Losordo DW, Stupp SI. Acta Biomater. 2010;6:3–11. doi: 10.1016/j.actbio.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrington DA, Cheng EY, Guler MO, Lee LK, Donovan JL, Claussen RC, Stupp SI. J Biomed Mater Res. 2006;78A:157–167. doi: 10.1002/jbm.a.30718. [DOI] [PubMed] [Google Scholar]
- 45.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 46.Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, Stupp SI, Kessler JA. J Neurosci. 2008;28:3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramachandran S, Tseng Y, Yu YB. Biomacromolecules. 2005;6:1316–1321. doi: 10.1021/bm049284w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramachandran S, Flynn P, Tseng Y, Yu YB. Chem Mater. 2005;17:6583–6588. [Google Scholar]
- 49.Ramachandran S, Trewhella J, Tseng Y, Yu YB. Chem Mater. 2006;18:6157–6162. [Google Scholar]
- 50.Feng Y, Lee M, Taraban MB, Yu YB. Chem Comm. 2011;47:10455–10457. doi: 10.1039/c1cc13943f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hyland LL, Twomey JD, Vogel S, Hsieh AH, Yu YB. Biomacromolecules. 2013;14:406–412. doi: 10.1021/bm301598g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamada Y, Hozumi K, Aso A, Hotta A, Toma K, Katagiri F, Kikkawa Y, Nomizu M. Biomaterials. 2012;33:4118–4125. doi: 10.1016/j.biomaterials.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 53.Yamada Y, Hozumi K, Katagiri F, Kikkawa Y, Nomizu M. Biopolymers. 2010;94:711–720. doi: 10.1002/bip.21429. [DOI] [PubMed] [Google Scholar]
- 54.Chinen N, Tanihara M, Nakagawa M, Shinozaki K, Yamamoto E, Mizushima Y, Suzuki Y. J Biomed Mater Res A. 2003;67:61–68. doi: 10.1002/jbm.a.10061. [DOI] [PubMed] [Google Scholar]
- 55.Fujita M, Ishihara M, Simizu M, Obara K, Ishizuka T, Saito Y, Yura H, Morimoto Y, Takase B, Matsui T, Kikuchi M, Maehara T. Biomaterials. 2004;25:699–706. doi: 10.1016/s0142-9612(03)00557-x. [DOI] [PubMed] [Google Scholar]
- 56.Nakamura S, Ishihara M, Obara K, Masuoka K, Ishizuka T, Kanatani Y, Takase B, Matsui T, Hattori H, Sato T, Kariya Y, Maehara T. J Biomed Mater Res A. 2006;78:364–371. doi: 10.1002/jbm.a.30688. [DOI] [PubMed] [Google Scholar]
- 57.Sakiyama-Elbert SE, Hubbell JA. J Control Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 58.Tanihara M, Suzuki Y, Yamamoto E, Noguchi A, Mizushima Y. J Biomed Mater Res. 2001;56:216–221. doi: 10.1002/1097-4636(200108)56:2<216::aid-jbm1086>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 59.Liao S, Yu TB, Guan Z. J Am Chem Soc. 2009;131:17638–17646. doi: 10.1021/ja907097t. [DOI] [PubMed] [Google Scholar]
- 60.Capito RM, Azevedo HS, Velichko YS, Mata A, Stupp SI. Science. 2008;319:1812–1816. doi: 10.1126/science.1154586. [DOI] [PubMed] [Google Scholar]
- 61.Hyland LL, Taraban MB, Feng Y, Hammouda B, Yu YB. Biopolymers. 2012;97:177–88. doi: 10.1002/bip.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Macosko CW. Rheology: principles, measurements and applications. New York, NY: Wiley-VCH, Inc; 1994. [Google Scholar]
- 63.Mezger TG. The rheology handbook: for users of rotational and oscillatory rheometers. 2nd. Hannover: Vincentz Network GmbH and Co KG; 2006. [Google Scholar]
- 64.Yan C, Pochan D. J Chem Soc Rev. 2010;39:3528–3540. doi: 10.1039/b919449p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nitta Y, Nishinari K. J Biol Macromol. 2005;5:47–52. [Google Scholar]
- 66.Lapasin R, Prid S. Rheology of Industrial Polysaccharides: Theory and Applications. London: Blackie Academic and Professional; 1995. [Google Scholar]
- 67.Ross-Murphy SB, Shatwell KP. Biorheology. 1993;30:217–227. doi: 10.3233/bir-1993-303-407. [DOI] [PubMed] [Google Scholar]
- 68.Greenleaf JF, Fatemi M, Insana M. Annu Rev Biomed Eng. 2003;5:57–78. doi: 10.1146/annurev.bioeng.5.040202.121623. [DOI] [PubMed] [Google Scholar]
- 69.Simis KS, Bistolfi A, Bellare A, Pruitt LA. Biomaterials. 2006;27:1688–1694. doi: 10.1016/j.biomaterials.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 70.Bigi A, Panzavolta S, Rubini K. Biomaterials. 2004;25:5675–5680. doi: 10.1016/j.biomaterials.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 71.Langdon SE, Chernecky R, Pereira CA, Abdulla D, Lee JM. Biomaterials. 1999;20:137–153. doi: 10.1016/s0142-9612(98)00142-2. [DOI] [PubMed] [Google Scholar]
- 72.Svergun D, Koch MH. J Rep Prog Phys. 2003;66:1735–1782. [Google Scholar]
- 73.Glatter O, Kratky O. Small-Angle Scattering of the X-Rays. Academic Press Inc.; New York: 1982. [Google Scholar]
- 74.http://www.embl-hamburg.de/biosaxs/software.html
- 75.Kline SR. J Appl Cryst. 2006;39:895–900. [Google Scholar]
- 76.Ilavsky J, Jemian PR. J Appl Cryst. 2009;42:347–353. [Google Scholar]
- 77.Hule RA, Nagarkar RP, Hammouda B, Schneider JP, Pochan DJ. Macromolecules. 2009;42:7137–7145. doi: 10.1021/ma9003242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pedersen JS, Shurtenberger P. Macromolecules. 1996;29:7602–7612. [Google Scholar]
- 79.MacKintosh FC, Käs J, Janmey PA. Phys Rev Lett. 1995;75:4425–4428. doi: 10.1103/PhysRevLett.75.4425. [DOI] [PubMed] [Google Scholar]
- 80.de Gennes PG. Scaling Concepts in Polymer Physics. Cornell University Press; Ithaca; 1979. [Google Scholar]
- 81.Whitten AE, Jeffries CM, Harris SP, Trewhella J. Proc Natl Acad Sci USA. 2008;105:18360–18365. doi: 10.1073/pnas.0808903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spinozzi F, Beltramini M. Biophys J. 2012;103:511–521. doi: 10.1016/j.bpj.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spinozzi F, Mariani P, Mičetić I, Ferrero C, Pontoni D, Beltramini M. PLoS ONE. 2012;7:e49644. doi: 10.1371/journal.pone.0049644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fratzl P. J Appl Cryst. 2003;36:397–404. [Google Scholar]
- 85.Jacques DA, Trewhella J. Protein Sci. 2010;19:642–657. doi: 10.1002/pro.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Putnam CD, Hammel M, Hura GL, Tainer JA. Q Rev Biophys. 2007;40:191–285. doi: 10.1017/S0033583507004635. [DOI] [PubMed] [Google Scholar]
- 87.Guilbaud JB, Saiani A. Chem Soc Rev. 2011;40:1200–1210. doi: 10.1039/c0cs00105h. [DOI] [PubMed] [Google Scholar]
- 88.Gamani A, Paoletti S, Toffanin R, Micali F, Michielin L, Bevilacqua C. Biomaterials. 2002;23:1161–1167. doi: 10.1016/s0142-9612(01)00231-9. [DOI] [PubMed] [Google Scholar]
- 89.Stokke B, Draget K, Smidsrod O, Yuguchi Y, Urakawa H, Kajiwara K. Macromolecules. 2000;33:1853–1863. [Google Scholar]
- 90.Shinohara Y, Kayashima K, Okumura Y, Zhao C, Ito K, Amemiya Y. Macromolecules. 2006;39:7386–7391. [Google Scholar]
- 91.Tada T, Matsumoto T, Masuda T. Carb Pol. 1999;39:53–59. [Google Scholar]
- 92.Hiller JC, Thompson TJU, Evison MP, Chamberlain AT, Wess TJ. Biomaterials. 2003;24:5091–5097. doi: 10.1016/s0142-9612(03)00427-7. [DOI] [PubMed] [Google Scholar]
- 93.Choudhary S, Bhatia SR. Carbohyd Pol. 2012;87:524–530. doi: 10.1016/j.carbpol.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 94.Markarian M, Hariri H, Reisch A, Urban V, Schlenoff J. Macromolecules. 2012;45:1016–1024. [Google Scholar]
- 95.Wilson K, Allen A, Washburn N, Antonucci J. J Biomed Mat Res. 2007;81A:113–123. doi: 10.1002/jbm.a.30975. [DOI] [PubMed] [Google Scholar]
- 96.Luk A, Murthy N, Wang W, Rojas R, Kohn J. Acta Biomat. 2012;8:1459–1468. doi: 10.1016/j.actbio.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feuz L, Stunz P, Geue T, Textor M, Borisov O. Eur Phys J. 2007;23:237–245. doi: 10.1140/epje/i2007-10180-9. [DOI] [PubMed] [Google Scholar]
- 98.Weigandt KM, Porcar L, Pozzo DC. Soft Matter. 2011;7:9992–10000. [Google Scholar]
- 99.http://www.aps.anl.gov/Beamlines 12-ID-B
- 100.http://www.ill.eu/en/html/instruments-support/instruments-groups/; D22.
- 101.Egelhaaf SU, Schurtenberger P. Phys Rev Lett. 1999;82:2804–2807. [Google Scholar]
- 102.Feigin LA, Svergun DI. Structure Analysis by Small-Angle X-Ray and Neutron Scattering. Plenum Press; New York: 1987. [Google Scholar]
- 103.Rendon S, Fang J, Burghardt WR, Bubeck RA. Rev Sci Instrum. 2009;80:043902. doi: 10.1063/1.3108531. [DOI] [PubMed] [Google Scholar]
- 104.Caputo FE, Burghardt WR. Macromolecules. 2001;34:6684–6694. [Google Scholar]
- 105.Dykes LMC, Torkelson JM, Burghardt WR, Krishnamoorti R. Polymer. 2010;57:4916–4927. [Google Scholar]
- 106.Pujari S, Dougherty L, Mobuchon C, Carreau PJ, Heuzey MC, Burghardt WR. Rheologica Acta. 2011;50:3–16. [Google Scholar]
- 107.Newbloom GM, Weigandt KM, Pozzo DC. Soft Matter. 2012;8:8854–8864. [Google Scholar]
- 108.Barron LD. Space Sci Rev. 2008;135:187–201. [Google Scholar]
- 109.Taraban M, Feng Y, Hammouda B, Hyland LL, Yu YB. Chem Mater. 2012;24:2299–2310. doi: 10.1021/cm300422q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hyland LL, Taraban MB, Hammouda B, Yu YB. Biopolymers. 2011;95:840–851. doi: 10.1002/bip.21687. [DOI] [PMC free article] [PubMed] [Google Scholar]