Abstract
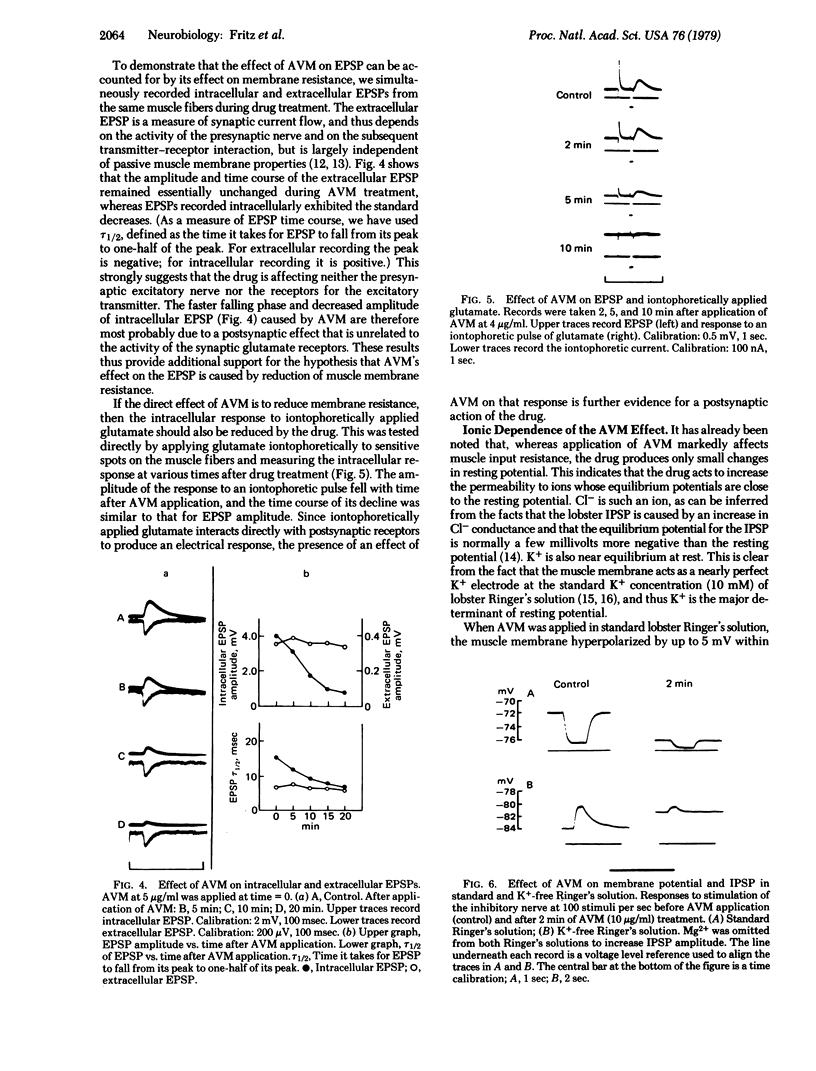
Avermectin B1a, a macrocyclic lactone with broad spectrum anthelmintic activity, affects neuromuscular transmission in the lobster stretcher muscle. Perfusion of the muscle with 1-10 microgram of the drug per ml eliminates inhibitory postsynaptic potentials within a few minutes. Intracellularly recorded excitatory postsynaptic potentials are gradually reduced in amplitude over 20-30 min, and their falling phases become faster; there is no effect, however, on extracellularly recorded excitatory potentials. Avermectin B1a reduced the input resistance of the muscle fibers with a time course similar to that of the reduction of excitatory potentials. Washing for up to 2 hr with drug-free solution fails to reverse the drug's effects. However, perfusion with 20 microgram of picrotoxin per ml results in recovery of the excitatory potentials and input resistance. Avermectin B1a also blocks the firing of the crayfish stretch receptor neuron, and this block is also reversed by picrotoxin. We hypothesize that the reduction in excitatory postsynaptic potentials after avermectin B1a treatment is caused solely by reduction in membrane resistance; additional experiments suggest that the reduction in membrane resistance is due to the opening of membrane Cl- channels, perhaps including those regulated by gamma-aminobutyric acid at the inhibitory synapse.
Full text
PDF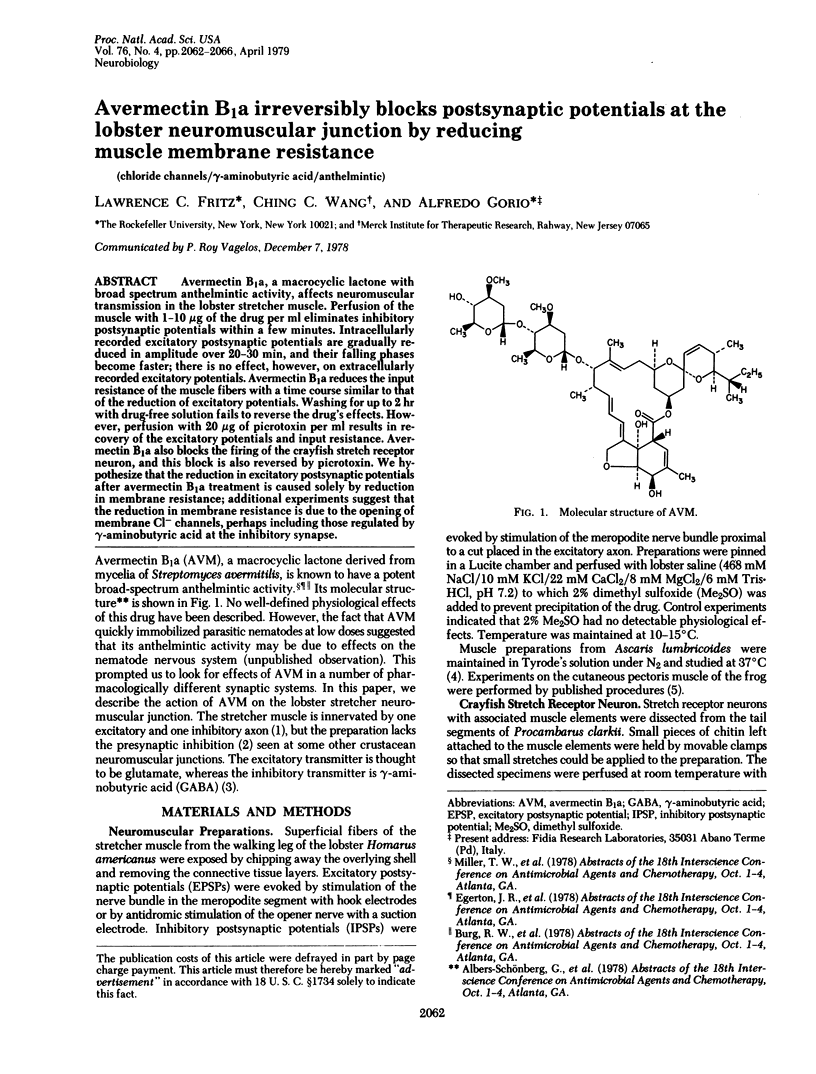



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash A. S., Tucker J. F. Inhibition of Ascaris muscle by gamma-aminobutyric acid: a possible new assay method. Nature. 1966 Jan 15;209(5020):306–307. doi: 10.1038/209306a0. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. Autoradiographic localization of gamma-aminobutyric acid receptors in the rat central nervous system by using [3H]muscimol. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1024–1028. doi: 10.1073/pnas.75.2.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A. The "mixed" effect of picrotoxin on the GABA dose/conductance relation recorded from lobster muscle. Neuropharmacology. 1978 Mar;17(3):159–167. doi: 10.1016/0028-3908(78)90095-3. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G. Two types of extrajunctional L-glutamate receptors in locust muscle fibres. J Physiol. 1976 Feb;255(2):449–464. doi: 10.1113/jphysiol.1976.sp011289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELCASTILLO J., DEMELLO W. C., MORALES T. THE PHYSIOLOGICAL ROLE OF ACETYLCHOLINE IN THE NEUROMUSCULAR SYSTEM OF ASCARIS LUMBRICOIDES. Arch Int Physiol Biochim. 1963 Nov;71:741–757. [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. The quantal nature of transmission and spontaneous miniature potentials at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:514–529. doi: 10.1113/jphysiol.1961.sp006644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J., De Mello W. C., Morales T. Inhibitory action of gamma-aminobutyric acid (GABA) on Ascaris muscle. Experientia. 1964 Mar 15;20(3):141–143. doi: 10.1007/BF02150701. [DOI] [PubMed] [Google Scholar]
- Dunham P. B., Gainer H. The distribution of inorganic ions in lobster muscle. Biochim Biophys Acta. 1968 Apr 29;150(3):488–499. doi: 10.1016/0005-2736(68)90149-1. [DOI] [PubMed] [Google Scholar]
- EYZAGUIRRE C., KUFFLER S. W. Processes of excitation in the dendrites and in the soma of single isolated sensory nerve cells of the lobster and crayfish. J Gen Physiol. 1955 Sep 20;39(1):87–119. doi: 10.1085/jgp.39.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNDFEST H., REUBEN J. P., RICKLES W. H., Jr The electrophysiology and pharmacology of lobster neuromuscular synapses. J Gen Physiol. 1959 Jul 20;42(6):1301–1323. doi: 10.1085/jgp.42.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W. Generation of end-plate potentials. Physiol Rev. 1976 Jan;56(1):177–247. doi: 10.1152/physrev.1976.56.1.177. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Gorio A., Hurlbut W. P., Ceccarelli B. Acetylcholine compartments in mouse diaphragm. Comparison of the effects of black widow spider venom, electrical stimulation, and high concentrations of potassium. J Cell Biol. 1978 Sep;78(3):716–733. doi: 10.1083/jcb.78.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorio A., Rubin L. L., Mauro A. Double mode of action of black widow spider venom on frog neuromuscular junction. J Neurocytol. 1978 Apr;7(2):193–202. doi: 10.1007/BF01217918. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., EYZAGUIRRE C. Synaptic inhibition in an isolated nerve cell. J Gen Physiol. 1955 Sep 20;39(1):155–184. doi: 10.1085/jgp.39.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- Kennedy D., Evoy W. H. The distribution of pre- and postsynaptic inhibition at crustacean neuromuscular junctions. J Gen Physiol. 1966 Jan;49(3):457–468. doi: 10.1085/jgp.49.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E., Paupardin-Tritsch D. The pharmacological properties of some crustacean neuronal acetylcholine, gamma-aminobutyric acid, and L-glutamate responses. J Physiol. 1978 Jul;280:213–236. doi: 10.1113/jphysiol.1978.sp012381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokizawa F., Reuben J. P., Grundfest H. Ionic permeability of the inhibitory postsynaptic membrane of lobster muscle fibers. J Gen Physiol. 1969 Oct;54(4):437–461. doi: 10.1085/jgp.54.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas I., Rahamimoff R., Sarney Tonic release of transmitter at the neuromuscular junction of the crab. J Physiol. 1975 Sep;250(2):275–286. doi: 10.1113/jphysiol.1975.sp011054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. A study of the action of picrotoxin on the inhibitory neuromuscular junction of the crayfish. J Physiol. 1969 Nov;205(2):377–391. doi: 10.1113/jphysiol.1969.sp008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. Anion permeability of the inhibitory post-synaptic membrane of the crayfish neuromuscular junction. J Physiol. 1967 Aug;191(3):575–590. doi: 10.1113/jphysiol.1967.sp008269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDMANN S. The electrical constants of Purkinje fibres. J Physiol. 1952 Nov;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERMAN R., GRUNDFEST H. Graded and all-or-none electrogenesis in arthropod muscle. II. The effects of alkali-earth and onium ions on lobster muscle fibers. J Gen Physiol. 1961 May;44:997–1027. doi: 10.1085/jgp.44.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]


