Abstract
Criteria for inclusion of diagnoses of Axis I disorders in the forthcoming Diagnostic and Statistical Manual (DSM-V) of the American Psychiatric Association are being considered. The 5 criteria that were proposed by Blashfield et al as necessary for inclusion in DSM-IV are reviewed and are met by the night eating syndrome (NES). Seventy-seven publications in refereed journals in the last decade indicate growing recognition of NES. Two core diagnostic criteria have been established: evening hyperphagia (consumption of at least 25% of daily food intake after the evening meal) and/or the presence of nocturnal awakenings with ingestions. These criteria have been validated in studies that used self-reports, structured interviews, and symptom scales. Night eating syndrome can be distinguished from binge eating disorder and sleep-related eating disorder. Four additional features attest to the usefulness of the diagnosis of NES: (1) its prevalence, (2) its association with obesity, (3) its extensive comorbidity, and (4) its biological aspects. In conclusion, research on NES supports the validity of the diagnosis and its inclusion in DSM-V.
1. Introduction
The Diagnostic and Statistical Manual (DSM-IV-TR) [1] of the American Psychiatric Association is currently being revised, and with it the specific criteria for Axis I and Axis II disorders. During the last DSM revision, in an influential article in this journal, Blashfield et al [2] proposed 5 criteria as necessary for inclusion of an Axis I disorder in DSM-IV. These criteria were recently reviewed by Striegel-Moore et al [3] in an assessment of the night eating syndrome (NES) [4]. The current report reviews the extensive research on NES that indicates that each of the criteria of Blashfield et al for a valid diagnosis is met by NES. Four additional features of NES that support the validity of the diagnosis follow.
2. Criteria of Blashfield et al [2]
2.1. Criterion I: “There should be at least 50 journal articles on the proposed diagnostic category in the last 10 years,… at least 25 of them should be empirical. “ (p17)
This criterion is designed to ensure that “a diagnostic category is sufficiently recognized to become part of the official language of psychopathology” [2]. A PubMed search of night eating in 2008 revealed 77 articles on NES during the past 10 years, most of which were empirical. Night eating syndrome has been the subject of reports in such widely read journals as JAMA [5,6], The American Journal of Psychiatry [7–9], and Obesity/Obesity Research [10–14].
2.2. Criterion II: that there be (a) a set of diagnostic criteria that (b) include self-report measures, structured interviews, and rating scales (p17)
The establishment of core diagnostic criteria for NES was based on weekly diaries of all food consumed and sleep records of more than 148 night eaters and 69 control subjects [15]. They are as follows.
evening hyperphagia (the consumption of at least 25% of daily food intake after the evening meal),1
three or more nocturnal awakenings with ingestions per week, or
These core criteria have been assessed by 4 measures, as recommended by Blashfield et al. First, week-long food and sleep diaries have been used. Second, self-reports of symptoms have been specified by the Night Eating Questionnaire developed at the University of Pennsylvania [17,18] and the Night Eating Diagnostic Questionnaire developed at Columbia University [10]. Third, the semi-structured interview Night Eating Syndrome History and Inventory (NESHI, unpublished clinical interview) has been used in several studies [11,14,16]. Fourth, the Night Eating Symptom Scale measures change of symptoms during the previous week [19]. Associated NES features include morning anorexia, insomnia, and depressed mood [4,5]. Some writers, in the past, have used widely differing criteria to diagnose NES. Their results are difficult to compare with recent studies that have used the core criteria described here that are increasingly accepted.
Proposed criteria for NES were evaluated by the use of item response theory analysis [20]. It was based on 6 studies that yielded 1481 night eating questionnaires. They were coded to reflect the presence/absence of 5 night eating behaviors that have been associated with NES. Behaviors were evaluated by the clinical usefulness of their diagnostic information in identifying persons with NES and on the assumptions of the item response theory analysis. Reports of (1) nighttime eating and/or evening hyperphagia, (2) initial insomnia, and (3) nighttime awakenings showed high precision in discriminating persons with night eating problems, whereas morning anorexia and delayed morning meal provided little additional information. The evening hyperphagia and/or nocturnal eating factor conveyed by far the largest item information.
Recently, the First International Night Eating Symposium (Minneapolis, MN, April 26, 2008) established a consensus among researchers on the diagnostic criteria for NES; and the final set of criteria is forthcoming. It is based on the 2 core criteria described here, with 1 exception. The frequency of nocturnal ingestions will be changed from 3 to 2 per week, the same frequency that is required for binges in bulimia nervosa (BN) and binge eating disorder (BED).
2.3. Criterion III: there should be at least 2 empirical studies by independent research groups demonstrating high interclinician correlations (p17)
Two outpatient [5,11] and 2 inpatient (General Clinical Research Center) studies of NES [5,21] have used diagnostic ratings made by different clinicians. The results are summarized in Table 1. In each of the 4 studies, different clinicians reported that night eaters had significantly more nighttime awakenings than did control subjects. In the 3 studies in which nighttime eating was allowed, different clinicians reported that persons with NES, compared with control participants, had significantly more nighttime ingestions and evening energy intake [5,11,21]. In the 2 studies in which sleep was measured, different clinicians reported that the times of sleep onset and waking of night eaters and control subjects (ie, circadian rhythm) were very similar [11,22]. Interclinician agreement in these studies was high, but it was between collaborating investigators. Multicenter trials are needed for independent assessments. Inclusion of NES in the DSM would encourage such trials.
Table 1.
Eating and sleep characteristics in outpatient (OP) and inpatient studies (IP) of NES
| Study | n | No. of awakenings per night |
Nocturnal ingestions |
Daily food intake (kcal) |
Food intake after dinner (kcal) |
% of total food intake after dinner |
Sleep onset |
Sleep offset |
Sleep duration |
|---|---|---|---|---|---|---|---|---|---|
| OP 1 | |||||||||
| NES | 10 | 3.6 | 1.3 | 2930 | 1641 | 56% | |||
| Control | 10 | 0.3 | 0 | 2334 | 347 | 15% | |||
| OP 2 | |||||||||
| NES | 46 | 1.5 | 1.1 | 2314 | 801 | 35% | 23:57 | 6:59 | 7:37 |
| Control | 43 | 0.5 | 0 | 2420 | 242 | 10% | 23:47 | 7:06 | 7:18 |
| IP 1 | |||||||||
| NES | 12 | 3.0 | a | a | a | a | |||
| Control | 21 | 0.1 | a | a | a | a | |||
| IP 2 | |||||||||
| NES | 15 | 4.5b | 1.2 | 2959 | 779 | 26% | 23:38 | 7:04 | 7:26 |
| Control | 14 | 3.1 | .05 | 2893 | 289 | 10% | 22:52 | 7:06 | 8:14 |
Outpatient 1 is Birketvedt et al [5], OP 2 is O’Reardon et al [11], IP 1 is Birketvedt et al [5], IP 2 is Allison et al [21] and Rogers et al [22].
Food intake was controlled in this study, with the administration of four 300-cal meals across the day, ending at 8:00 PM.
Awakenings in IP 2 were measured by PSG, which was more sensitive than diary recordings of awakenings.
2.4. Criterion IV: the proposed category represents a syndrome of frequently cooccurring symptoms, described in at least 2 independent studies (p17)
Blashfield et al suggest that, if patients meet at least 1 diagnostic criterion for the disorder, there be at least a .50 probability that they will meet another diagnostic criterion. de Zwaan and colleagues [23] reported that their sample of self-identified night eaters met this criterion. Of those who endorsed “usually” or “always” eating when they awoke, 80% reported consuming at least 25% of their daily energy intake after the evening meal, whereas 51% consumed at least 50% of their daily energy intake after the evening meal.
A second study involved a carefully screened sample of 148 persons with NES, as diagnosed with the Night Eating Questionnaire and NESHI. Food diaries showed that 67% had both evening hyperphagia (≥25%) and nocturnal ingestions (≥3 per week). By contrast, 17% had only evening hyperphagia; and 16% had only nocturnal ingestions [15].
2.5. Criterion V: there should be at least 2 independent empirical studies showing that the proposed category “can be differentiated from other categories with which it is likely to be confused” (p17)
Studies of NES have clearly differentiated it from 1 disorder with which it might be confused: BED. Two NES behaviors differ from those of BED: first, there is the limited energy intake of nocturnal ingestions (280 kcal per episode) [5] that are not “objectively large” and do not qualify as binge episodes. Second, the characteristic circadian delay in the food intake of night eaters has not been reported in BED. Further research should compare the timing of binges with the “grazing” throughout the evening more characteristic of night eaters. Carefully assessed samples of patients report an overlap of NES and BED in about 15% to 20% of cases [14,16,24,25].
In contrast to the relatively clear differentiation of NES from BED, there has been confusion regarding its differentiation from sleep-related eating disorder (SRED). The differences between the 2 disorders will be described in some detail.
The first objectively documented case of SRED was reported by Oswald and Adam [26] in a 37-year-old man who had 28 episodes of night eating during 6 days in the sleep laboratory. He had no awareness of the episodes or memory of them the next morning and consumed inappropriate items such as safflower oil. The second report of SRED by Whyte and Kavey [27] described 3 patients with sleepwalking who ate during somnambulistic periods with little recall. As in the article by Oswald and Adam, they, too, consumed inappropriate, unappetizing substances.
The feature that first differentiated SRED from other eating disorders was the lower level of consciousness of eating and the subsequent lack of recall [28,29]. The largest SRED study was that described by Schenck et al [28] and Schenck and Mahowald [29]. This study also found decreased awareness of eating with subsequent amnesia and ingestion of inappropriate objects in 38 patients out of 8000 individuals seen over an 8-year period in a sleep disorders program [28,29]. Most (84%) of the patients ate during total or partial sleep by polysomnography (PSG). In Winkelman’s [30] 1998 study of 23 patients, 91% were “mostly” or “half” asleep while eating and 91% were “consistently” or “occasionally” amnesic for the episode. In contrast to these reports of reduced consciousness, 3 reports in the literature have described patients fully awake by PSG criteria [31–33]. The first 2 studies [31,32] used the criteria for the Nocturnal Eating and Drinking Syndrome (NEDS), which were similar to the NES criteria; but this category was removed from the International Classification of Sleep Disorders nomenclature with the 2005 revision [34].
Manni et al [31] described PSG studies of 120 insomniac referrals of whom 7 subjects, or 5.8%, were diagnosed with NEDS on the basis of full consciousness of eating and memory of it the next day. The NEDS patients of Spaggiari et al [32] represented 5% of the 200 referrals to their sleep laboratory during an 18-month period. All subjects were fully awake during eating episodes and clearly remembered the episodes the next morning. The serotonin agonist d-fenfluramine produced a marked reduction in night eating episodes. The role of serotonergic pathway has recently been shown to be critical in NES [7,19,35]. Finally, Vertrugno et al [33] described 35 patients who were fully conscious while eating and remembered it the next day.
The greatly diminished awareness of eating episodes was the most striking difference between these studies of SRED [28–30] and those of NES [5,11]. Our program reported full consciousness of eating in NES patients by PSG in a controlled inpatient study [22] and by actigraphy in an outpatient study [11]. The disorders also differ on 8 other issues noted in Table 2:
Ingestion of “inappropriate objects” (raw bacon and buttered cigarettes) has been reported as typical of SRED, but has not been reported in NES.
Patients with SRED manifest other parasomnias: periodic leg movements/restless legs syndrome in 47% [30] and 13% [29]. Sleepwalking was reported in as many as 61% of patients by Schenck and Mahowald [29] and 70% by Winkelman [30]. It has rarely been reported in NES, with only a 3% comorbidity reported by Vertrugno et al [33].
Night eating syndrome appears to be far more prevalent than SRED. Studies of the prevalence of SRED have been limited and confined to special populations. The classic study of SRED by Schenck and Mahowald described 0.5% of those who presented to a sleep disorders laboratory over a period of 8 years [28,29], although this prevalence estimate may be closer to 1% in retrospect because of unrecognized cases (personal communication with Carlos Schenck, March 30, 2007). A later report by Winkelman et al [36] described a higher prevalence of SRED among patients with anorexia nervosa and BN: 17% of inpatients and 9% of outpatients. These values, however, were based on a single questionnaire with a low response rate and no validation by interview or PSG. There have been no estimates of SRED in the general population. Reports of prevalence of NES in the general population have ranged from 1.5% [37] to 5.7% [38] and are higher in special populations, as discussed below.
Nocturnal eating episodes were described as “binges” in 68% of SRED patients seen by Schenck and Mahowald [29] and in 67% of SRED patients by Winkelman [30]. Persons with NES rarely reported binge eating, and their nighttime ingestions average 280 kcal [5].
The SRED studies do not report evening hyperphagia (a minimum 25% of daily caloric intake after supper). As noted above, this criterion is one of the two that define NES.
The delayed circadian food intake in NES is also associated with a delay in circadian aspects of neuroendocrine function [39], which has not been reported in SRED.
There have been several reports of SRED associated with hypnotics. Schenck et al [40] have recently described 19 cases of SRED associated with the use of zolpidem, which was relieved with discontinuation of the medication. A similar report described 5 patients, who also had restless leg syndrome, whose SRED began with zolpidem and was relieved with its discontinuation [41]. Onset of SRED has also been reported in association with risperidone [42], olanzapine [43], and triazolam [28,44]. Some NES patients, just as those who had never had night eating episodes previously, have reported occasional sleep eating after the use of hypnotics. They are, however, usually conscious when they eat at night in the absence of hypnotics.
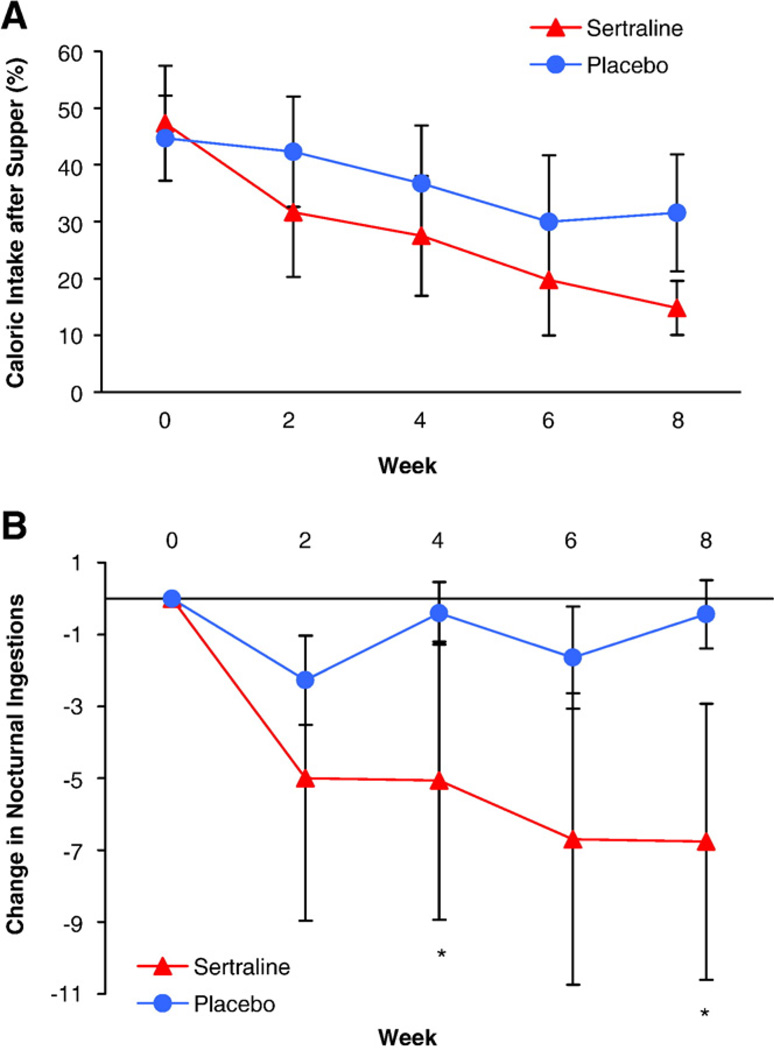
Finally, the most practical difference between these disorders is in treatment. Selective serotonin reuptake inhibitors (SSRIs) proved to be very effective in 4 reports of treatment of NES [7,19,45,46] (see Figure 3). They have rarely been reported in SRED. Sleep-related eating disorder associated with sleepwalking and idiopathic subtypes has responded to dopaminergics, and/or codeine, and/or clonazepam [28,47]. These differences in treatment make discrimination between SRED and NES vitally important. Two studies have shown 65% [48] and 68% [49] responses to topiramate in SRED. Topiramate has been reported to be effective in 2 cases of NES [50]; thus, this medication may be the exception that proves effective for both disorders.
Table 2.
Comparison of features associated with NES and SRED
| NES | SRED | |
|---|---|---|
| Consciousness during nocturnal ingestions |
+ | 0 |
| Memory for nocturnal ingestions | + | 0 |
| Inappropriate eating | 0 | + |
| Comorbidity with parasomnias | 0 | + |
| Prevalence | > | < |
| Binge eating during nocturnal ingestions | 0 | + |
| Evening hyperphagia | + | 0 |
| Delayed circadian pattern of eating | + | ? |
| Induced by hypnotics | ? | + |
| Pharmacologic treatments | SSRI | Topiramate |
| Topiramate | Dopaminergic agents |
|
| Codeine | ||
| Clonazepam |
Fig. 3.
Changes in percentage of energy intake after the evening meal (panel A; F = 3.5; df = 4, 106; P = .009) and frequency of nocturnal ingestions per week (panel B; F = 3.7; df = 4, 80; P = .01; *P <.0125) in participants with NES randomly assigned to 8 weeks of double-blind treatment with sertraline or placebo [7]. Reprinted by permission from The American Journal of Psychiatry. © 2006, American Psychiatric Association.
These clear differences between NES and SRED were not recognized in the 2005 International Classification of Sleep Disorders–2 [34]. It designated the primary criterion of SRED as “recurrent episodes of involuntary eating and drinking occur during the main sleep period.” This criterion does not mention clouded awareness or memory of eating and, in effect, incorporates NES into SRED. The critical issue of the relationship between the 2 disorders is ignored. Inclusion of appropriately defined NES in the DSM would encourage efforts to distinguish between these 2 disorders.
3. Four additional aspects of NES
Four additional aspects of NES have added to our understanding of the disorder. They are as follows: (1) its prevalence, (2) its relationship to obesity, (3) its psychiatric comorbidity, and (4) its biological features (delayed circadian rhythms, a strong genetic influence, 3 animal models, and strong responsivity to stress). The wide variety of findings from so many domains strongly suggests the coherence of the disorder and the usefulness of the criteria that selected subjects for these studies.
3.1. Prevalence of NES
Prevalence of NES has been reported as 1.5%, 1.6%, and 5.7% [37,38,51] in general population samples. These rates are at least as great as the lifetime estimates of the classic eating disorders: anorexia nervosa, 0.9% for women and 0.3% for men; BN, 1.5% for women and 0.5% for men [52]. Prevalence rates of NES have also been determined in special populations. For example, NES prevalence has been estimated at 6% to 16% in weight loss samples of class I and II obesity [25,53].
Prevalence of NES among bariatric surgery candidates in a prospective study was 9% [12]. A prevalence of 3.8% has been found among older adults in a large multicenter study of type 2 diabetes [14] and 12% in an outpatient psychiatric population [8].
3.2. Relationship to obesity
The study by Colles et al [38] (n = 431) showed a great increase in NES prevalence across 5 body mass index categories from 18 to 22 through 40 kg/m2 (χ2 = 22.7, P <.001). The earlier study of Aronoff et al [54] also showed a strong relationship between overweight status and NES (χ2 = 7.1, P = .008).
The large Danish Multinational MONItoring of trends and determination CArdiovascular disease (MONICA) study assessed the relationship between a screening question on night eating and weight change over the next 6 years [55]. Nine percent of women and 7.4% of men endorsed the question, “Do you get up at night to eat?” Obese women who endorsed it gained 5.2 kg over a period of 6 years, whereas obese women who did not endorse it gained only 0.9 kg. There was no relationship between endorsing the question and weight gain among men or nonobese women. In addition, in a study in psychiatric clinics, obese patients were 5.2 times more likely to be diagnosed with NES than were normal-weight patients [8].
Three studies suggest that night eating may be a pathway to obesity. Marshall et al [58] reported that their normal-weight night eaters were younger (33.1 ± 10.7 years) than their obese night eaters (43.1 ± 9.6 years, P < .01). Furthermore, 52% of the obese night eaters reported that their night eating had preceded their obesity. Napolitano et al [56] also reported that nonobese night eaters were younger than obese night eaters, and Spaggiari et al [32] reported that night eating preceded the onset of obesity in most of their night eaters.
In distinction to these findings, 2 studies by Striegel-Moore et al [51,57] failed to find a relationship between night eating and obesity. Differences in assessment procedures may account for this difference from most studies of NES [8,38,54,58]. Thus, of the 2 core criteria, the Striegel-Moore studies did not assess nighttime awakening with ingestions [35,47] and estimated evening hyperphagia from a single 24-hour food recall [51] or a 3-day recall [57].
Another distinctive aspect of NES is that obese night eaters have more difficulty losing weight than do non-night eaters, both in behavioral [10] and psychodynamic treatment [4].
3.3. Psychiatric comorbidity
Night eating syndrome has been linked with psychiatric comorbidity in several studies. Persons with NES report depressed mood more often than persons without NES [5,10,16]. An unusual aspect of this depression is the contrast to the common lessening of depressed mood in the evening in classic depression. Many night eaters become more depressed in the evening [4,5]. A lifetime history of major depressive disorder among night eaters is also high—56% of 1 sample, with an additional 11% reporting dysthymia [23]. Lifetime histories of anxiety disorders in this same sample are also high—17.5% for generalized anxiety disorder and 18% for posttraumatic stress disorder [23]. Lundgren et al [59] reported that 52% of nonobese night eaters met lifetime criteria for unipolar mood disorders and that 47% met lifetime criteria for anxiety disorders, both significantly higher than lifetime prevalence of these disorders among nonobese control subjects. Rates of current and lifetime substance use disorders were significantly higher among night eaters than among non–night eaters in the psychiatric clinic study [8], and low self-esteem was also related to NES in obesity clinic samples [10,60].
Eating disorder psychopathology is also elevated in persons with NES. In recent studies, night eaters, compared with persons without NES, showed elevation on all 4 subscales of the Eating Disorder Examination: dietary restraint, eating concern, shape concern, and weight concern [16,59]. Furthermore, on the Eating Inventory [61], the subscales of disinhibition and hunger (but not restraint) were higher than those of persons in a comparison group [16].
3.4. Biological features
3.4.1. Circadian rhythms and neuroendocrine function
A central aspect of NES is its delayed circadian rhythm. Circadian rhythms of NES and control subjects were assessed using cosinor analysis [39]. It confirmed that, in contrast to those of control subjects, night eaters’ circadian rhythms of energy, carbohydrate, and fat intake were significantly delayed by about 1.5 hours and that of protein was nonsignificantly delayed by about the same amount. The night eaters’ delay in meal timing was accompanied by a similar delay in the circadian rhythm of the food regulatory hormones, insulin and leptin, as well as 4 additional measures: melatonin, cortisol, prolactin, and thyroid-stimulating hormone. An exception was ghrelin, which showed a phase advance of 5.2 hours among night eaters, a finding that deserves further study.
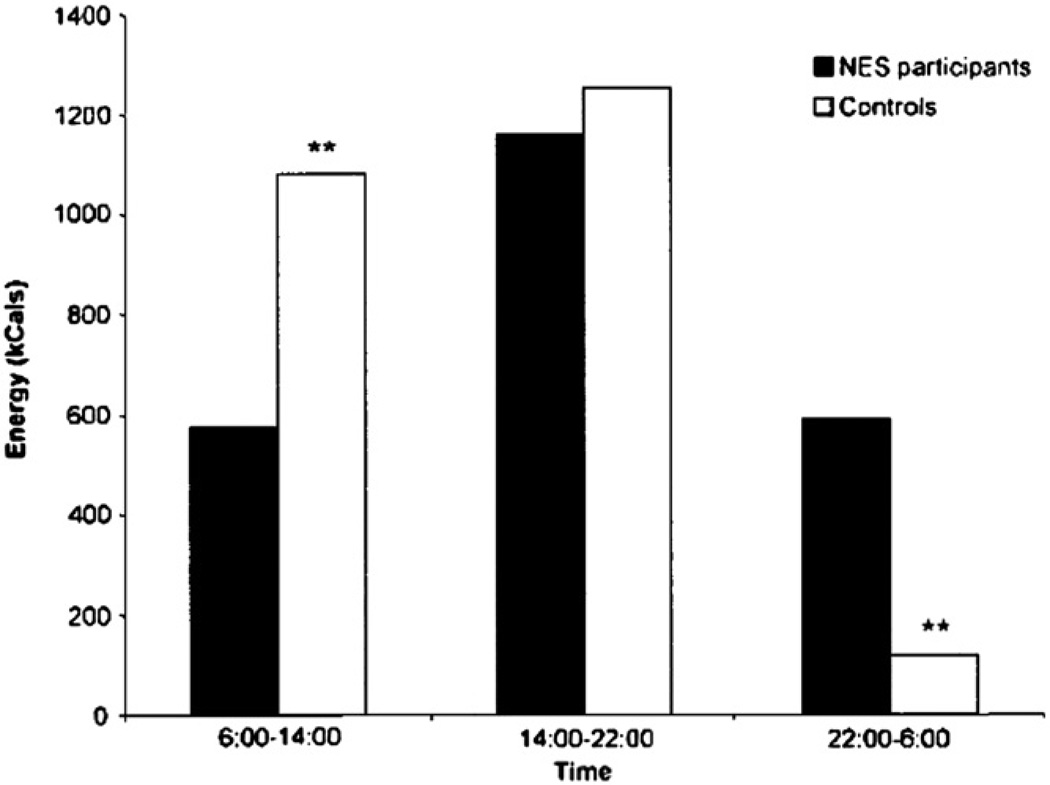
The studies that allowed ad libitum food intake showed that night eaters consumed significantly more (26% to 56%) of their daily energy intake after the evening meal than the 10% to 15% consumed by control subjects [5,11,21]. Compared with control subjects, night eaters had lower energy intake in the first third of the day and greater energy intake during the last third of the day in a free-living environment (Fig. 1) [11].
Fig. 1.
Figure shows energy intake of night eaters and control subjects during three 8-hour periods of the day. This carefully monitored study was based on food and sleep diaries kept by participants for a period of 1 week. Note that food intake is lower in the first part of the day and higher in the evening and night in the NES group. **P <.01. Reprinted by permission of Obesity Research (O’Reardon et al [11]).
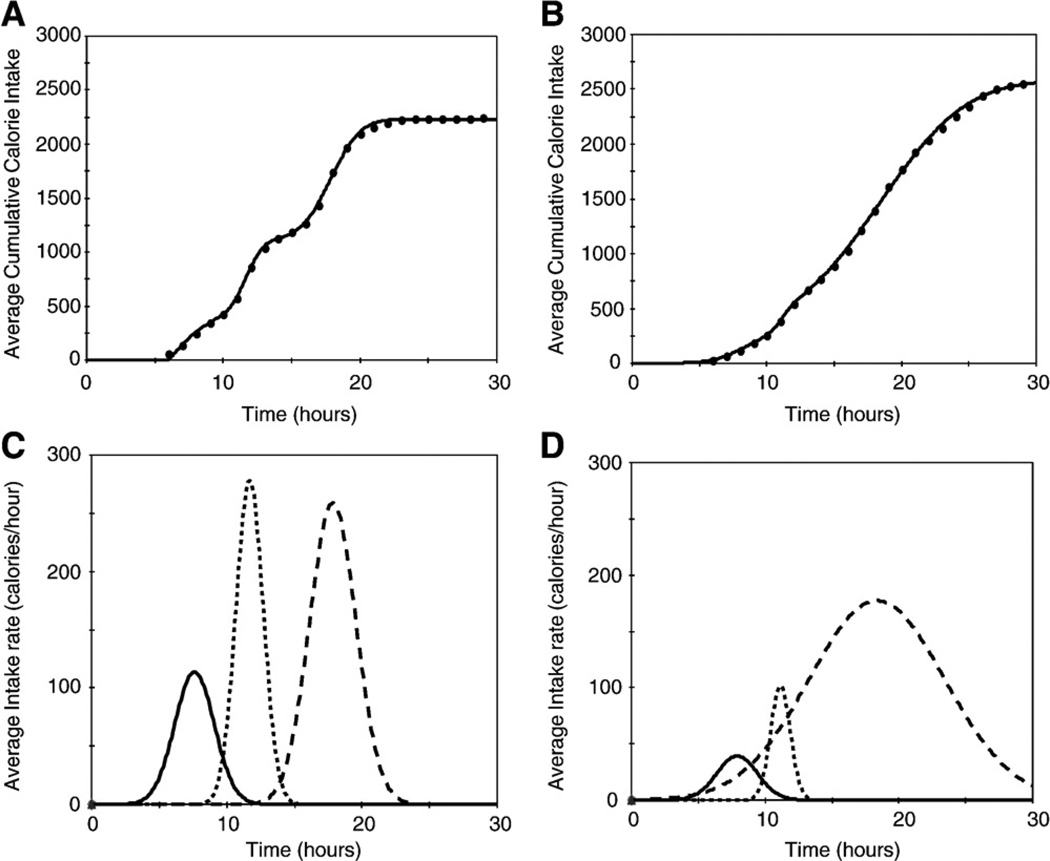
These findings were subsequently also analyzed by a novel equation designed to describe the 24-hour temporal eating patterns of 148 night eaters and 68 control subjects [62]. Fig. 2 shows the average cumulative energy intake of control subjects (Fig. 2A) and of NES subjects (Fig. 2B). The average rate and duration of eating during each of 3 separate meals for control subjects is shown in Fig. 2C and, for NES subjects, in Fig. 2D. The figures show that meals of night eaters are significantly delayed and are spread out over a greater period than those of controls. Persons with NES also consumed more energy than did the controls.
Fig. 2.
Use of the model of Boston et al to describe the average cumulative energy intake of control participants (panel A, means of 68 participants) and NES patients (panel B, means of 148 patients). Dots represent data, and the solid lines are the model predictions. The R2 for prediction equations shown in panels A and B were both in excess of 0.99; and the root mean square errors were 19 and 24 cal for A and B, respectively. Panel C (control participants) and panel D (NES participants) depict the individual Gaussian curves that describe the average rate and amount of eating during each of 3 separate meals. Reprinted by permission from The American Journal of Clinical Nutrition (Boston et al [62]).
A surprising similarity between night eaters and control subjects in sleep onset and offset was found by actigraphy among outpatients [11] and by PSG among inpatients [22] (Table 1). Despite their comparable circadian sleep rhythms, night eaters manifested significantly greater sleep disturbance than control subjects, with more frequent awakenings and lower sleep efficiency (72% ± 17%) than controls (83% ± 7%, P < .03). Sleep apnea was not found [22]. In a sleep laboratory, prevalence of sleep apnea was no greater among night eaters than among non–night eaters [63] (Fig. 3).
3.4.2. Animal models
Animal models of a clinical disorder speak not only to its biological plausibility, but also to critical elements of the disorder. This is true of 3 mouse models that showed an altered circadian rhythm of food intake [64–66]. Each had an unusually high percentage of eating during the light phase. Further differences between the “day eating” and control mice were increased daily energy intake and, ultimately, obesity. An important behavioral similarity of these different mouse models is a failure to suppress the rate of eating during the light period. These 3 different models suggest that altered circadian rhythm of food intake may lead to hyperphagia, perhaps constituting a final common pathway for disorders of various etiologies.
3.4.3. Genetics
A genetic influence on NES has been supported by 2 studies. A family study found an odds ratio of 4.9 for the presence of NES among first-degree relatives of persons diagnosed with NES by the NESHI and food diaries as compared with control subjects [67]. Preliminary results of a large study using the Swedish Twin Register also support the influence of genetic factors [68]. Genetic factors may involve vulnerability to stress.
3.4.4. Stress
Reports of stress initiating NES were noted in the first article on the topic [4]. Patients have since reported the onset of NES during periods of stress, and questionnaires show a higher level of ongoing stress among night eaters [17,69]. These verbal responses have been supplemented by 2 laboratory studies. Birketvedt et al [5] found cortisol levels to be significantly higher among night eaters than among control subjects, and Geliebter et al [24] reported that a cold pressor test produced significantly higher cortisol levels among night eaters than among control subjects.
4. Discussion
Diagnosis is critical in psychiatry, and particularly for NES. For some disorders, the diagnosis is straightforward: abnormalities in anatomy or blood tests tell the story. For the eating disorders, however, diagnosis cannot rely on a simple measure and requires the multiple overlapping measures discussed above. Criteria developed for the diagnosis of NES have been successful in what is perhaps the most rigorous test of their validity: selection of persons who manifest the specific behavioral and biological signs of the disorder.
Treatment depends on diagnosis and may, in turn, influence diagnosis. The history of the treatment of classic manic-depressive psychosis illustrates such interplay between treatment and diagnosis. The discovery of the effectiveness of lithium in the treatment of mania made it critical to distinguish mania from disorders for which lithium was not effective. In this latter category, Cade [70] included “dementia praecox” and “depressive psychosis.” The effectiveness of lithium in mania also led to the recognition that it was effective in disorders other than mania. These disorders include classic bipolar I disorder and the soft “bipolar spectrum” disorders (which comprise bipolar II, bipolar disorder not otherwise specified, and cyclothymia), which have a far higher prevalence than bipolar I disorder.
The efficacy of SSRIs for NES may show a similar importance to that of the therapeutic impact of lithium on bipolar I in differentiating the disorder as a distinct diagnostic category. The weight loss produced in overweight and obese persons by SSRIs seems unique to night eaters [7]. The regulation of feeding and circadian rhythms provides a focus for understanding the etiology of NES. Expansion of its diagnosis beyond current behavioral criteria for NES may define a wider spectrum of disorders of food intake and circadian rhythms.
Inclusion of new diagnoses in the official nomenclature concerned the editors of the DSM-IV-TR: “In general, new diagnoses should be included in the system only after research has established that they should be included.” In the 14th century, William of Occam dealt with similar concerns with his “razor”: “plurality should not be posited without necessity” [71]. In the case of NES, the “necessity” is the diagnosis that precedes and prescribes an effective treatment.
Night eating syndrome currently falls in the category of “Eating Disorders Not Otherwise Specified.” Separating it as an independent psychiatric diagnosis is justified only if it is valid to do so and if the best interests of persons with the disorder are served. The weight of the evidence presented here suggests that this is the case.
Footnotes
“Evening hyperphagia” begins at the end of the evening meal to accommodate different meal times, particularly in different cultures.
References
- 1.American Psychiatric Association. (DSM-IV-TR) Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Press, Inc; 2000. text revision. [Google Scholar]
- 2.Blashfield RK, Sprock J, Fuller AK. Suggested guidelines for including or excluding categories in the DSM-IV. Comp Psychiat. 1990;31:15–19. doi: 10.1016/0010-440x(90)90049-x. [DOI] [PubMed] [Google Scholar]
- 3.Striegel-Moore RH, Franko DL, May A, Ach E, Thompson D, Hook JM. Should night eating syndrome be introduced in the DSM? Int J Eat Disord. 2006;39:544–549. doi: 10.1002/eat.20302. [DOI] [PubMed] [Google Scholar]
- 4.Stunkard AJ, Grace WJ, Wolff HG. The night-eating syndrome: a pattern of food intake among certain obese patients. Am J Med. 1955;19:78–86. doi: 10.1016/0002-9343(55)90276-x. [DOI] [PubMed] [Google Scholar]
- 5.Birketvedt G, Florholmen J, Sundsfjord J, Osterud B, Dinges D, Bilker W, et al. Behavioral and neuroendocrine characteristics of the night-eating syndrome. JAMA. 1999;282:657–663. doi: 10.1001/jama.282.7.657. [DOI] [PubMed] [Google Scholar]
- 6.Lamberg L. All night diners: researchers take a new look at night eating syndrome. JAMA. 2003;290:1442. doi: 10.1001/jama.290.11.1442. [DOI] [PubMed] [Google Scholar]
- 7.O’Reardon JP, Allison KC, Martino NS, Lundgren JD, Heo M, Stunkard AJ. A randomized placebo-controlled trial of sertraline in the treatment of the night eating syndrome. Am J Psychiatry. 2006;164:893–898. doi: 10.1176/ajp.2006.163.5.893. [DOI] [PubMed] [Google Scholar]
- 8.Lundgren JD, Allison KC, Crow S, O’Reardon JP, Berg KC, Galbraith J, et al. Prevalence of the night eating syndrome in a psychiatric population. Am J Psychiatry. 2006;163:156–158. doi: 10.1176/appi.ajp.163.1.156. [DOI] [PubMed] [Google Scholar]
- 9.Stunkard AJ, Allison KC, Lundgren JD. Night eating syndrome in DSM-V. Am J Psychiatry. 2008;165:174. doi: 10.1176/appi.ajp.2007.07081351. [DOI] [PubMed] [Google Scholar]
- 10.Gluck ME, Geliebter A, Satov T. Night eating syndrome is associated with depression, low self-esteem, reduced daytime hunger, and less weight loss in obese outpatients. Obes Res. 2001;9:264–267. doi: 10.1038/oby.2001.31. [DOI] [PubMed] [Google Scholar]
- 11.O’Reardon JP, Ringel BL, Dinges DF, Allison KC, Rogers NL, Martino NS, et al. Circadian eating and sleeping patterns in the night eating syndrome. Obes Res. 2004;12:1789–1796. doi: 10.1038/oby.2004.222. [DOI] [PubMed] [Google Scholar]
- 12.Allison KC, Wadden TA, Sarwer DB, Fabricatore AN, Crerand C, Gibbons L, et al. Night eating syndrome and binge eating disorder among persons seeking bariatric surgery: prevalence and related features. Obesity. 2006;4:77S–82S. doi: 10.1038/oby.2006.286. [DOI] [PubMed] [Google Scholar]
- 13.Aronoff N, Geliebter A, Hashim SA, Zammit G. The relationship between daytime and nighttime intake in an obese night eater. Obes Res. 1994;2:145–151. doi: 10.1002/j.1550-8528.1994.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 14.Allison KC, Crow S, Reeves RR, West DS, Foreyt JP, DiLillo VG, et al. The prevalence of binge eating disorder and night eating syndrome in adults with type 2 diabetes mellitus. Obesity. 2007;15:1285–1291. doi: 10.1038/oby.2007.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allison K, Lundgren J, O’Reardon J, Moore R, Stunkard A. Comparing the clinical significance of the different typologies of night eating syndrome. Obesity. 2007;15:A9–A10. [Google Scholar]
- 16.Allison KC, Grilo CM, Masheb RM, Stunkard AJ. Binge eating disorder and night eating syndrome: a comparative study of disordered eating. J Consult Clin Psychol. 2005;73:1107–1115. doi: 10.1037/0022-006X.73.6.1107. [DOI] [PubMed] [Google Scholar]
- 17.Allison KC, Stunkard AJ, Thier SL. Overcoming the night eating syndrome: a step-by-step guide to breaking the cycle. Oakland (Calif: New Harbinger; 2004. [Google Scholar]
- 18.Allison KC, Lundgren JD, O’Reardon JP, Martino NS, Sarwer DB, Wadden TA, et al. The Night Eating Questionnaire (NEQ): psychometric properties of a measure of severity of the night eating syndrome. Eat Beh. 2008;9:62–72. doi: 10.1016/j.eatbeh.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 19.O’Reardon JP, Stunkard AJ, Allison KC. A clinical trial of sertraline in the treatment of the night eating syndrome. Int J Eat Disord. 2004;35:16–26. doi: 10.1002/eat.10224. [DOI] [PubMed] [Google Scholar]
- 20.Allison KC, Engel SG, Crosby RD, de Zwaan M, O’Reardon JP, Wonderlich ST, et al. Evaluation of diagnostic criteria for night eating syndrome using item response theory analysis. Eat Beh. 2008 doi: 10.1016/j.eatbeh.2008.04.004. http://dx.doi.org/10.1016/j.eatbeh.2008.04.004. [DOI] [PMC free article] [PubMed]
- 21.Allison KC, Ahima RS, O’Reardon JP, Dinges DF, Sharma V, Cummings DE, et al. Neuroendocrine profiles associated with energy intake, sleep, and stress in the night eating syndrome. J Clin Endocr Metab. 2005;90:6214–6217. doi: 10.1210/jc.2005-1018. [DOI] [PubMed] [Google Scholar]
- 22.Rogers NL, Dinges DF, Allison KC, Maislin G, Martino N, O’Reardon JP, et al. Assessment of sleep in women with night eating syndrome. Sleep. 2006;29:814–819. doi: 10.1093/sleep/29.6.814. [DOI] [PubMed] [Google Scholar]
- 23.de Zwaan M, Roerig DB, Crosby RD, Karaz S, Mitchell JE. Nighttime eating: a descriptive study. Int J Eat Disord. 2006;39:224–232. doi: 10.1002/eat.20246. [DOI] [PubMed] [Google Scholar]
- 24.Geliebter A. New developments in binge eating disorder and the night eating syndrome. Appetite. 2002;38:1–3. doi: 10.1006/appe.2001.0472. [DOI] [PubMed] [Google Scholar]
- 25.Stunkard AJ, Berkowitz RT, Tanrikut C, Reiss E, Young L. Binge eating disorder and the night-eating syndrome. Int J Obes Relat Metab Disord. 1996;20:1–6. [PubMed] [Google Scholar]
- 26.Oswald I, Adam K. Rhythmic raiding of refrigerator related to rapid eye movement sleep. Br Med J. 1986;292:589. doi: 10.1136/bmj.292.6520.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whyte J, Kavey NB. Somnambulistic eating: a report of three cases. Int J Eat Disord. 1990;9:577–581. [Google Scholar]
- 28.Schenck CD, Hurwitz TD, Bundlie SR, Mahowald MW. Sleep-related eating disorders—polysomnographic correlates of a heterogeneous syndrome distinct from daytime eating disorders. Sleep. 1991;14:419–431. doi: 10.1093/sleep/14.5.419. [DOI] [PubMed] [Google Scholar]
- 29.Schenck CH, Mahowald MW. Review of nocturnal sleep-related eating disorders. Int J Eat Disord. 1994;15:343–356. doi: 10.1002/eat.2260150405. [DOI] [PubMed] [Google Scholar]
- 30.Winkelman JW. Clinical and polysomnographic features of sleep-related eating disorder. J Clin Psychiatry. 1998;59:14–19. doi: 10.4088/jcp.v59n0104. [DOI] [PubMed] [Google Scholar]
- 31.Manni R, Ratti MT, Tartara A. Nocturnal eating: prevalence and features in 120 insomniac referrals. Sleep. 1997;20:734–738. doi: 10.1093/sleep/20.9.734. [DOI] [PubMed] [Google Scholar]
- 32.Spaggiari MC, Granella F, Parrino L, Marchesi C, Melli I, Terzano MG. Nocturnal eating syndrome in adults. Sleep. 1994;17:339–344. doi: 10.1093/sleep/17.4.339. [DOI] [PubMed] [Google Scholar]
- 33.Vetrugno R, Manconi M, Ferini-Strambi L, Provini F, Plazzi G, Montagna P. Nocturnal eating: sleep-related eating disorder or night eating syndrome? A videopolysomnographic study. Sleep. 2006;29:949–954. doi: 10.1093/sleep/29.7.949. [DOI] [PubMed] [Google Scholar]
- 34.Sateia MJ, editor. International classification of sleep disorders. 2nd ed. Westchester (Ill: American Academy of Sleep Medicine; 2005. pp. 174–175. [Google Scholar]
- 35.Lundgren JD, Newberg AB, Allison KC, Wingering NA, Plossel K, Stunkard AJ. SPECT imaging of serotonin transporter binding in patients with night eating syndrome. Psychiatry Res Neuroimaging. 2008;162:214–220. doi: 10.1016/j.pscychresns.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkelman JW, Herzog DB, Fava M. The prevalence of sleep-related eating disorder in psychiatric and non-psychiatric populations. Psycholog Med. 1999;29:1461–1466. doi: 10.1017/s0033291799008272. [DOI] [PubMed] [Google Scholar]
- 37.Rand CSW, Macgregor AM, Stunkard AJ. The night eating syndrome in the general population and among post-operative obesity surgery patients. Int J Eat Disord. 1997;22:65–69. doi: 10.1002/(sici)1098-108x(199707)22:1<65::aid-eat8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Colles SL, Dixon JB, O’Brien PE. Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int J Obes. 2007;31:1722–1730. doi: 10.1038/sj.ijo.0803664. [DOI] [PubMed] [Google Scholar]
- 39.Goel N, Stunkard AJ, Rogers NL, Van Dongen HPA, Allison KC, O’Reardon JP, et al. Circadian rhythm profiles in night eating syndrome. Soc Light Treat Biol Rhythms Abst. 2007;19:10. doi: 10.1177/0748730408328914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schenck CH, Connoy DA, Castellanos M, Johnson B, Werner R, Wills L, et al. Zolpidem-induced sleep-related eating disorder (SRED) in 19 patients. Sleep. 2005;28(Suppl):A259. [Google Scholar]
- 41.Morgenthaler TI, Silber MG. Amnestic sleep-related eating disorder associated with zolpidem. Sleep Med. 2002;3:323–327. doi: 10.1016/s1389-9457(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 42.Lu ML, Shen WW. Sleep-related eating disorder induced by risperidone. J Clin Psychiatry. 2004;65:273–274. doi: 10.4088/jcp.v65n0220a. [DOI] [PubMed] [Google Scholar]
- 43.Paquet V, Strul J, Servais L, Pelc I, Fossion P. Sleep-related eating disorder induced by olanzapine. J Clin Psychiatry. 2002;63:579. doi: 10.4088/jcp.v63n0710d. [DOI] [PubMed] [Google Scholar]
- 44.Menke DB. Triazolam-induced nocturnal bingeing with amnesia. Aust N Z J Psychiatry. 1992;26:320–321. doi: 10.3109/00048679209072046. [DOI] [PubMed] [Google Scholar]
- 45.Miyaoka T, Yasukawa R, Tsubouchi K, Miura S, Shimizu Y, Sukegawa T, et al. Successful treatment of nocturnal eating/drinking syndrome with selective serotonin reuptake inhibitors. Int Clin Psychopharmacol. 2003;18:175–177. doi: 10.1097/01.yic.0000068440.56680.8e. [DOI] [PubMed] [Google Scholar]
- 46.Stunkard AJ, Allison KC, Lundgren JD, Martino NS, Heo M, Etemad B. A paradigm for facilitating pharmacotherapy at a distance: treatment of the night eating syndrome. J Clin Psychiatry. 2006;67:1568–1572. doi: 10.4088/jcp.v67n1011. [DOI] [PubMed] [Google Scholar]
- 47.Schenck CD, Mahowald MW. Dopaminergic and opiate therapy of nocturnal sleep-related eating disorder associated with sleepwalking or unassociated with another nocturnal disorder. Sleep. 2002;25(Suppl S):A249–A250. [Google Scholar]
- 48.Schenck CH, Mahowald MW. Topiramate therapy of sleep related eating disorder (SRED) Sleep. 2006;29:A268. [Google Scholar]
- 49.Winkelman JW. Efficacy and tolerability of open-label topiramate in the treatment of sleep-related eating disorder: a retrospective case series. J Clin Psychiatry. 2006;67:1729–1734. doi: 10.4088/jcp.v67n1109. [DOI] [PubMed] [Google Scholar]
- 50.Winkelman JW. Treatment of nocturnal eating syndrome and sleep-related eating disorder with topiramate. Sleep Med. 2003;4:243–246. doi: 10.1016/s1389-9457(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 51.Striegel-Moore RH, Franko DL, Thompson D, Affenito S, Kraemer HC. Night eating: prevalence and demographic correlates. Obes Res. 2006;14:139–147. doi: 10.1038/oby.2006.17. [DOI] [PubMed] [Google Scholar]
- 52.Hudson JI, Hiripi E, Pope HG, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adami GF, Campostano A, Marinari GM, Ravera G, Scopinaro N. Night eating in obesity: a descriptive study. Nutrition. 2002;18:587–589. doi: 10.1016/s0899-9007(02)00761-x. [DOI] [PubMed] [Google Scholar]
- 54.Aronoff NJ, Geliebter A, Zammit G. Gender and body mass index as related to the night eating syndrome. J Am Diet Assoc. 2001;101:102–104. doi: 10.1016/S0002-8223(01)00022-0. [DOI] [PubMed] [Google Scholar]
- 55.Andersen GS, Stunkard AJ, Sørensen TIA, Petersen L, Heitmann BL. Night eating and weight change in middle-aged men and women. Int J Obes. 2004;28:1138–1143. doi: 10.1038/sj.ijo.0802731. [DOI] [PubMed] [Google Scholar]
- 56.Napolitano MA, Head S, Babyak MA, Blumenthal JA. Binge eating disorder and night eating syndrome: psychological and behavioral characteristics. Int J Eat Disord. 2001;30:193–203. doi: 10.1002/eat.1072. [DOI] [PubMed] [Google Scholar]
- 57.Striegel-Moore RH, Dohn FA, Hook JM, Schreiber GB, Crawford PB, Daniels SR. Night eating syndrome in young adult women: prevalence and correlates. Int J Eat Disord. 2005;37:200–206. doi: 10.1002/eat.20128. [DOI] [PubMed] [Google Scholar]
- 58.Marshall HM, Allison KC, O’Reardon JP, Birketvedt G, Stunkard AJ. Night eating syndrome among nonobese persons. Int J Eat Disord. 2004;35:217–222. doi: 10.1002/eat.10241. [DOI] [PubMed] [Google Scholar]
- 59.Lundgren JD, Allison KC, O’Reardon JP, Stunkard AJ. A descriptive study of non-obese persons with night eating syndrome and a weight-matched comparison group. Eat Behav. 2008;9:352–359. doi: 10.1016/j.eatbeh.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adami GF, Meneghelli A, Scopinaro N. Night eating and binge eating disorder in obese patients. Int J Eat Disord. 1999;25:335–338. doi: 10.1002/(sici)1098-108x(199904)25:3<335::aid-eat12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 61.Stunkard AJ, Messick S. The Three-Factor Eating Questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 62.Boston RC, Moate PJ, Allison KC, Lundgren JD, Stunkard AJ. Modeling of parametric deconvolution: results of studies of the night eating syndrome. Am J Clin Nutr. 2008;87:1672–1677. doi: 10.1093/ajcn/87.6.1672. [DOI] [PubMed] [Google Scholar]
- 63.Gluck ME, Hollister J, Geliebter A, Lorence M, Rice K, Zammit G. Prevalence and symptom report of obese individuals with night eating syndrome (NES) presenting to a clinical sleep laboratory. Obes Res. 2004;12S:A96. [abstract] [Google Scholar]
- 64.Masaki T, Chiba S, Yasuda T, Noguchi H, Kakuma T, Watanabe T, et al. Involvement of hypothalamic histamine H1 receptor in the regulation of feeding rhythm and obesity. Diabetes. 2004;53:2250–2260. doi: 10.2337/diabetes.53.9.2250. [DOI] [PubMed] [Google Scholar]
- 65.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanchez-Alvarez M, Klein I, Brownell SE, Tabarean JVT, Davis CN, Conti B, et al. Night eating and obesity in the EP3R deficient mouse. Proc Natl Acad Sci U S A. 2007;104:3009–3014. doi: 10.1073/pnas.0611209104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lundgren J, Allison KC, Stunkard AJ. Familial aggregation in the night eating syndrome. Int J Eat Disord. 2006;39:516–518. doi: 10.1002/eat.20269. [DOI] [PubMed] [Google Scholar]
- 68.Rasmussen F, Tholin F, Tynelius PO. Importance of genes and environmental factors for symptoms of night eating among 5209 pairs of twins from Sweden. Int J Obes. 2007 T4:PO.21. [Google Scholar]
- 69.Takeda E, Terao J, Nakaya Y, Miyamoto K, Baba Y, Chuman H, et al. Stress control and human nutrition. J Med Invest. 2004;51:139–145. doi: 10.2152/jmi.51.139. [DOI] [PubMed] [Google Scholar]
- 70.Cade JFJ. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;36:349–352. doi: 10.1080/j.1440-1614.1999.06241.x. [DOI] [PubMed] [Google Scholar]
- 71.Hyman A, Walsh JJ. Philosophy in the middle ages: the Christian, Islamic, and Jewish Traditions. 2nd ed. Indianapolis: Hackett Publishing Company; 1973. [Google Scholar]