Abstract
Objective
Polycystic kidney disease (PKD), a monogenic disease with an autosomal dominant or an autosomal recessive form of inheritance (ARPKD), is the most common genetic cause of renal dysfunction and end stage renal failure. In addition to the development of cysts, the autosomal form of PKD is associated with vascular endothelial dysfunction, a marker of vascular disease. Whether vascular endothelial dysfunction is also present in ARPKD, and the relationship with renal dysfunction remains to be determined.
Methods
ARPKD rats (PCK model) and controls were studied at 6 and 10 weeks of age, and mean arterial pressure (MAP) and renal function were measured. Aortic endothelial function was assessed using organ chamber techniques. Aortic endothelial cells (ECs) were isolated, characterized and their function studied.
Results
Compared to controls, ARPKD animals had a decrease in the vasorelaxation to endothelium-dependent vasodilators, even prior to changes in MAP or renal function. The abnormal vasoreactivity was corrected with L-arginine (precursor to nitric oxide), while the expression of endothelial nitric oxide synthase (eNOS) was unchanged. Furthermore, isolated ECs from 6 week-old ARPKD animals showed increased oxidative stress, with preserved eNOS expression and abnormal patterns of migration and angiogenic capacity (measured by the scratch and tube formation assays, respectively).
Conclusion
ARPKD leads to impaired aortic vascular function and ECs at an early stage, which can have significant functional consequences, potentially representing a novel therapeutic target in this disease.
Keywords: polycystic kidney disease, cardiovascular, kidney, endothelial dysfunction, acetylcholine
Introduction
Polycystic kidney disease (PKD) is the most common renal monogenic disorder and an important cause of end-stage renal disease.[1,2] PKD is characterized by abnormal production of cytoskeletal proteins that are important for the normal function of the primary cilia, cellular structures that determine the orientation, shape and function of somatic cells.[1,2] PKD is characterized by a defect in the cilia-associated protein polycystins −1 and −2 in its autosomal dominant form, while the autosomal recessive form of the disease has been linked to a defect in the Pkhd1 gene and in the expression of the cilia-associated protein fibrocystin.[2,3] PKD is a chronic disease mainly characterized by the development of parenchymal cysts of tubular origin, what leads to impaired handling of urine concentration ability and end-stage renal function.[1,4] Despite differences in transmission and genes involved, both forms have been associated with cyst development and renal dysfunction, albeit with some differences in the rate of progression of the disease.[2,3]
In addition to the renal tubular defects, PKD has also been associated with abnormalities of the vasculature.[5,6] Previous studies have shown that patients and different animal models of PKD have abnormal response to hyperemia and endothelial-dependent vasorelaxation,[5,6] which can contribute to the hypertension observed in PKD patients. Furthermore, this defect has been linked to a deficiency in nitric oxide (NO) bioavailability by endothelial cells (ECs).[6] Because abnormalities in vascular function have been identified as precursors of more advanced vascular disease,[7,8] identification of the mechanisms that may lead to vascular dysfunction and early treatment can be of significant importance. Until now, these abnormalities have been demonstrated mainly in the autosomal dominant form of PKD. Whether the autosomal recessive form of PKD (ARPKD) is also associated with vascular abnormalities, i.e. endothelial dysfunction, and its relation to the appearance of renal dysfunction remains to be determined. Understanding the pathophysiology and timing of vascular changes in PKD may provide novel therapeutic targets for the treatment of this disease.
Thus, in this study we tested the hypothesis that the PCK rat model of ARPKD has vascular endothelial dysfunction, its relationship with renal dysfunction, and investigated the role of ECs in the pathophysiology of the disease.
Materials and Methods
Study outline
All procedures using animals were reviewed and approved by the Mayo Foundation Institutional Animal Care and Use Committee. Female PKD (PCK rat model, n=9, which is on a Sprague-Dawley background)[9] and wild type controls (Sprague-Dawley, n=10) were classified in early (5-6 weeks of age) and late (10-12 weeks of age). Systolic blood pressure was measured non-invasively using the tail cuff method[10] while renal blood flow will be measured non-invasively using high resolution ultrasound (Vevo770, Visualsonics, Toronto, CA) [11]. Aortic rings were obtained for studies of vascular reactivity using organ chamber techniques.[12] Another set of animals was used to isolate aortic ECs for cell culture experiments. The isolation of ECs was performed using the previously described explant technique, and the phenotype of ECs was performed using standard RT-PCR and western blotting techniques. Cell viability and cell proliferation were done using the LDH release and MTT assays, respectively. The angiogenic capacity and migration of ECs were assessed by the tube formation assay and the “scratch” assay, respectively. Methods details can be found in the supplementary information.
Statistical Analysis
Data are expressed as mean±SEM or as percent change from the maximal contraction (in-vitro vascular reactivity). Within each group, repeated measurements were analyzed with repeated measures ANOVA followed by the Bonferroni t-test, or by unpaired Student’s t-test between groups. Statistical significance was accepted for a p value of <0.05.
Results
Systemic hemodynamics and renal function
At 5-6 weeks of age there was no difference in systolic blood pressure (control: 109.90±4.93 vs. PKD: 112.76±4.74 mmHg, p=0.35) or protein/creatinine ratio (control: 0.81±0.28 vs. PKD: 0.70±0.08, p=0.45). However, at 10-12 weeks of age PCK animals had a small but significant increase in systolic blood pressure compared to controls (126.22±2.73 vs. 116.45±3.53 mmHg, p=0.02), together with an increase in the protein/creatinine ratio (0.93±0.2 vs. 0.48±0.06, p=0.02). The renal perfusion of 5-6 week old animals was not different between control and PCK animals (3.20±0.24 and 2.76±0.20 mL/min/100cc3, respectively, p=0.3). We have previously shown that PCK animals at 12 weeks have lower global renal perfusion than controls.[11]
Vascular reactivity: role of NO
The response of the aortic rings to cumulative concentrations of phenylephrine was not statistically different between the groups studied (Supplemental Data, Figure S1), and there was no difference in the pre-contraction obtained with phenylephrine between control and PCK (p=NS).
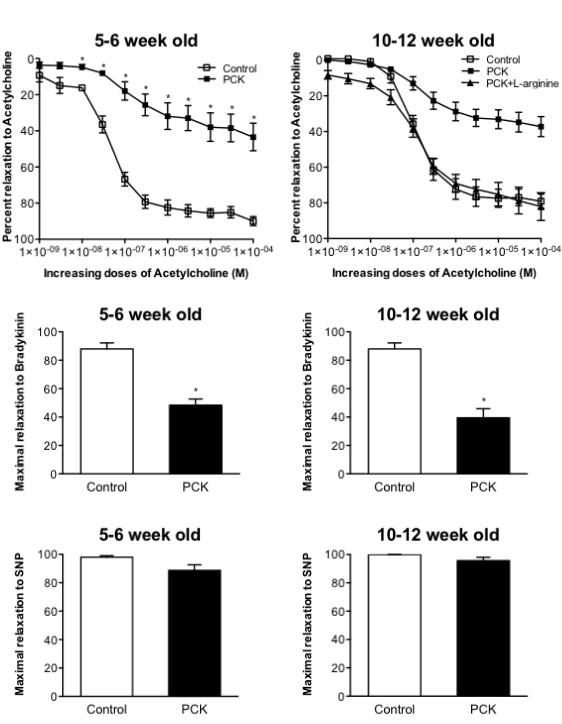
The vasorelaxation response to the endothelium-dependent vasodilator acetylcholine in aortic rings of PKD animals was significantly attenuated compared to normal animals (Figure 1 top). Importantly, the abnormal vasoreactivity response was observed not only in 10-12 week animals (Figure 1, top right), that already have a small increase in MAP and renal dysfunction, but was also observed in young PCK animals (Figure 1, top left), even prior to changes in creatinine clearance and protein/creatinine ratio. An abnormal response, in PCK animals of both ages, was also observed in response to increasing doses of bradykinin (Figure 1, middle panels). There was no difference in the vasorelaxation to the non-endothelium dependent vasodilator SNP between the groups at either age (Figure 1, bottom panels).
Figure 1.
Top, Aortic vasorelaxation response to increasing doses of Acetylcholine in control and PCK 5-6 week old animals (left) and 10-12 week old animals (right). Middle, maximal vasorelaxation to Bradykinin in 5-6 week old animals (left) and 10-12 week old animals (right). Bottom, maximal vasorelaxation to sodium nitroprusside in 5-6 week old animals (left) and 10-12 week old animals (right). *p<0.05 compared with control and PCK+L-arginine. PCK: rat PKD model.
There was not difference between groups in the aortic protein expression of eNOS (control: 2.10±0.87 vs. PCK: 2.95±0.90, p=NS) or iNOS (control: 2.04±0.75 vs. PCK: 1.80±0.65, p=NS). However, when aortic rings were pre-incubated with the NO precursor L-arginine, the vasorelaxation to acetylcholine was normalized (Figure 1, top right), suggesting that a decrease in NO bioavailability was implicated in the PKD abnormal response.
Isolation and characterization of endothelial cells
To specifically test the function of endothelial cells, ECs were successfully isolated from the aorta of control and PKD animals using the explant technique. Furthermore, both control and PKD ECs expressed the endothelial marker vWF and were positive for the uptake of Ac-LDL (Supplemental Data, Figure S2A), showing that the isolated cells are in fact ECs. Furthermore, ECs from both WT and PCK have the capacity to express fibrocystin (Supplemental Data, Figure S2B), although PCK rats will have a splicing mutation in the Pkhd1 gene, as previously described,[13] leading to an abnormal protein, and potentially abnormal cell function.
Nitric oxide system/Oxidative stress balance in isolated aortic endothelial cells
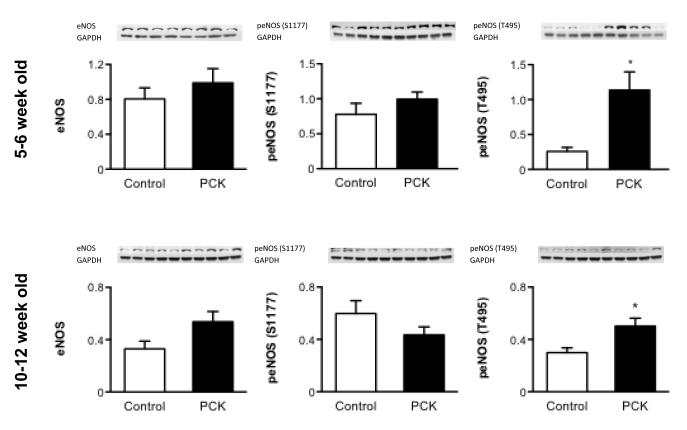
Endothelial cells of 5 and 10 week-old animals did not show a difference in the expression of eNOS between PCK and controls. Although there was not difference in the expression of the “active” form of eNOS (phosphorylated at Ser 1177) between the groups, the expression of the “inactive” form of eNOS (phosphorylated at Thr 495) was significantly higher in PCK than controls both in 5 and 10 week-old rats (Figure 2).
Figure 2.
Protein expression of eNOS and its active (eNOS phosphorylated at Ser 1177) and inactive (eNOS phosphorylated at Thr 495) forms in 5 week old and 10 week old isolated endothelial cells, showing an increase in the expression of inactive eNOS in PCK compared to control
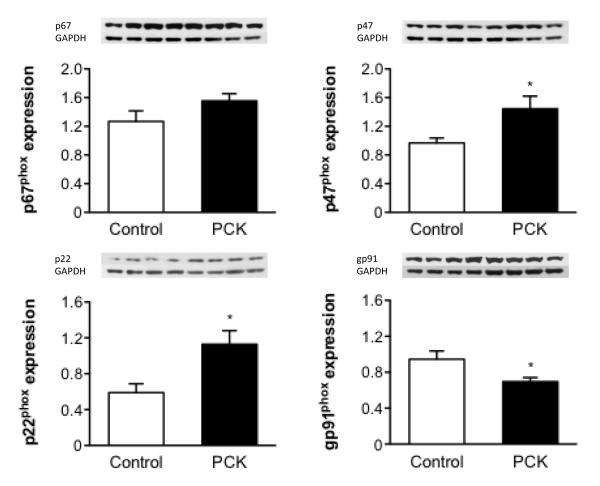
An imbalance in the expression of pro-oxidant enzymes was observed in 5 week-old PCK-ECs compared to controls but not in ECs isolated from older animals. Specifically, 5 week-old PCK-ECs had an increase in the expression of NAD(P)H p22phox, NAD(P)H p47phox, with no change in the expression of NAD(P)H p67phox and a decrease in NAD(P)H gp91phox (Figure 3).
Figure 3.
Protein expression of pro-oxidant enzymes in 5 week old isolated endothelial cells, showing an imbalance towards a pro-oxidant status in PCK vs. control. *p<0.05 compared to WT. eNOS: endothelial nitric oxide synthase, Ser: Serine, Thr: Threonine.
Functional studies on endothelial cells
Proliferation/Death
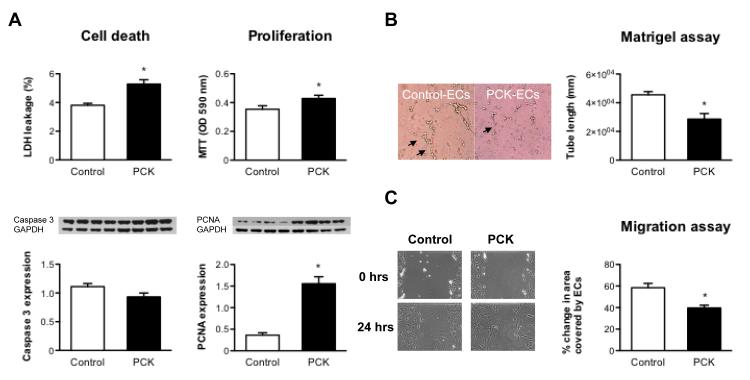
Compared to controls, PCK-ECs had an increase in cell proliferation (MTT assay and expression of PCNA and Akt, Figure 4A) and cell death (LDH release assay), although the expression of Caspase 3 was not different between PKD and controls (Figure 4A).
Figure 4.
A, Cell viability (LDH release assay and protein expression of Caspase 3) and proliferation (MTT assay and protein expression of PCNA) in PCK and control ECs, showing that PCK-ECs have increased cell death and cell proliferation compared to control. B, Tube formation assay in WT and PCK ECs. Left panel shows a representative picture of control and PCK ECs tube formation and the right panel shows the quantification of tube length in mm. Note that PCK-ECs have decreased capacity to form tubes compared to control. C, Migration capacity of ECs in response to injury. ECs from PCK animals have decreased capacity for migration compared to control, as evidenced by representative images (left panel) and quantification of area covered by ECs (right panel). *p<0.05 compared to WT. LDH: lactate dehydrogenase, MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. PCNA: proliferating cell nuclear antigen.
Angiogenic capacity
Endothelial cells from PKD animals had abnormal function, as demonstrated by an impaired angiogenic capacity, compared to control cells. This abnormal capacity is evidenced by the lower tube length formation of PCK-ECs in the first 3 hours after plating (Figure 4B).
Migration
In response to injury, ECs from PCK animals had significant decrease in migration compared to WT, as shown by PCK-ECs covering a smaller area after injury (Figure 4C).
Discussion
This study shows that the aorta of PCK animals, as a model of PKD, has abnormal endothelial-dependent vascular reactivity probably due to a primary defect in ECs.
The pathogenesis of PKD has been mainly associated with a primary renal tubular dysfunction, although variables of vascular disease have been described (e.g., vascular aneurysms)[4,14]. In this study we show that the PCK rat model of PKD, a model that is autosomal recessive by inheritance pattern, also possesses an abnormal pattern of endothelial dependent vascular reactivity. Furthermore, we show that it is corrected by the addition of the NO precursor L-arginine, despite no changes in NOS expression, implying that the problem relates to substrate availability, rather than availability of NOS. Furthermore, PKD has been previously shown to have elevated levels of asymmetric dimethylarginine (ADMA),[15] what competes with L-arginine for eNOS and the production of NO. This hypothesis is supported by our finding of an increased expression of “inactive” peNOS in ECs isolated from both 5 and 10 week-old animals. Although we have expected a decrease in the active form, it is important to keep in mind that this balance could be tenuous and that the in-vivo activation of eNOS or iNOS could somewhat vary from the in-vitro setting. Moreover, it should be noted that the expression of NOS is dynamic, so we cannot exclude the possibility that at other time points the expression pattern of eNOS may be different. Importantly, we show that the aortic vascular dysfunction is not primarily related to renal dysfunction, preceding changes in global renal perfusion or urinary creatinine/protein ratio. The importance of the findings in this study is that we show for the first time that the PCK rat model also presents abnormalities in non-renal vascular function and provide insights on the timing of the appearance of these abnormalities.
In this study the abnormalities in vascular reactivity were mainly related to the endothelial-dependent vasorelaxation, while the endothelium-independent, i.e. smooth muscle, relaxation was not impaired. This finding of early abnormal vascular reactivity led us to investigate the status of the ECs in PKD, where we demonstrated that ECs from PCK animals have increased cell death and proliferation rate, as previously described for other cells types in PKD.[16,17] Furthermore, we also showed that ECs express fibrocystin, a protein that has been implicated in the structure and function of the primary cilia, an organelle that has been associated with regulation in vascular function.[18,19] Taking into account that the main defect in the PCK model, compared to WT, is a splicing mutation in fibrocystin with potential defect in their structure and function,[13] this defect in primary cilia may in part account for the vascular abnormalities described. Whether there are other cilia abnormalities in this model and if they are related to the impaired vasoreactivity observed in this study is still unclear and further studies are needed.
Nitric oxide has been shown to play a key role in vascular relaxation, and ECs to be a key component in NO production. At the same time, NO interacts with other biological pathways what in turn can decrease the bioavailability of NO. In fact, changes in oxidative stress balance have been identified as one of those pathways.[20] In this study, we extend these observations to the PKD population and show that ECs isolated from 5 week-old PCK rats have an imbalance towards a prooxidant phenotype. This result, together with other factors, could support the hypothesis that the impaired vascular relaxation potentially is due to a reduced bioavailability of NO, with an increase in the expression of the “inactive” eNOS (phosphorylated at Thr 495), although the total eNOS expression is not decreased. The oxidative stress pathway involves a large number of genes which expression is dynamic. This could explain the fact that we didn’t observe any significant differences in the expression of pro-oxidant enzymes between control and PCK ECs isolated from 10 week-old rats. Moreover, other pathways can interact with NO, especially in older animals in which for example elevated levels of ADMA have been previously described. These data are supported by our in vivo findings, in which the addition of the NO precursor L-arginine normalized the vasorelaxation in PKD animals.
Polycystic kidney disease research has been mainly directed towards investigation of renal and liver disease. In this study we show that PKD affects main vessels like the aorta, and that it does so prior to the development of renal dysfunction, as measured by clinically used parameters. In addition, and taking into account the abnormalities seen in young cultured ECs, it can be hypothesized that the vascular abnormalities in PCK are not purely due to renal dysfunction, but also to primary systemic alterations in PKD-ECs. In fact, in this study we showed that ECs from young PCK animals have abnormal angiogenic activity and migration, two common assays to assess EC functionality.
This study provides important information regarding the vascular changes in ARPKD, identifying potentially novel targets for the treatment of this disease. We have previously shown that this model of PKD is associated with decreased renal vascular density,[11] and in this paper we demonstrate that the vascular changes are not limited to the renal vasculature and that they appear prior to changes in MAP and changes in clinically used parameters of renal dysfunction.
In summary, in this study we show that ARPKD is associated with systemic endothelial dysfunction, in a non-renal vascular territory. Furthermore, the similarity of ARPKD with ADPKD in the development of vascular endothelial dysfunction suggests that this model of disease can be used to test novel therapies that address this phenotype of polycystic disease.
Limitations
The present study focuses on the development of disease in ARPKD, and although similarities between ARPKD and ADPKD have been described by our group,[9] caution should be exerted when extrapolating results. Secondly, we chose a “young” group of animals at 5-6 weeks of age, and described that vascular defects were already present at that time, when no changes in BP, creatinine clearance or protein/creatinine ratio were observed, inferring that changes in vascular function are not only due to changes in renal function. However, we cannot exclude the presence of smaller degrees of renal dysfunction that may be undetectable by clinically used methods. Thirdly, the LDH and MTT assays provide an overall assessment of cell death and proliferation, respectively, but cannot specifically identify the mechanisms involved in the abnormal death and proliferation rates seen in PKD, questions that will be addressed in future studies.
Supplementary Material
Figure S1. Aortic vasoconstriction response to increasing doses of Phenylephrine in control and PCK 5-6 week old animals. Differences are not significant. The dose responses to phenylephrine clearly show that the vasculature in both groups was reactive to PE at comparable doses, although there was a small difference in the grams of tension achieved.
Figure S2. A, Endothelial cells (ECs) from both control and PCK express the endothelial marker vWF (left) and uptake of Acetylated LDL (right). B, Expression of fibrocystin and transferrin in control and PCK derived endothelial cells (ECs). vWF: vonWillebrand factor
Perspectives.
This study describes that the vascular abnormalities in PKD appear even before clinical measures of renal dysfunction. Furthermore, we show that they are not limited to the kidney and suggest that ECs in PKD may have primary functional defects that, in combination with the renal dysfunction in this disease, could potentiate the vascular derangements in PKD. Lastly, it further supports the concept that the PCK rat model of PKD shares many vascular features with ADPKD and could be used to evaluate novel treatments of this disease.
Acknowledgements
The authors want to thanks Drs. Vicente E. Torres and Christopher J. Ward, and Mrs. Xiaofang Wang for her assistance with the PCK model.
Funding: National Institutes of Health grants DK90728 and HL88048, and the Mayo Foundation.
Footnotes
Conflict of interest: None
Bibliography
- 1.Harris PC, Torres VE. Polycystic kidney disease. Annual review of medicine. 2009;60:321–37. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres VE. New insights into polycystic kidney disease and its treatment. Curr Opin Nephrol Hypertens. 1998;7:159–69. doi: 10.1097/00041552-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Torres VE, Harris PC. Polycystic kidney disease: genes, proteins, animal models, disease mechanisms and therapeutic opportunities. J Intern Med. 2007;261:17–31. doi: 10.1111/j.1365-2796.2006.01743.x. [DOI] [PubMed] [Google Scholar]
- 4.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney international. 2009;76:149–68. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Iversen J, Strandgaard S. Endothelium-dependent relaxation of small resistance vessels is impaired in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2000;11:1371–6. doi: 10.1681/ASN.V1181371. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Iversen J, Wilcox CS, Strandgaard S. Endothelial dysfunction and reduced nitric oxide in resistance arteries in autosomal-dominant polycystic kidney disease. Kidney international. 2003;64:1381–8. doi: 10.1046/j.1523-1755.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- 7.Lavi S, Bae JH, Rihal CS, Prasad A, Barsness GW, Lennon RJ, Holmes DR, Jr., Lerman A. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart. 2009;95:1525–30. doi: 10.1136/hrt.2009.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lerman A, Holmes DR, Jr., Bell MR, Garratt KN, Nishimura RA, Burnett JC., Jr. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation. 1995;92:2426–31. doi: 10.1161/01.cir.92.9.2426. [DOI] [PubMed] [Google Scholar]
- 9.Lager DJ, Qian Q, Bengal RJ, Ishibashi M, Torres VE. The pck rat: a new model that resembles human autosomal dominant polycystic kidney and liver disease. Kidney international. 2001;59:126–36. doi: 10.1046/j.1523-1755.2001.00473.x. [DOI] [PubMed] [Google Scholar]
- 10.Stroope A, Radtke B, Huang B, Masyuk T, Torres V, Ritman E, LaRusso N. Hepato-renal pathology in pkd2ws25/- mice, an animal model of autosomal dominant polycystic kidney disease. The American journal of pathology. 2010;176:1282–91. doi: 10.2353/ajpath.2010.090658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu R, Franchi F, Miller B, Crane JA, Peterson KM, Psaltis PJ, Harris PC, Lerman LO, Rodriguez-Porcel M. Polycystic kidneys have decreased vascular density: a microCT study. Microcirculation. 2012 Nov 20; doi: 10.1111/micc.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Porcel M, Lerman LO, Holmes DR, Richardson D, Napoli C, Lerman A. Chronic antioxidant supplementation attenuates nuclear factor-kappaB activation and preserves endothelial function in hypercholesterolemic pigs. Cardiovascular research. 2002;53:1010–8. doi: 10.1016/s0008-6363(01)00535-1. [DOI] [PubMed] [Google Scholar]
- 13.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nature genetics. 2002;30:259–69. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 14.Nagao S, Yamaguchi T. PPAR-gamma agonists in polycystic kidney disease with frequent development of cardiovascular disorders. Curr Mol Pharmacol. 2012;5:292–300. doi: 10.2174/1874467211205020292. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Strandgaard S, Borresen ML, Luo Z, Connors SG, Yan Q, Wilcox CS. Asymmetric dimethylarginine and lipid peroxidation products in early autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2008;51:184–91. doi: 10.1053/j.ajkd.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Tao Y, Kim J, Faubel S, Wu JC, Falk SA, Schrier RW, Edelstein CL. Caspase inhibition reduces tubular apoptosis and proliferation and slows disease progression in polycystic kidney disease. Proc Natl Acad Sci U S A. 2005;102:6954–9. doi: 10.1073/pnas.0408518102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanoix J, D’Agati V, Szabolcs M, Trudel M. Dysregulation of cellular proliferation and apoptosis mediates human autosomal dominant polycystic kidney disease (ADPKD) Oncogene. 1996;13:1153–60. [PubMed] [Google Scholar]
- 18.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–71. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nauli SM, Jin X, Hierck BP. The mechanosensory role of primary cilia in vascular hypertension. International journal of vascular medicine. 2011;2011:376–281. doi: 10.1155/2011/376281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas SR, Chen K, Keaney JF., Jr. Oxidative stress and endothelial nitric oxide bioactivity. Antioxid Redox Signal. 2003;5:181–94. doi: 10.1089/152308603764816541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Aortic vasoconstriction response to increasing doses of Phenylephrine in control and PCK 5-6 week old animals. Differences are not significant. The dose responses to phenylephrine clearly show that the vasculature in both groups was reactive to PE at comparable doses, although there was a small difference in the grams of tension achieved.
Figure S2. A, Endothelial cells (ECs) from both control and PCK express the endothelial marker vWF (left) and uptake of Acetylated LDL (right). B, Expression of fibrocystin and transferrin in control and PCK derived endothelial cells (ECs). vWF: vonWillebrand factor