Abstract
Background
The COP9 signalosome (CSN) is an evolutionarily conserved protein complex composed of 8 unique protein subunits (CSN1 through CSN8). We have recently discovered in perinatal mouse hearts that CSN regulates not only proteasome-mediated proteolysis but also macroautophagy. However, the physiological significance of CSN in a pots-mitotic organ of adult vertebrates has not been determined. We sought to study the physiological role of CSN8/CSN in adult mouse hearts.
Methods and Results
Csn8 was conditionally ablated in the cardiomyocytes of adult mice (CSN8CKO) using a temporally controlled Cre-LoxP system. Loss of CSN8 accumulated the neddylated forms of cullins and non-cullin proteins, increased ubiquitinated proteins, and stabilized a surrogate substrate of the proteasome in the heart. Autophagic flux was significantly decreased while autophagosomes were markedly increased in CSN8CKO hearts, indicative of impaired autophagosome removal. Furthermore, we observed increased oxidized proteins, massive necrotic cardiomyocytes, and morphological and functional changes characteristic of dilated cardiomyopathy in CSN8CKO mice.
Conclusions
CSN deneddylates substrates more than cullins and is indispensable to cardiomyocyte survival in not only perinatal hearts but also adult hearts. CSN8/CSN regulates both proteasome-mediated proteolysis and the autophagic-lysosomal pathway, critical to the removal of oxidized proteins in the heart.
Keywords: COP9 signalosome, autophagy, proteasome, NEDD8, heart
Protein quality control (PQC) is pivotal to proteostasis in the cell. Targeted removal of terminally misfolded/damaged proteins is the last line of defense in PQC and is performed by the ubiquitin proteasome system (UPS) and macroautophagy (hereafter referred to as autophagy), two main intracellular proteolytic pathways.1 UPS-mediated proteolysis involves ubiquitination of a protein molecule and the subsequent proteasome-mediated degradation of the ubiquitinated protein. Autophagy uses specialized double-membrane structures to sequester a portion of the cytoplasm, proteins aggregates, or damaged organelles into autophagosomes for fusion with and degradation by lysosomes.2, 3 UPS and autophagy dysfunctions are implicated in the pathogenesis of many cardiac disorders.1–11 However, molecular mechanisms by which the UPS and autophagy are regulated in the heart remain poorly understood.
The COP9 signalosome (CSN), identified first in Arabidopsis thaliana,12 is an evolutionarily conserved multi-protein complex consisting of eight unique subunits (CSN1 through CSN8).13 The attachment of a ubiquitin-like protein NEDD8 to cullin via a process known as neddylation is critical for the activation of cullin-based RING ligases (CRLs).14 The bona fide biochemical activity of CSN is to remove NEDD8 from cullin (i.e., deneddylation);15 therefore, CSN is believed to play an important role in regulating CRLs. Studies from lower organisms and cultured mammalian cells have suggested that CSN participates in a variety of biological processes, including invertebrate development, DNA repair, cell cycle, kinase signaling, nuclear transport, and T cell proliferation.16 These observed roles are more or less tied to the deneddylation activity of CSN; but this does not preclude other unidentified functions that CSN might possess. Despite these observations, the physiological role of the CSN in mammals has just begun to be investigated. Germ-line deletion of the genes encoding CSN subunits in mice all resulted in embryonic lethality, at least partially due to defect in cell proliferation, underscoring its essential role in embryonic development.16, 17
CSN8 is the smallest and least conserved subunit of CSN.17 By conditional gene targeting at the perinatal stage of murine development, we have recently found that CSN8regulates both the UPS and the autophagic-lysosomal pathway in perinatal hearts and is essential to early postnatal cardiomyocyte survival and cardiac function.9, 18 However, the biochemical function and physiological significance of CSN has never been tested in a post-mitotic organ of vertebral animals. Given that CSN is required for cell division and rodent cardiomyocytes do not stop proliferating until several days after birth,13, 19 the phenotypes, including increased cell death which is often intimately linked to cell cycle perturbation, and resultant cardiac failure observed in mice with perinatal cardiomyocyte-restricted Csn8 knockout may be unique to the perinatal stage. In other words, the heart with cardiomyocytes undergoing active proliferation at the perinatal stage may respond to CSN8/CSN deficiency differently from an adult heart in which cardiomyocyte proliferation has ceased. Furthermore, neddylation and CSN are emerging therapeutic targets in adult malignancies.20, 21 Understanding the impact of Csn8/CSN deficiency on adult hearts should help unveil the potential adverse impact of these new therapeutic strategies on adult hearts. Hence, the present study has determined the impact of cardiomyocyte-restricted ablation of the csn8 gene initiated in adult mice (CSN8CKO) on cardiac PQC and heart structure and function. The results demonstrate for the first time in a post-mitotic organ of intact adult vertebral animals that CSN8 is required for the deneddylation of cullins and unknown non-cullin proteins and regulates both the UPS and autophagy and thereby is essential to PQC and the functioning and survival of cardiomyocytes.
Methods
Animal models and experimental protocols
CSN8-floxed mice (CSN8flox/+),17 transgenic (tg) mice with cardiac expression of the mutant estrogen receptor fused Cre driven by the mouse Mhc6 promoter (MerCreMertg),22 GFPdgn tg mice,23 and GFP-LC3 tg mice24 were previously described. To generate mice suitable for CSN8CKO, CSN8flox/flox mice were cross-bred with MerCreMertg. The resultant MerCreMertg::Csn8flox/+ mice were then mated with Csn8flox/flox mice, which gave rise to littermate mice that have a genotype of (A) MerCreMerntg::Csn8flox/+, (B) MerCreMerntg::Csn8flox/flox, (C) MerCreMertg::Csn8flox/+, or (D) MerCreMertg::Csn8flox/flox in the expected Mendelian frequency and appear healthy and indistinguishable from one another. To induce the MerCreMer mediated ablation of the floxed csn8 alleles, 8- to 10-week-old littermate mice with the 4 different genotypes described above, as well as age- and gender- matched wild type mice were treated with daily intraperitoneal injection of tamoxifen (Sigma, 100µg/g/day) for 3 consecutive days. During the initial tests, no significant difference in CSN8 and neddylated cullin protein levels was detected among mice with genotypes (A), (B), and wild type mice after tamoxifen or mock treatments. Hence, mice of genotype (B) and (D) after tamoxifen treatments were respectively used as the control group (CTL) and the CSN8CKO group.
The protocol for the care and use of animals in this study was approved by the Institutional Animal Care and Use Committee of the University of South Dakota and conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Immunofluorescence confocal microscopy
The fixation, embedding, immunostaining, and image acquisition were performed as previously described.18
Western blot analyses
Protein extraction from myocardium, protein concentration determination with BCA reagents (Pierce), SDS-PAGE, western blot analyses, and densitometry were performed as previously described.9
RNA analyses
Total RNA was extracted from ventricular tissue using the Tri-Reagent (Molecular Research Center). Northern blot analysis for Csn8 mRNA and RNA dot blot analyses for GFPdgn and genes of the fetal gene program were performed as previously described.9, 25
Proteasome peptidase activity assays
The synthetic fluorogenic substrate Suc-LLVY-minomethycoumarin (25µM) and Z-LLE-β naphthylamide (25µM, BIOMOL) were respectively used for measuring chymotrypsin-like and caspase-like activities in crude protein extracts from ventricular myocardium.18
Transmission electron microscopy
Transmission electron microscopy (TEM) of perfusion fixed myocardium was performed as previously described.25
Assessing autophagic flux in the heart
Mice were intraperitoneally injected with 2 doses of bafilomycin-A1 (BFA, 3µmol/kg, Sigma) or vehicle control with 1 hour in between. At 2 hours after the first dose, the mice were euthanized and the ventricular myocardium was sampled for western blot analyses of LC3-II levels. BFA induced increases of LC3-II reflect autophagic flux.8
Echocardiography
Mice were anesthetized by inhalation of isoflurane (2.5% for induction and 1.5% for maintenance) via a nose cone. The adequacy of anesthesia was monitored by toe pinch. Transthoracic echocardiograph was recorded using the Vevo770™ echocardiography system and a 30MHz transducer with a focal length of 12.7mm (Visual Sonics). The LV morphometric and functional parameters were analyzed off-line as previously described.18
Left ventricle (LV) hemodynamics
Mice anesthetized by inhalation of 2.5% isoflurane were intubated through the mouth and mechanically ventilated. The LV catheterization and hemodynamic assessments were performed as previously described.26
Statistical Analysis
All quantitative data are presented as dot plots with mean ± SD superimposed. Differences between two experimental groups were evaluated for statistical significance using the Wilcoxon rank sum test. Differences among multiple groups were evaluated using the Kruskal-Wallis test followed by the Dunn test. The P value <0.05 were considered statistically significant.
Results
Targeted ablation of the Csn8 gene in cardiomyocytes of adult mice
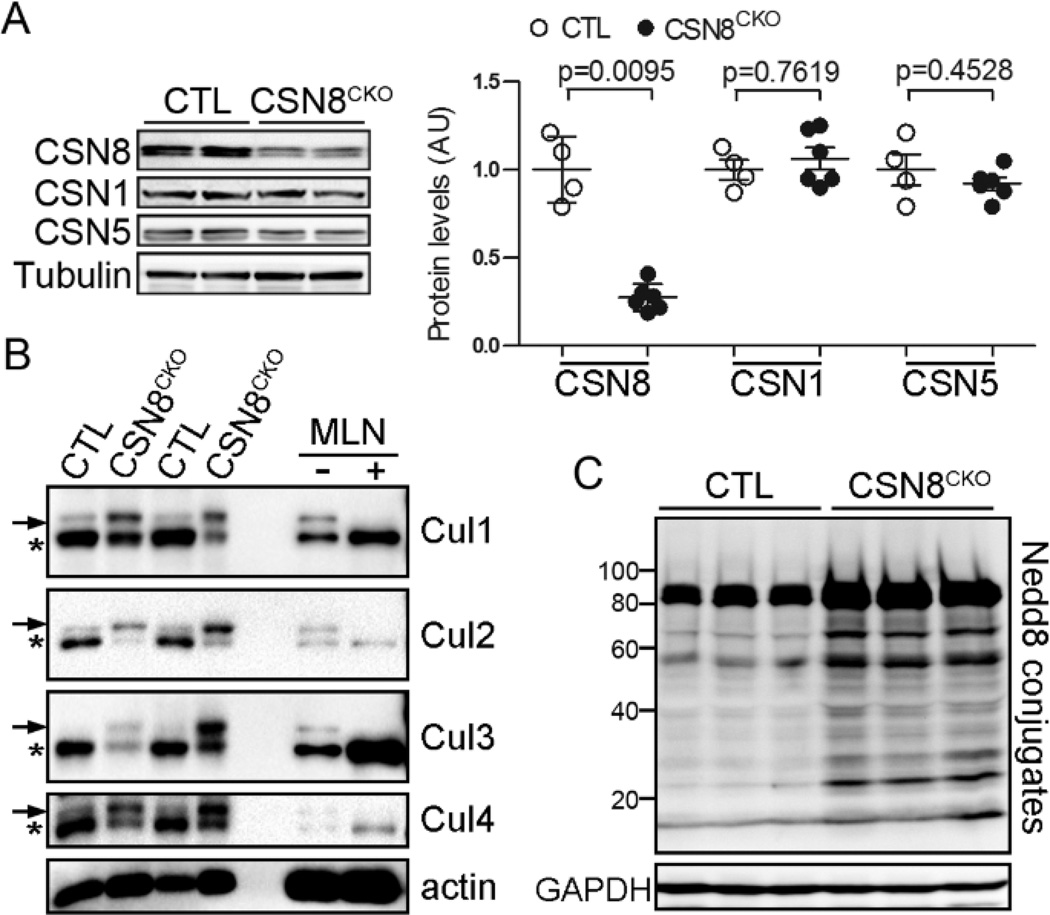
We targeted the Csn8 gene in adult mouse hearts using the MerCreMer mediated inducible Cre-LoxP system (Supplemental Figure S1A). Deletion of the Csn8 gene in adult hearts was induced by administration of tamoxifen to 8- to 10-week-old mice. Tamoxifen can bind to MerCreMer and moves the MerCreMer from cytoplasm to the nucleus where the MerCreMer performs the recombination of the floxed alleles.22 The recombination were verified by PCR analyses, using genomic DNA extracted from myocardium of the CTL and CSN8CKO mice at 3 days after the first tamoxifen injection. The recombined CSN8flox allele was detected only in the CSN8CKO hearts but not in the CTL hearts (Supplemental Figure S1B). At 3 days after the first tamoxifen injection, the CSN8 mRNA level in the ventricle of CSN8CKO hearts fell nearly below the limit of northern blot analysis (Supplemental Figure S1C), indicative of efficient excision of CSN8flox alleles. Approximately 70% of CSN8 proteins were lost in the CSN8CKO hearts by 5 days after the first tamoxifen injection (Figure 1A). Consistent with the findings from western blot analyses, CSN8 immunostaining was significantly diminished in both the nucleus and cytoplasm of cardiomyocytes in CSN8CKO hearts (Supplemental Figure S1D). Together, these data indicate that highly efficient deletion of the Csn8 gene was achieved in the cardiomyocytes of CSN8CKO adult mice within 5 days after the first tamoxifen injection.
Figure 1. Cardiomyocyte-restricted Csn8 knockout initiated in adult mice impairs deneddylation activities in the heart.
At 5 days after the first tamoxifen injection, ventricular myocardium was sampled from Csn8flox/flox (CTL) or Csn8flox/flox::MerCreMertg (CSN8CKO) littermate mice for western blot analyses. Representative images are shown with each lane representing a mouse. α-Tubulin or GAPDH was probed for loading control. (A) Changes in CSN8, CSN1 and CSN5. AU: arbitrary unit. (B) Increases in the neddylated form (arrow) and decreases in the native form (*) of cullin1 (Cul1), Cul 2, Cul3, and Cul4A in CSN8CKO hearts. To verify the identity of the neddylated cullins, cultured neonatal rat cardiomyocytes were treated with a specific NEDD8 E1 inhibitor MLN4924 (MLN, 1µM) (Active Biochem, Maplewood, NJ) or vehicle (−) for 24 hours. MLN4924, which inhibits neddylation, abolished the slow-migrating band of all cullins, confirming their identity as neddylated cullins. (C) Increased Nedd8 conjugates in CSN8CKO hearts.
Loss of CSN deneddylation activity in CSN8CKO hearts
The bona fide cullin deneddylation activity of CSN resides in CSN5.13 Neither CSN5 nor CSN1 protein abundance were altered in CSN8CKO hearts (Figure 1A), but the neddylated form of cullin (Cul) proteins, including Cul1, Cul2, Cul3, and Cul4A was markedly increased in the CSN8CKO hearts (Figure 1B). A number of non-cullin proteins can also be neddylated.27 Interestingly, increases in the neddylated form of unknown proteins were also detected in CSN8CKO hearts (Figure 1C). These data demonstrate that deletion of the Csn8 gene suffices to compromise CSN deneddylation activity in adult cardiomyocytes.
Impairment of UPS proteolytic function in CSN8CKO hearts
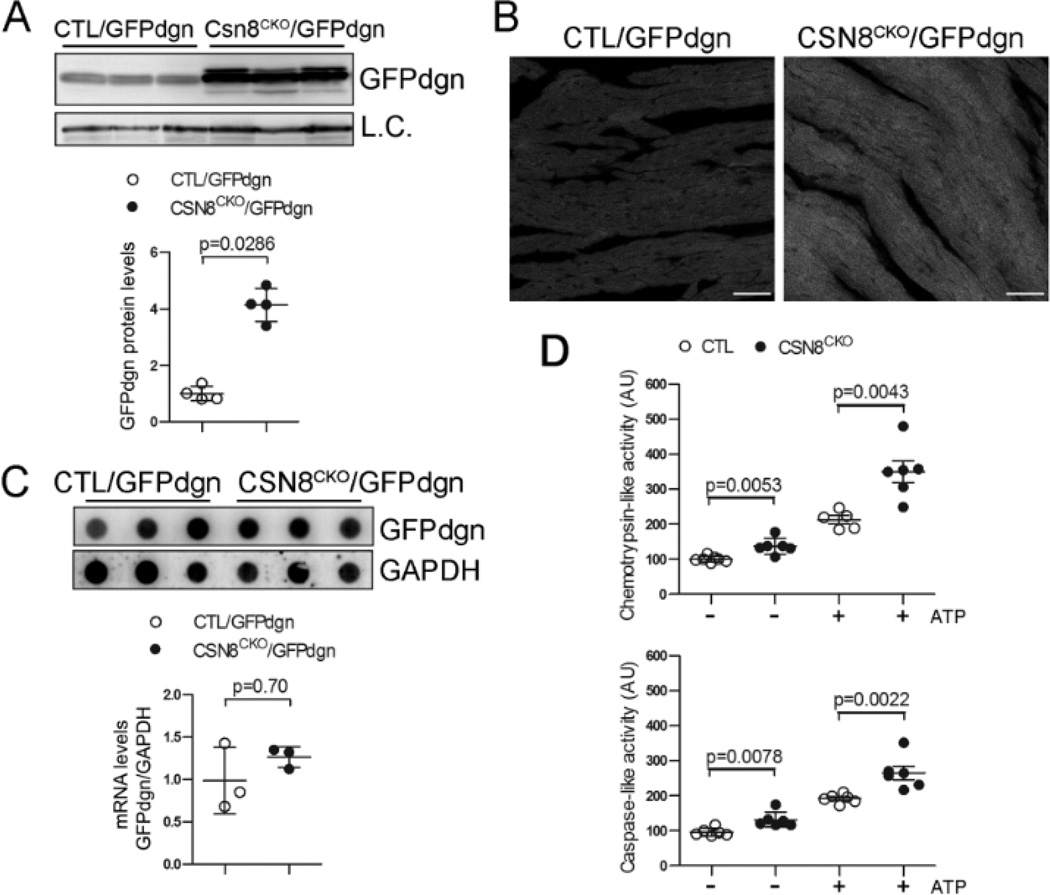
We recently showed that perinatal loss of CSN8 in cardiomyocytes caused cardiac PFI but this has not been tested in adult hearts.18 To monitor UPS proteolytic function in vivo, a reporter mouse model was previously generated and validated.23 In this tg mouse, a UPS surrogate substrate (GFPdgn) was ubiquitously and constitutively expressed and GFPdgn protein levels inversely reflect UPS proteolytic function. Tg GFPdgn was introduced into CSN8CKO and littermate CTL mice through cross-breeding. GFPdgn protein levels in CSN8CKO::GFPdgn mouse hearts were significantly increased at 3 days after the first tamoxifen injection, compared with the littermate CTL::GFPdgn mice (Figure 2A). Consistently, fluorescent confocal microscopy showed that GFPdgn fluorescence in cardiomyocytes was brighter in the CSN8CKO::GFPdgn mice than in CTL::GFPdgn mice (Figure 2B). RNA dot blot analysis revealed that the GFPdgn transcripts were not significantly different between the CTL::GFPdgn mice and the CSN8CKO::GFPdgn mice (Figure 2C), indicating that the increased GFPdgn protein levels likely result from decreased UPS-mediated degradation. These results reveal that cardiac UPS proteolytic function is severely impaired by CSN8CKO. This impairment does not appear to be intrinsic to the proteasome because the proteasomal chymotrypsin-like and caspase-like activities both in presence or absence of ATP were significantly increased in the CSN8CKO mice compared with the CTL (Figure 2D).
Figure 2. Proteasome functional insufficiency in CSN8CKO hearts.
(A~C) CSN8CKO hearts accumulate GFPdgn, a surrogate substrate of the UPS. GFPdgn was introduced into the CTL and CSN8CKO mice via cross-breeding. At 3 days after the first injection of tamoxifen, ventricular myocardium of littermate mice was collected for western blot analyses (A) and immunofluorescence/confocal microscopy (B) for GFPdgn or RNA dot blot analyses (C). L.C., loading control; Scale bar = 40µm. (D) Proteasomal peptidase activity assays of ventricular myocardium at 3 days after the first tamoxifen injection, in the presence (+) or absence (−) of ATP.
Accumulation of autophagosomes in CSN8CKO hearts
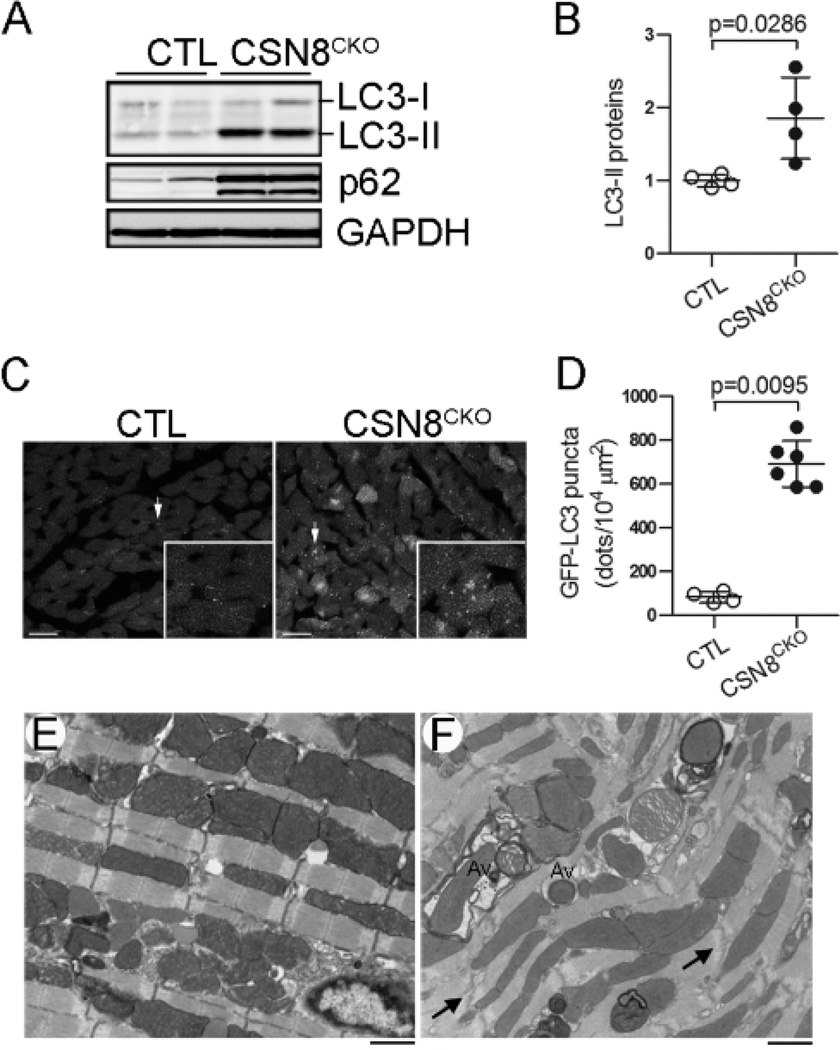
Targeted protein degradation in the cell is carried out mainly by the UPS and the autophagic-lysosomal pathway. Proteasome functional insufficiency (PFI) can activate autophagy in cardiomyocytes;5, 28 but chronic inhibition of autophagy has been shown to hinder the degradation of UPS substrates.29 Since we have observed severe impairment in UPS-mediated protein degradation in CSN8CKO hearts, we sought to investigate whether autophagy was altered. The conversion of LC3 from the cytosolic form (LC3-I) to the lipidated form (LC3-II) targets LC3-II to autophagosome membrane during autophagosome formation. Therefore, LC3-II protein levels are extensively used as a marker for autophagic vesicles. We found LC3-II were markedly increased in the CSN8CKO hearts (Figure 3A, 3B), suggesting that Csn8 deficiency increases autophagosomes in adult mouse hearts. This was confirmed using the GFP-LC3 reporter and TEM. We cross-bred the GFP-LC3 tg mice with the CSN8CKO mice. Direct fluorescent confocal microscopy revealed more GFP puncta in LV myocardium from the CSN8CKO mice, compared with the CTL mice (Figure 3C). Quantification showed that GFP-LC3 puncta increased by a factor of >8 in CSN8CKO compared with the littermate GFP-LC3 control mice (Figure 3D). Morphological detection of autophagic vacuoles via TEM remains the gold standard to visualize autophagosomes in the cell. Our TEM analyses further confirmed the markedly increase in autophagic vacuoles, especially the early stage ones (i.e., autophagosomes) in the cardiomyocytes of CSN8CKO hearts (Figure 3E, 3F). These autophagosomes often contain organelles such as degenerative mitochondria.
Figure 3. Marked increases of autophagic vacuoles in CSN8CKO hearts.
(A, B) Representative images of western blot analyses of LC3 and p62 (A) and a summary of LC3-II densitometry data (B) in CTL and CSN8CKO hearts at 5 days after the first tamoxifen injection. (C, D) Probing autophagy in CSN8CKO hearts using GFP-LC3. GFP-LC3 was introduced into the CTL and CSN8CKO mice via cross-breeding. Perfusion-fixed ventricular myocardium from CTL::GFP-LC3 mice and CSN8KO::GFP-LC3 littermate mice at 5 days after the first injection of tamoxifen was subjected to GFP-LC3 direct fluorescence confocal microscopy. Images from a 2.1 µm-thick slide of tissue were projected (C, representative images) and analyzed for the GFP-LC3 puncta density (D). The inset in panel C shows the area indicated by the arrow in a higher magnification. Bar=10 µm. (E, F) Electron micrographs of myocardium from CTL (E) and CSN8CKO (F) hearts. Abundant autophagosomes (Av) containing degenerating mitochondria and/or other cytoplasmic contents are evident in CSN8CKO hearts.
Diminished autophagy flux in CSN8CKO mouse hearts
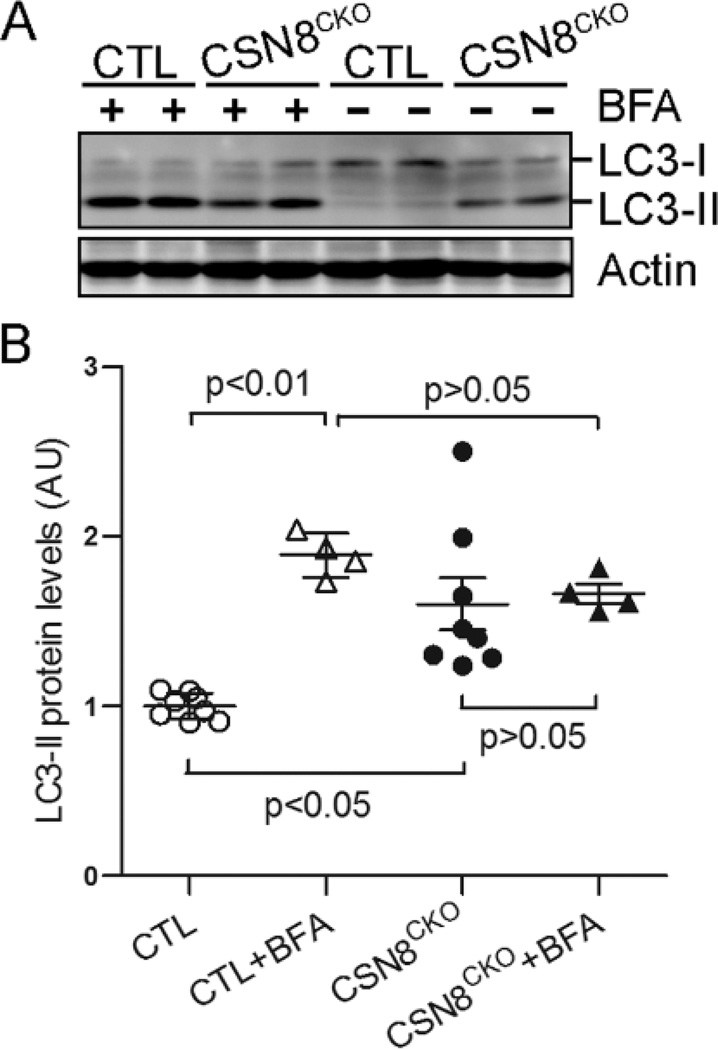
The increase in autophagosomes could result from elevated formation or defective removal. Interestingly, the protein levels of p62, an autophagy substrate, were also significantly increased in CSN8CKO hearts. This finding prompted us to test if autophagic flux is compromised. We used intraperitoneal injection of BFA to inhibit lysosomes. As expected, BFA treatment substantially accumulated LC3-II in the CTL mouse hearts. However, the BFA treatment failed to induce a significant increase in LC3-II in the CSN8CKO hearts (Figure 4). These data indicate that the increase of autophagosomes in CSN8CKO hearts is due to decreased lysosomal removal.
Figure 4. Impaired autophagic flux in CSN8CKO hearts.
Three days after the first tamoxifen injection, CTL and CSN8CKO littermate mice were treated with two doses of BFA (3µmol/kg, i.p.) or vehicles with 1 hour in between, and sacrificed 2 hour after the first dose. LC3-II protein levels in ventricular myocardium were quantified using western blot analysis. Representative western blot images (A) and densitometric quantification (B) of LC3 proteins are presented.
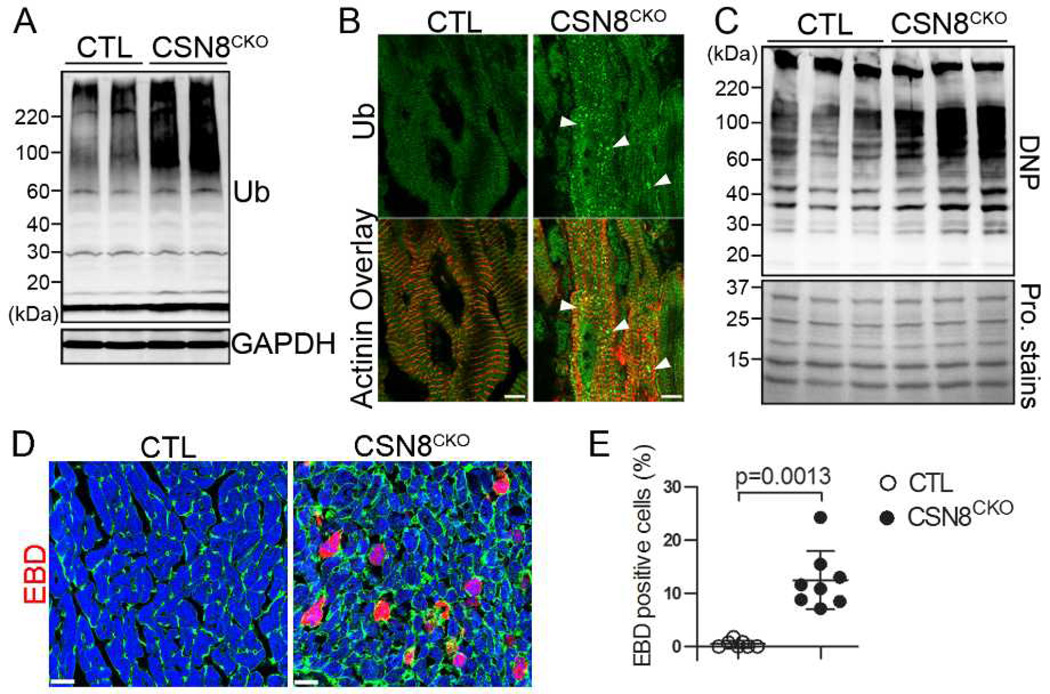
Accumulation of abnormal proteins in CSN8CKO hearts
Terminally damaged/misfolded proteins are degraded by the UPS and autophagy. Consistent with PFI and the impaired autophagic-lysosomal pathway, the total ubiquitinated proteins (Figure 5A) in the form of aggregates (Figure 5B) and the level of protein carbonyls, a common feature of oxidized proteins (Figure 5C), were greatly increased in CSN8CKO hearts. These ubiquitin-positive protein aggregates are frequently co-localized with GFP-LC3 puncta and enriched in p62 in CSN8CKO hearts (Supplemental Figure S2).
Figure 5. CSN8 deficiency in adult hearts accumulates abnormal proteins and increases cardiomyocyte necrosis.
Ventricular myocardium from CTL and CSN8CKO littermate mice at 5 days after the first tamoxifen injection were used for the following analyses. (A) Western blot analysis of myocardial total ubiquitinated proteins (Ub). (B) Immunofluorescence analysis of the subcellular localization of Ub (green) in cardiomyocytes. Sarcomeric α-actinin was counter-immunostained (red) to identify cardiomyocytes. Note increased ubiquitin-positive aggregates (arrowheads) in CSN8CKO cardiomyocytes. (C) Western blot analysis of DNP-derivatized protein carbonyls. The carbonyls of oxidized proteins in the total myocardial protein extracts were first derivatized by DNP and then detected by immunoblot for DNP. The representative image is shown as the upper panel. Before the immunoblotting, the proteins on the same membrane were visualized by MemCode protein (Pro.) stains (Pierce) (bottom panel). (D, E) Increased Evans Blue Dye (EBD, red) incorporation into the cardiomyocytes of CSN8CKO hearts. Perfusion-fixed ventricular myocardium was collected 18 hours after an intraperitoneal injection of EBD (100 mg/kg) and processed for cryosections. FITC-conjugated wheat germ agglutinin and rhodamine-conjugated phalloidin were used to stain cell membrane (green) and myofibrils (blue), respectively. The percentage of EBD positive cardiomyocytes in 2 or 3 random fields per heart and 3 hearts per group was measured and presented in panel E.
Massive cardiomyocyte necrosis in CSN8CKO hearts
A hallmark of necrotic cells is the loss of cell membrane integrity.30 To assess the prevalence of cardiomyocyte necrosis, we first tested the uptake of intraperitoneally injected Evan’s blue dye (EBD) by the cardiomyocytes in intact mice. Nearly none of the cardiomyocytes of CTL mice showed EBD uptake but over 10% of cardiomyocytes in the CSN8CKO mice did (Figure 5D, 5E), suggesting that Csn8 deficiency causes cardiomyocytes to die in the necrotic form in adult mice. Consistently, TEM also revealed evidence of necrotic cardiomyocytes in the CSN8CKO mice, including markedly cell swollen and mitochondrial swollen, decreased overall electron density, and myofibril fragmentation (Supplemental Figure S3).
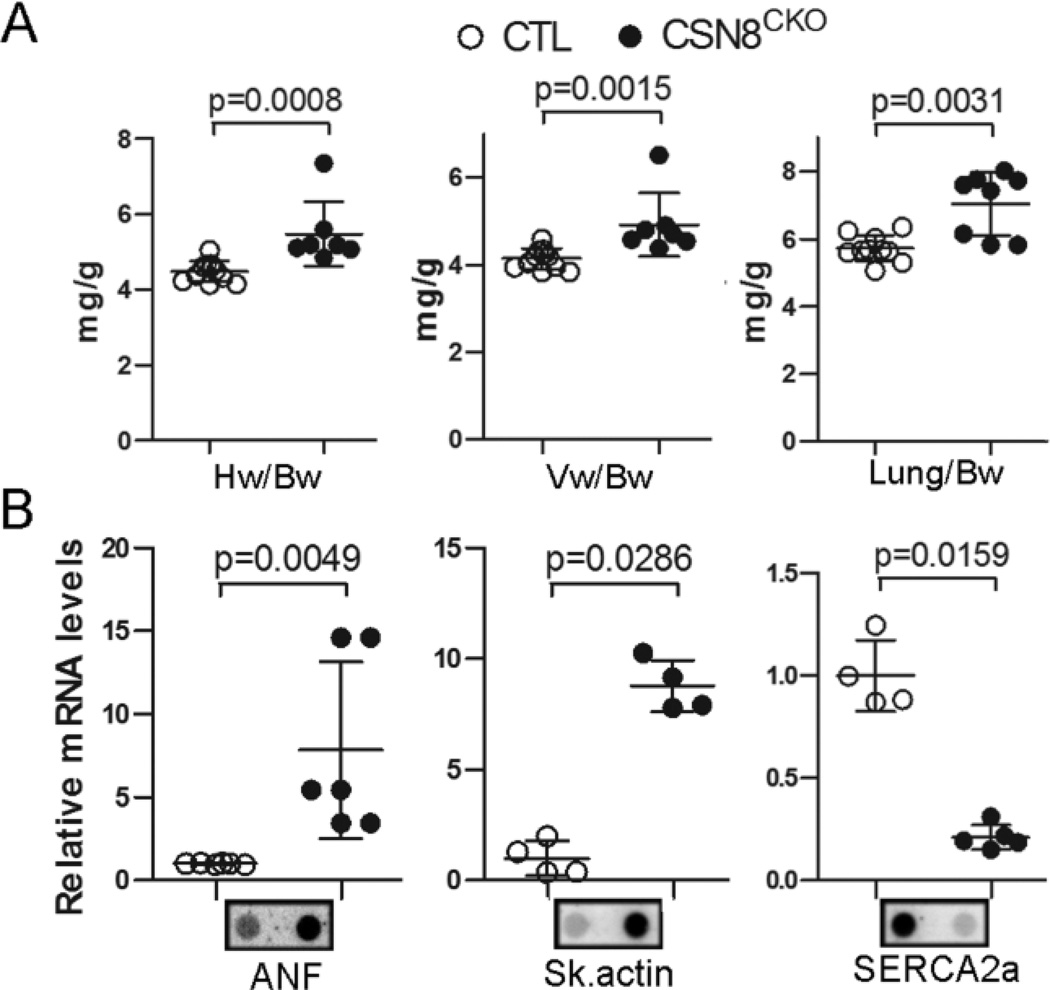
CSN8CKO mice developed dilated cardiomyopathy (DCM)
Five days after the first tamoxifen injection, the heart of CSN8CKO mice appeared to be enlarged, compared to the CTL (Supplemental Figure S4A). Gravimetric analyses showed that CSN8CKO mice had modest but statistically significant increases in the heart weight to body weight ratio, the ventricle weight to body weight ratio, and the lung weight to body weight ratio (Figure 6A) but not the kidney weight to body weight ratio (data not shown), compared with the CTL mice. The cross-sectional area of LV cardiomyocytes increased by 30%, compared with the CTL mice (Supplemental Figure S4B, S4C). A hallmark of cardiac hypertrophy/pathology is re-activation of the fetal gene program. The steady state transcript levels of atrial natriuretic factor (ANF) and skeletal α-actin in CSN8CKO hearts were up-regulated by a factor of 8 and 9, respectively, whereas the SERCA2a mRNA levels were down-regulated by a factor of 4 (Figure 6B). These results show that Csn8 ablation in adult cardiomyocytes triggers hypertrophic responses.
Figure 6. Gravimetric and cardiac fetal gene expression changes in CSN8CKO mice.
CTL and CSN8CKO mice were used 5 days after the first injection of tamoxifen. (A) Gravimetric measurements. (B) Changes in the transcript levels of atrial natriuretic factor (ANF), skeletal (Sk.) α-actin, and sarcoplasmic reticulum calcium ATPase 2a (SERCA2a) in ventricular myocardium. RNA dot blot analyses were performed using radioactively labeled transcript-specific oligonucleotide probes.
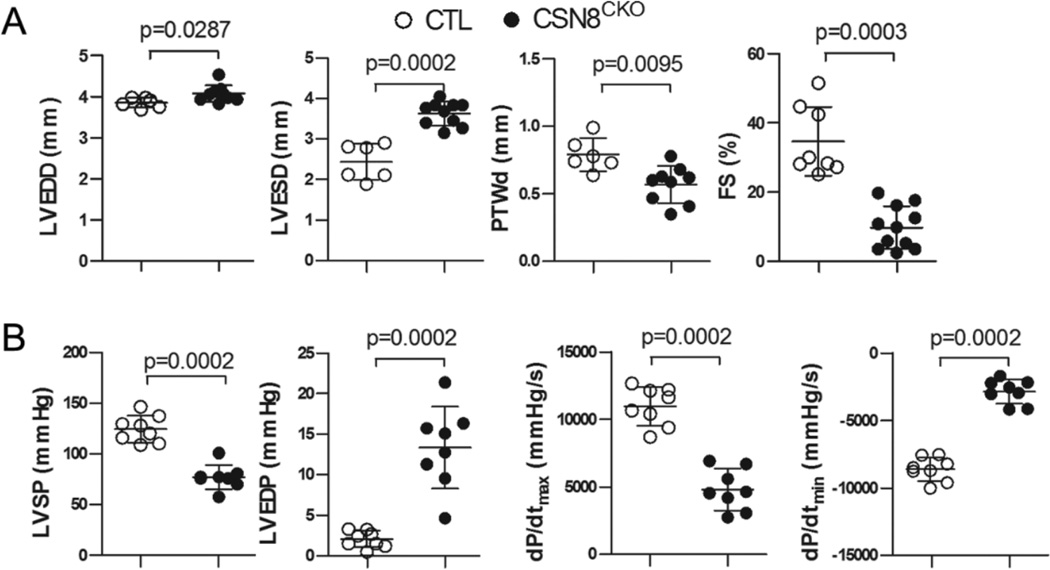
Morphometric and cardiac functional assessments further revealed that Csn8 deficiency initiated in adult hearts caused DCM. Echocardiography at 5 days after the first tamoxifen injection revealed significant increases in the LV end-diastolic dimension and end-systolic dimension and significant decreases in the posterior wall thickness at the end of diastole and LV fractional shortening in CSN8CKO mice (Figure7A, Supplemental Figure S5). Hemodynamic analysis via monitoring LV pressure (Figure 7B) revealed a marked reduction of LV systolic pressure and the maximum dP/dt in CSN8CKO mice, indicative of reduced cardiac contractility. The accompanying marked increase of LV end diastolic pressure and a significant reduction of the minimum dP/dt in CSN8CKO hearts indicate that LV relaxation is also impaired. Taken together, these findings demonstrate that loss of CSN8 in the cardiomyocytes of adult mice causes rapidly severe congestive heart failure.
Figure 7. Changes in left ventricle (LV) geometry and function in CSN8CKO mice.
CTL and CSN8CKO mice were used 5 days after the first injection of tamoxifen. (A) Echocardiographic assessments. LV end-diastolic dimension (LVEDD), end-systolic dimension (LVESD), posterior wall thickness in diastole (PWTd), and fraction shortening (FS) were determined using transthoracic M-mode echocardiography. (B) Hemodynamic assessments. LV systolic pressure (LVSP), LV end diastolic pressure (LVEDP), the maximum first derivative of LV pressure (dP/dtmax) and the minimum first derivative of LV pressure (dP/dtmin) were measured by LV catheterization via carotid artery.
Discussion
The physiological significance of CSN has not been demonstrated in a post-mitotic organ of intact adult vertebral animals. Here we have shown that CSN8 deficiency initiated in adult mouse hearts caused severe duo-impairment of both the UPS and autophagy, resulted in cardiomyocyte necrosis, and led to rapid left heart failure. Along with the similar findings from perinatal mice,18, 31 it is now comprehensively demonstrated that CSN8/CSN regulates protein degradation by not only the UPS but also lysosomes in the heart during both the early development and adulthood. Moreover, we show here for the first time in intact animals that CSN8/CSN may modulate the redox state in the heart.
CSN8 is required for CSN-mediated deneddylation of a variety of substrates
Using the same Csn8 floxed allele but temporally less controllable conditional gene targeting, we and others have previously showed that CSN-mediated cullin deneddylation in mouse hepatocytes, neonatal cardiomyocytes, and T cells was compromised by CSN8 deficiency.17, 18, 32 In those previous studies, the accompanied down-regulation of other CSN subunits, such as CSN5 which harbors the deneddylase, could not be dissociated from the impaired deneddylation. Because the strategy used for the inducible Csn8 targeting in the present study allows the timing of gene ablation to be controlled and pinpointed, we were able to observe severe impairment of CSN deneddylation in CSN8 ablated hearts before reduction of other key CSN subunits becomes evident, providing compelling evidence that CSN8 is required for CSN5-mediated deneddylation. Cullins are the first and best studied proteins that undergo neddylation but the list of the latter is growing.27, 33 It is unknown whether CSN plays a role in the deneddylation of proteins other than cullins. Besides neddylated cullins, a large number of neddylated proteins of unknown idetities were also accumulated in CSN8CKO hearts. This is in agreement with our recent findings in postnatal CSN8-deficient heart.18 Taken together, it is now strongly demonstrated that CSN-mediated deneddylation in the heart has an array of substrates besides neddylated cullins. It will be interesting to identify these non-cullin substrates using the CSN8 deficient hearts.
CSN8/CSN is central to protein degradation and quality control in cardiomyocytes
CSN has long been purported as a regulator of UPS-mediated proteolysis, by controlling the catalytic dynamics of CRLs,34 serving as an alternate lid of the 19S proteasome,35 or stabilizing the ubiquitin conjugation enzyme.36 Our previous study showed impaired UPS function in postnatal CSN8-deficient hearts,18 which may be caused by uncoupling of ubiquitination from proteasome-mediated degradation.31 Consistent with this notion, we observed that loss of CSN8 in adult hearts rapidly accumulated the proteasome surrogate substrate GFPdgn but proteasomal peptidase activities were increased. Taken together, it is revealed that CSN8 is crucial to the functioning of the UPS in both neonatal and adult cardiomyocytes. Since DCM occurred immediately following the induction of CSN8CKO and cardiomyopathy can result in cardiac PFI,1, 5 it cannot be ruled out that the concomitant DCM may contribute to the UPS malfunction.
Another exciting contribution of the current study is to have confirmed an important role of CSN8/CSN in the autophagic-lysosomal pathway that was initially revealed by our studies on the perinatal Csn8 knockout.18 Here we found the co-existence of increased autophagosomes with decreased autophagic flux in the adult mouse hearts with Csn8 ablated in cardiomyocytes, confirming that CSN8/CSN is required for autophagosome removal. Impaired autophagosome removal can result from defective fusion between autophagosomes and lysosomes and/or from lysosome malfunction.
UPS-mediated protein degradation is a highly regulated process. Polyubiquitination of target proteins, the delivery of polyubiquitinated proteins to the proteasome, and proteasome activities can all impact on UPS functioning.37 Given that the proteasome peptidase activities were increased in Csn8 deficient myocardium, the impairment of UPS proteolytic function as evidenced by GFPdgn accumulation is likely caused by defective ubiquitination and/or impaired delivery of ubiquitinated proteins to the proteasome. The latter is consistent with the increases of total ubiquitinated proteins in Csn8CKO hearts. As discussed below, macroautophagy is impaired and p62 increased in Csn8CKO hearts, which may impair delivery of ubiquitinated proteins to the proteasome. Chronic inhibition of macroautophagy was shown to accumulate p62 which in turn binds ubiquitinated proteins and hinders their degradation by the proteasome.29
Selective autophagy contributes to quality control in the cell by targeted removal of defective organelles and protein aggregates. p62 is involved in the recruitment of autophagosome to misfolded proteins.8 Consistent with PFI and inadequate PQC, both ubiquitinated proteins and oxidized proteins were markedly increased in CSN8CKO hearts. The accumulation of ubiquitinated proteins in the form of aggregates and the co-localization of p62 and GFP-LC3 with the aggregates suggest that defective removal of autophagosomes at least contributes to, if is not the primary cause of, PQC inadequacy in CSN8CKO hearts.
The increased level of protein carbonyls in CSN8CKO hearts suggests that CSN8/CSN may regulate the redox state via supporting the removal of oxidized proteins and/or suppressing oxidative modifications. Indeed, a recent study on the transcriptome, proteome, and metabolome of the filamentous fungus Aspergillus nidulans with CSN5 gene deletion has revealed a critical role of CSN in mediating transcriptional and metabolic responses to oxidative stress.38 Hence, the current study presents the first evidence that CSN8/CSN may regulate the redox state in vertebral animals.
CSN8 deficiency causes cardiomyocyte necrosis and DCM
We have previously reported that CSN8 deficiency causes primarily apoptosis in hepatocytes but predominantly necrosis in neonatal cardiomyocytes.9, 18, 32 Here we observed massive cardiomyocyte necrosis in adult mice with CSN8CKO. These results suggest that CSN8/CSN is essential for cardiomyocyte survival but the type of cell death caused by CSN8 deficiency is tissue/organ-specific. Similarly to Csn8 knockout initiated at the perinatal stage,18 cardiomyocyte-restricted Csn8 ablation initiated in adult mice causes cardiac structural and functional changes that are characteristic of DCM. Interestingly, it takes a shorter time for cardiac Csn8 ablation to cause DCM in adults than in neonates. This is strikingly reminiscent of the cardiac ablation of Atg5,39 a protein required for autophagy, implicating that impairment of autophagy might be primarily responsible for the DCM in CSN8 deficient hearts. Massive cardiomyocyte necrosis is associated with, and likely an underlying cause of, the DCM in both perinatal and adult CSN8 deficient hearts. Indeed, inhibition of autophagosome removal is sufficient to cause cardiomyocyte necrosis in adult mice.9 Moreover, accumulation of autophagic vacuoles was also observed in necrotic cardiomyocytes in diseased human and mouse hearts,40, 41 raising an interesting possibility that impaired autophagosome removal may be a cause of cardiomyocyte necrosis in a diseased heart. It will be important to test this possibility and to investigate how impaired autophagosome removal causes necrotic cell death.
Supplementary Material
Clinical Summary.
Targeted removal of terminally misfolded/damaged proteins by the ubiquitin proteasome system (UPS) and the autophagic-lysosomal pathways represents the last line of defense in protein quality control. UPS and autophagy dysfunctions are implicated in cardiac pathogenesis. However, the regulation of the UPS and autophagy is poorly understood. The attachment of a ubiquitin-like protein NEDD8 to cullin via a process known as neddylation activates cullin-based RING ligases (CRLs), a large family of ubiquitin ligases. The proper functioning of CRLs also requires cullin deneddylation catalyzed by the COP9 signalosome (CSN), a protein complex formed by eight unique proteins (CSN1 through CSN8). Hence, CSN may regulate cardiac UPS. By conditional gene targeting, we previously discovered that CSN8/CSN regulates both cardiac UPS and autophagy and is essential to cardiomyocyte survival at the perinatal stage when cardiomyocyte proliferation contributes actively to cardiac development. Here we report that CSN8/CSN also plays indispensable roles in adult hearts. Similarly to perinatal csn8 knockout, cardiac ablation of the csn8 gene initiated in adult mice impairs both UPS proteolytic function and autophagosome removal, leading to massive cardiomyocyte necrosis and progressive dilated cardiomyopathy and heart failure. This has immediate clinical relevance because neddylation inhibition via NEDD8 activating enzyme (NAE) inhibitors (e.g., MLN4924) is in clinical trial to treat cancer and CSN inhibition is also being tested in the laboratory as a new therapeutic strategy. Similar to CSN8 deficiency, both NAE and CSN inhibitors effect to inhibit CRLs. Our findings caution that the use of these inhibitors may damage the heart.
Acknowledgments
Sources of Funding
This work was in part supported by NIH grants R01HL085629 and R01HL072166 and the American Heart Association grants 0740025N (to X. W.) and 0625738Z to (H.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Schlossarek S, Carrier L. The ubiquitin-proteasome system in cardiomyopathies. Curr Opin Cardiol. 2011;26:190–195. doi: 10.1097/HCO.0b013e32834598fe. [DOI] [PubMed] [Google Scholar]
- 2.Gustafsson AB, Gottlieb RA. Recycle or die: The role of autophagy in cardioprotection. J Mol Cell Cardiol. 2008;44:654–661. doi: 10.1016/j.yjmcc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Pattison JS, Su H. Posttranslational modification and quality control. Circ Res. 2013;112:367–381. doi: 10.1161/CIRCRESAHA.112.268706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drews O, Tsukamoto O, Liem D, Streicher J, Wang Y, Ping P. Differential regulation of proteasome function in isoproterenol-induced cardiac hypertrophy. Circ Res. 2010;107:1094–1101. doi: 10.1161/CIRCRESAHA.110.222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Li J, Zheng H, Su H, Powell SR. Proteasome functional insufficiency in cardiac pathogenesis. Am J Physiol Heart Circ Physiol. 2011;301:H2207–H2219. doi: 10.1152/ajpheart.00714.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day SM. The ubiquitin proteasome system in human cardiomyopathies and heart failure. Am J Physiol Heart Circ Physiol. 2013;304:H1283–H1293. doi: 10.1152/ajpheart.00249.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie M, Morales CR, Lavandero S, Hill JA. Tuning flux: Autophagy as a target of heart disease therapy. Curr Opin Cardiol. 2011;26:216–222. doi: 10.1097/HCO.0b013e328345980a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Q, Su H, Ranek MJ, Wang X. Autophagy and p62 in cardiac proteinopathy. Circ Res. 2011;109:296–308. doi: 10.1161/CIRCRESAHA.111.244707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Horak KM, Su H, Sanbe A, Robbins J, Wang X. Enhancement of proteasomal function protects against cardiac proteinopathy and ischemia/reperfusion injury in mice. J Clin Invest. 2011;121:3689–3700. doi: 10.1172/JCI45709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian Z, Zheng H, Li J, Li Y, Su H, Wang X. Genetically induced moderate inhibition of the proteasome in cardiomyocytes exacerbates myocardial ischemia-reperfusion injury in mice. Circ Res. 2012;111:532–542. doi: 10.1161/CIRCRESAHA.112.270983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, Condorelli G, Sadoshima J. Rheb is a critical regulator of autophagy during myocardial ischemia: Pathophysiological implications in obesity and metabolic syndrome. Circulation. 2012;125:1134–1146. doi: 10.1161/CIRCULATIONAHA.111.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei N, Deng XW. Cop9: A new genetic locus involved in light-regulated development and gene expression in arabidopsis. Plant Cell. 1992;4:1507–1518. doi: 10.1105/tpc.4.12.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei N, Deng XW. The cop9 signalosome. Annu Rev Cell Dev Biol. 2003;19:261–286. doi: 10.1146/annurev.cellbio.19.111301.112449. [DOI] [PubMed] [Google Scholar]
- 14.Kawakami T, Chiba T, Suzuki T, Iwai K, Yamanaka K, Minato N, Suzuki H, Shimbara N, Hidaka Y, Osaka F, Omata M, Tanaka K. Nedd8 recruits e2-ubiquitin to scf e3 ligase. Embo J. 2001;20:4003–4012. doi: 10.1093/emboj/20.15.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyapina S, Cope G, Shevchenko A, Serino G, Tsuge T, Zhou C, Wolf DA, Wei N, Deshaies RJ. Promotion of nedd-cul1 conjugate cleavage by cop9 signalosome. Science. 2001;292:1382–1385. doi: 10.1126/science.1059780. [DOI] [PubMed] [Google Scholar]
- 16.Kato JY, Yoneda-Kato N. Mammalian cop9 signalosome. Genes Cells. 2009;14:1209–1225. doi: 10.1111/j.1365-2443.2009.01349.x. [DOI] [PubMed] [Google Scholar]
- 17.Menon S, Chi H, Zhang H, Deng XW, Flavell RA, Wei N. Cop9 signalosome subunit 8 is essential for peripheral t cell homeostasis and antigen receptor-induced entry into the cell cycle from quiescence. Nat Immunol. 2007;8:1236–1245. doi: 10.1038/ni1514. [DOI] [PubMed] [Google Scholar]
- 18.Su H, Li J, Menon S, Liu J, Kumarapeli AR, Wei N, Wang X. Perturbation of cullin deneddylation via conditional csn8 ablation impairs the ubiquitin-proteasome system and causes cardiomyocyte necrosis and dilated cardiomyopathy in mice. Circ Res. 2011;108:40–50. doi: 10.1161/CIRCRESAHA.110.230607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li F, Wang X, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol. 1996;28:1737–1746. doi: 10.1006/jmcc.1996.0163. [DOI] [PubMed] [Google Scholar]
- 20.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, Cullis CA, Doucette A, Garnsey JJ, Gaulin JL, Gershman RE, Lublinsky AR, McDonald A, Mizutani H, Narayanan U, Olhava EJ, Peluso S, Rezaei M, Sintchak MD, Talreja T, Thomas MP, Traore T, Vyskocil S, Weatherhead GS, Yu J, Zhang J, Dick LR, Claiborne CF, Rolfe M, Bolen JB, Langston SP. An inhibitor of nedd8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 21.Lee YH, Judge AD, Seo D, Kitade M, Gomez-Quiroz LE, Ishikawa T, Andersen JB, Kim BK, Marquardt JU, Raggi C, Avital I, Conner EA, MacLachlan I, Factor VM, Thorgeirsson SS. Molecular targeting of csn5 in human hepatocellular carcinoma: A mechanism of therapeutic response. Oncogene. 2011;30:4175–4184. doi: 10.1038/onc.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 23.Kumarapeli AR, Horak KM, Glasford JW, Li J, Chen Q, Liu J, Zheng H, Wang X. A novel transgenic mouse model reveals deregulation of the ubiquitin-proteasome system in the heart by doxorubicin. Faseb J. 2005;19:2051–2053. doi: 10.1096/fj.05-3973fje. [DOI] [PubMed] [Google Scholar]
- 24.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Osinska H, Dorn GW, 2nd, Nieman M, Lorenz JN, Gerdes AM, Witt S, Kimball T, Gulick J, Robbins J. Mouse model of desmin-related cardiomyopathy. Circulation. 2001;103:2402–2407. doi: 10.1161/01.cir.103.19.2402. [DOI] [PubMed] [Google Scholar]
- 26.Kumarapeli AR, Su H, Huang W, Tang M, Zheng H, Horak KM, Li M, Wang X. Alpha b-crystallin suppresses pressure overload cardiac hypertrophy. Circ Res. 2008;103:1473–1482. doi: 10.1161/CIRCRESAHA.108.180117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson IR, Irwin MS, Ohh M. Nedd8 pathways in cancer, sine quibus non. Cancer cell. 2011;19:168–176. doi: 10.1016/j.ccr.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Q, Wang X. Autophagy and the ubiquitin-proteasome system in cardiac dysfunction. Panminerva Med. 2010;52:9–25. [PMC free article] [PubMed] [Google Scholar]
- 29.Korolchuk VI, Mansilla A, Menzies FM, Rubinsztein DC. Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Molecular cell. 2009;33:517–527. doi: 10.1016/j.molcel.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kung G, Konstantinidis K, Kitsis RN. Programmed necrosis, not apoptosis, in the heart. Circ Res. 2011;108:1017–1036. doi: 10.1161/CIRCRESAHA.110.225730. [DOI] [PubMed] [Google Scholar]
- 31.Su H, Li F, Ranek MJ, Wei N, Wang X. Cop9 signalosome regulates autophagosome maturation. Circulation. 2011;124:2117–2128. doi: 10.1161/CIRCULATIONAHA.111.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lei D, Li F, Su H, Tian Z, Ye B, Wei N, Wang X. Cop9 signalosome subunit 8 is required for postnatal hepatocyte survival and effective proliferation. Cell Death Differ. 2011;18:259–270. doi: 10.1038/cdd.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xirodimas DP. Novel substrates and functions for the ubiquitin-like molecule nedd8. Biochem Soc Trans. 2008;36:802–806. doi: 10.1042/BST0360802. [DOI] [PubMed] [Google Scholar]
- 34.Wei N, Serino G, Deng XW. The cop9 signalosome: More than a protease. Trends Biochem Sci. 2008;33:592–600. doi: 10.1016/j.tibs.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Deng XW. The cop9 signalosome: An alternative lid for the 26s proteasome? Trends Cell Biol. 2003;13:507–509. doi: 10.1016/j.tcb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez-Sanchez ME, Sechet E, Margottin-Goguet F, Rogge L, Bianchi E. The human cop9 signalosome protects ubiquitin-conjugating enzyme 3 (ubc3/cdc34) from beta-transducin repeat-containing protein (betatrcp)-mediated degradation. J Biol Chem. 2010;285:17390–17397. doi: 10.1074/jbc.M109.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Terpstra EJ. Ubiquitin receptors and protein quality control. J Mol Cell Cardiol. 2013;55:73–84. doi: 10.1016/j.yjmcc.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nahlik K, Dumkow M, Bayram O, Helmstaedt K, Busch S, Valerius O, Gerke J, Hoppert M, Schwier E, Opitz L, Westermann M, Grond S, Feussner K, Goebel C, Kaever A, Meinicke P, Feussner I, Braus GH. The cop9 signalosome mediates transcriptional and metabolic response to hormones, oxidative stress protection and cell wall rearrangement during fungal development. Mol Microbiol. 2010;78:964–979. doi: 10.1111/j.1365-2958.2010.07384.x. [DOI] [PubMed] [Google Scholar]
- 39.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 40.Kostin S, Pool L, Elsasser A, Hein S, Drexler HC, Arnon E, Hayakawa Y, Zimmermann R, Bauer E, Klovekorn WP, Schaper J. Myocytes die by multiple mechanisms in failing human hearts. Circ Res. 2003;92:715–724. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- 41.Miyata S, Takemura G, Kawase Y, Li Y, Okada H, Maruyama R, Ushikoshi H, Esaki M, Kanamori H, Li L, Misao Y, Tezuka A, Toyo-Oka T, Minatoguchi S, Fujiwara T, Fujiwara H. Autophagic cardiomyocyte death in cardiomyopathic hamsters and its prevention by granulocyte colony-stimulating factor. Am J Pathol. 2006;168:386–397. doi: 10.2353/ajpath.2006.050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.