Abstract
BACKGROUND
Global efforts to eliminate lymphatic filariasis are based on the annual mass administration of antifilarial drugs to reduce the microfilaria reservoir available to the mosquito vector. Insecticide-treated bed nets are being widely used in areas in which filariasis and malaria are coendemic.
METHODS
We studied five villages in which five annual mass administrations of antifilarial drugs, which were completed in 1998, reduced the transmission of Wuchereria bancrofti, one of the nematodes that cause lymphatic filariasis. A total of 21,899 anopheles mosquitoes were collected for 26 months before and 11 to 36 months after bed nets treated with long-lasting insecticide were distributed in 2009. We evaluated the status of filarial infection and the presence of W. bancrofti DNA in anopheline mosquitoes before and after the introduction of insecticide-treated bed nets. We then used a model of population dynamics to estimate the probabilities of transmission cessation.
RESULTS
Village-specific rates of bites from anopheline mosquitoes ranged from 6.4 to 61.3 bites per person per day before the bed-net distribution and from 1.1 to 9.4 bites for 11 months after distribution (P<0.001). During the same period, the rate of detection of W. bancrofti in anopheline mosquitoes decreased from 1.8% to 0.4% (P = 0.005), and the rate of detection of filarial DNA decreased from 19.4% to 14.9% (P = 0.13). The annual transmission potential was 5 to 325 infective larvae inoculated per person per year before the bed-net distribution and 0 after the distribution. Among all five villages with a prevalence of microfilariae of 2 to 38%, the probability of transmission cessation increased from less than 1.0% before the bed-net distribution to a range of 4.9 to 95% in the 11 months after distribution.
CONCLUSIONS
Vector control with insecticide-treated bed nets is a valuable tool for W. bancrofti elimination in areas in which anopheline mosquitoes transmit the parasite. (Funded by the U.S. Public Health Service and the National Institutes of Health.)
Lymphatic filariasis is a parasitic-worm infection caused by Wuchereria bancrofti, Brugia malayi, and B. timori that affects approximately 120 million people in Africa, Asia, the Pacific, and the Americas.1 Adult filarial worms live in the lymphatic system, causing lymph-edema of the limbs, elephantiasis, and hydrocele. Fecund adult female worms release microfilariae, which ultimately enter the bloodstream, where they are ingested by anthropophilic mosquitoes of various genera. Microfilariae develop through several stages in mosquito vectors until they become infective larvae (L3), which continue transmission by establishing infection in humans through the bite site created during blood feeding. Safe, single-dose, inexpensive drug regimens have been developed that significantly reduce blood loads of microfilariae in humans for more than a year. For this reason, lymphatic filariasis has been targeted for global elimination by the year 2020 on the basis of annual mass administration of single-dose albendazole combined with either ivermectin or diethylcarbamazine for 5 or more years, the estimated reproductive life span of adult worms, which is anticipated to break the transmission of lymphatic filariasis from humans to mosquitoes.2,3
Although this effort has had successes, including the distribution of drugs to 570 million people in 48 countries,4 it is faced with several challenges.5 Annual treatment of at least 80% of eligible persons is key to elimination, but this level of population coverage has proved to be difficult to achieve in some areas because of health-system constraints6 and human migration.7 Financial and political limitations constrain the sustainability of control programs for lymphatic filariasis,8 and there is the possibility of drug resistance developing in the parasite population.9 Finally, elimination thresholds are site-specific and unknown in most areas.10–12 Therefore, program managers in countries in which lymphatic filariasis is endemic may lack the evidence necessary to make informed decisions regarding whether to conclude, continue, or reinstitute mass drug-administration campaigns.
Heterogeneities in elimination thresholds for lymphatic filariasis are due largely to differences in vector–parasite relationships. In anopheline mosquitoes, the proportion of microfilariae that develop to become infective larvae decreases as the number that are ingested decreases, making this vector less efficient as the microfilaria reservoir diminishes, whereas the converse occurs in lymphatic filariasis transmitted by culicine mosquitoes.13 In both systems, a decrease in the rate of mosquito bites will increase the worm breakpoint (i.e., the threshold below which the prevalence of microfilariae spontaneously moves to zero).10 Therefore, the elimination of lymphatic filariasis becomes more attainable if vector control accompanies mass drug-administration campaigns. Elimination end points may also be affected by differences in local endemicity, infection aggregation, and the magnitude of acquired immunity.10 For example, the worm breakpoint has been estimated to differ among neighboring villages in Papua New Guinea, where Anopheles punctulatus is the primary vector.11 These difficulties suggest that a uniform global strategy for permanent cessation of transmission of lymphatic filariasis may not be resilient and that a lack of vector control may hinder progress toward this goal.14
The possibility of including vector control as part of programs to eliminate lymphatic filariasis has received increased attention.15,16 In sub-Saharan Africa and Papua New Guinea, where anopheles species transmit both W. bancrofti and malaria, there is the opportunity to integrate the elimination of lymphatic filariasis with national malaria-control programs in which vector interventions are an essential component.17 Observations made during malaria-eradication efforts in the Solomon Islands from 1974 through 1977 support the efficacy of vector control, since indoor residual spraying with insecticides decreased the prevalence of microfilariae from 22% to 0% without the use of antifilarial drugs.18 Currently, the most widely implemented vector intervention used by malaria-control programs is universal coverage with insecticide-treated bed nets. However, only one study in Kenya has examined the effect of conventional permethrin-impregnated nets on W. bancrofti transmission by anophelines,19 and there are no data that quantify how the use of bed nets treated with long-lasting insecticide will complement the mass administration of antifilarial drugs in reducing transmission of lymphatic filariasis and the probability of cessation of transmission.
We measured the transmission of lymphatic filariasis in five villages in the East Sepik Province of Papua New Guinea before and after a nationwide bed-net distribution effort in 2009. Communities in this area had received the last of five annual treatments with antifilarial drugs more than 10 years earlier (1998) with no subsequent interventions until 2009. Since entomologic and human-infection data from this earlier time were available,20 we were able to compare mass drug administration alone and the distribution of insecticide-treated bed nets alone with respect to the effect on the rate of transmission of lymphatic filariasis in the same region.
METHODS
STUDY AREA
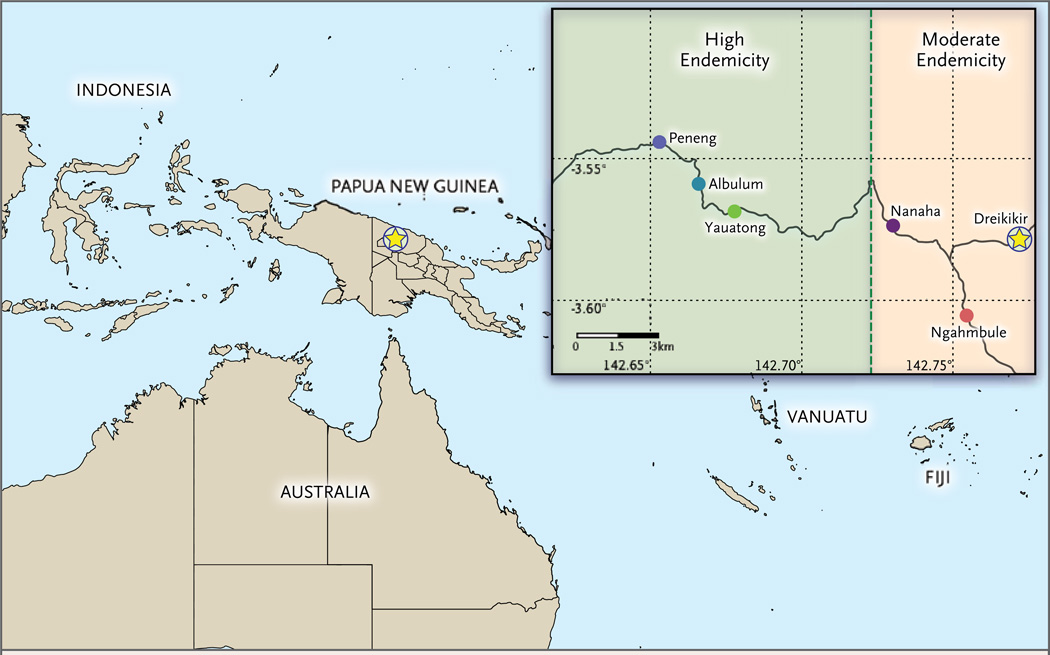
Lymphatic filariasis is highly endemic in Papua New Guinea, where it is estimated that 4.4 million of the country’s 6.3 million residents live in areas that qualify for disease elimination.21 We selected five villages in the Ambunti-Dreikikir District of East Sepik Province for entomologic surveys and quantification of the prevalence of microfilariae (Fig. 1). Transmission of lymphatic filariasis in these villages has been well characterized.20,22–24 We present our findings in the context of historical endemicity levels on the basis of annual transmission potentials (the number of infective larvae that were inoculated per person per year), which were measured before a trial of mass administration of antifilarial drugs conducted from 1993 through 1998.22
Figure 1. Location of Study Villages in East Sepik Province, Papua New Guinea.
STUDY PARTICIPANTS
Mosquito collectors and village residents who participated in surveys of the prevalence of microfilariae and bed nets provided written informed consent after protocols were approved by the institutional review boards at University Hospitals Case Medical Center in Cleveland, the Institute of Medical Research, and the Medical Research Advisory Committee in Papua New Guinea.
PREVALENCE OF MICROFILARIAE
We measured the prevalence of microfilariae in 2008, before the distribution of insecticide-treated bed nets, by counting the number of microfilariae in a 1-ml sample of nocturnally collected venous blood after passing it through a 5-µm polycarbonate filter.
MOSQUITO COLLECTION AND ANALYSIS
Mosquitoes were collected monthly after landing on human adult collectors from July 2007 through July 2010. The collectors sat outdoors within several meters of the household entrance from 6 p.m. to 6 a.m. with their lower legs and feet exposed. Collectors worked in teams, with one member collecting from 6 p.m. to midnight and the other from midnight to 6 a.m. Mosquitoes landing in search of a blood meal were captured with an aspirator and stored according to the hour they were collected. Each village was divided into four hamlets, with monthly collections in each hamlet. The total effort each month varied from 40 to 48 collection nights. Mosquitoes were morphologically identified as A. punctulatus, A. koliensis, or A. farauti sensu lato, according to criteria established previously,25 and were stored according to species, location, and hour collected. Half the mosquitoes were stored in 70% ethanol for later dissection and the other half on silica gel for DNA diagnostic evaluation, as described below. A total of 10,578 mosquitoes were stained individually with Mayer’s hemalum,26 separated into body sections on a glass slide, and dissected with forceps and needles under a microscope. Dissected specimens were examined for W. bancrofti in the infective larval stage and other developing larval stages (L1 and L2) with the use of standard criteria.27
Genomic DNA was extracted from unfed dried mosquitoes singly or in pools of two (Qiagen). Identification of the species of 2867 mosquitoes was confirmed with the use of polymerase-chain-reaction (PCR) amplification of the internal transcribed spacer 2 region of mosquito ribosomal DNA and either digested with MSP1 enzyme28 or used in a ligase detection reaction–fluorescent microsphere assay.29 A total of 1009 samples were also screened for W. bancrofti DNA by PCR amplification of the long DNA repeat region to be used as a xenomonitoring tool.30
At the close of the study, one village with a moderate transmission level (Nanaha) and one with a high transmission level (Yauatong) were selected for long-term assessment of rates of mosquito biting. Mosquito collections were conducted quarterly in the second and third year after the bed-net distribution.
INSECTICIDE-TREATED BED NETS
PermaNet 2.0, an insecticide-treated bed net impregnated with 55 mg of deltamethrin per square meter (Vestergaard Frandsen), was distributed to study communities by the East Sepik Province Division of Health in August 2009. At that time, the national target for bed-net coverage was 80% of household ownership and 80% use for children under the age of 5 years and for pregnant women. Surveys regarding bed-net use were conducted in November 2008 and again in September through December 2009 by asking adults (≥18 years of age) and parents or guardians of children if they had slept under a bed net the previous night.
STATISTICAL ANALYSIS
We estimated the daily mosquito-biting rates on the basis of the mean number of host-seeking anopheline mosquitoes that were collected in a 12-hour period. We calculated annual transmission potentials by multiplying the mean daily biting rate in the community by 365, which was then multiplied by the proportion of bites that were infective and by the mean number of L3 larvae per infective bite. We used the Mann–Whitney U test to compare biting rates before and after bed-net distribution and Fisher’s exact test to compare rates of mosquito infection and infectivity before and after bed-net distribution. All statistical analyses were performed with the use of PASW Statistics, version 17.0.3 (IBM).
We used a numerical-modeling and Bayesian analysis method that was based on the mosquito-biting rate and the prevalence of microfilariae, stratified according to the age of residents in the 2008 survey, to estimate the likelihood of transmission cessation before and after bed-net distribution.10,11 (Details are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.)
RESULTS
PREVALENCE OF HUMAN INFECTION
Descriptive characteristics of the study population20 are summarized in Table 1. In 2008, the prevalence of microfilariae in the three study villages in the high-transmission zone ranged from 23.7% to 38.6%. These values were significantly higher than in 1998, when the values ranged from 3.7 to 10.8% (P<0.001 by Fisher’s exact test), 1 year after the fourth annual mass administration of antifilarial drugs and immediately before the fifth and final mass treatment. In contrast, the prevalence of microfilariae in the two villages in the moderate-transmission zone, Nanaha and Ngahmbule, remained low and did not change significantly during the 10-year period, with prevalences of 3.4% or less in both villages in 2008 (P = 0.78 and P = 0.39, respectively, for comparisons with 1998 values). Notably, the prevalence of microfilariae in 2008 did not increase in any of the villages to the level in 1994, before mass drug administration (Table 1).
Table 1.
Status of Lymphatic Filariasis and Use of Insecticide-Treated Bed Nets in the Study Villages.*
| Level of Transmission |
Village Name |
Microfilariae Prevalence | Population of Village |
Bed-Net Use before Distribution in 2009 |
Bed Nets Distributed |
Bed-Net Use after Distribution |
||
|---|---|---|---|---|---|---|---|---|
| 1994 | 1998 | 2008 | ||||||
| % | no. | % | no. | % | ||||
| High | Yauatong | 79.5 | 10.8 | 38.6 | 408 | 12.4 | 190 | 84.3 |
| High | Albulum | 78.3 | 7.4 | 38.4 | 526 | NA | 234 | 81.7 |
| High | Peneng | 61.5 | 3.7 | 23.7 | 233 | 3.8 | 142 | 75.0 |
| Moderate | Nanaha | 48.3 | 2.4 | 2.0 | 507 | NA | 222 | 84.1 |
| Moderate | Ngahmbule | 36.2 | 1.7 | 3.4 | 256 | NA | 109 | 90.6 |
Transmission levels are based on annual transmission potentials determined in 1993 and 1994. Five annual mass antifilarial treatments consisting of diethylcarbamazine alone or diethylcarbamazine plus ivermectin were administered from 1994 through 1998. (This regimen differed from the standard treatment in the global program to eliminate lymphatic filariasis.) Insecticide-treated bed nets were distributed in August 2009, with a target coverage of at least 80% of households per village. NA denotes not available.
USE OF INSECTICIDE-TREATED BED NETS
Immediately before bed-net distribution, 3.8% and 12.4% of households of the two study villages surveyed used bed nets of any type. Four to 5 months after distribution, self-reported household use of bed nets in the five study villages ranged from 75.0 to 90.6% (Table 1).
MOSQUITO VECTORS
A total of 20,345 anopheline mosquitoes were collected in the 26 months before bed-net distribution and 1554 in the 11 months after distribution. The subgroups of mosquitoes in which the species was confirmed included 78% of A. punctulatus and 21% of A. koliensis; the remaining 1% was a mix of A. hinesorum, A. farauti 4, and A. farauti sensu stricto. Among A. koliensis mosquitoes, 94% were caught in Nanaha and Ngahmbule; molecular confirmation of the morphologic identification of A. punctulatus resulted in 95% concordance. Only mosquitoes that were identified as A. punctulatus on morphologic analysis harbored W. bancrofti infective larvae. Subsequent data are therefore based on morphologically identified A. punctulatus.
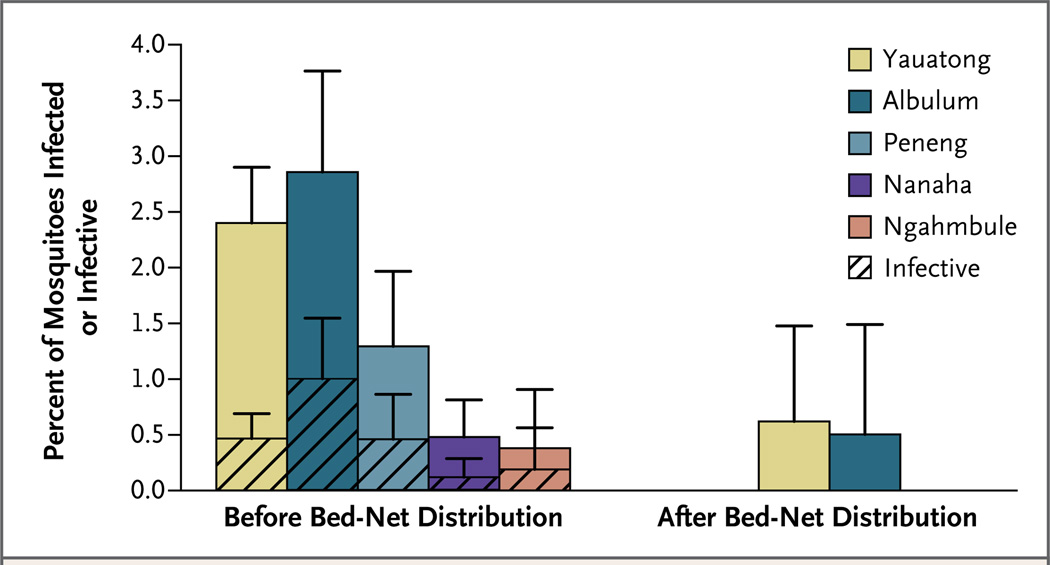
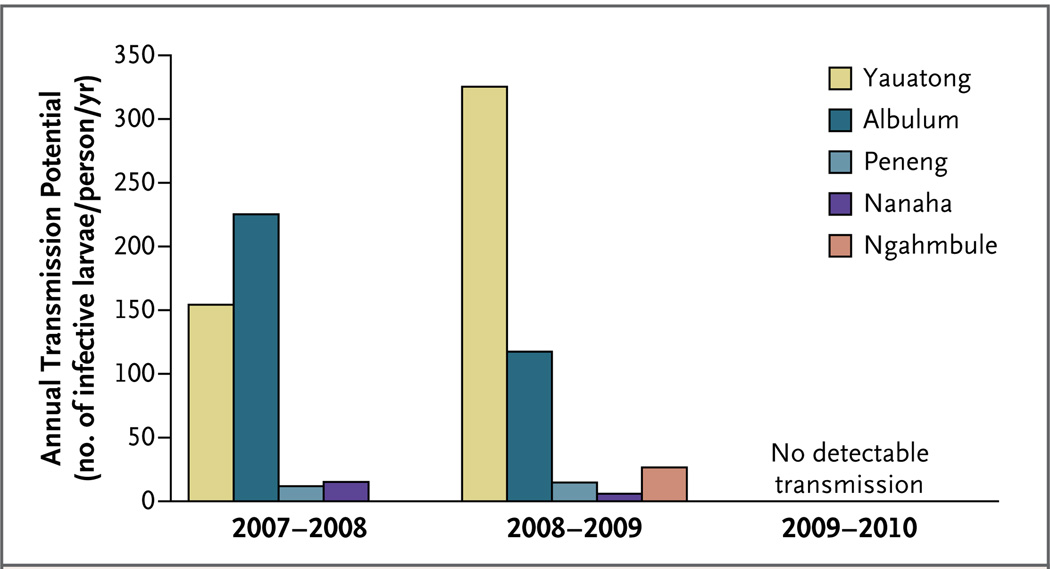
The proportion of A. punctulatus mosquitoes that were infected with any stage of larvae decreased from 1.8% to 0.4% after bed-net distribution (P = 0.005 by Fisher’s exact test) (Fig. 2). None of the mosquitoes that were collected in Peneng, Nanaha, or Ngahmbule after bed-net distribution contained larvae of any stage. Notably, no mosquitoes harboring infective larvae were identified in any of the villages after bed-net distribution (P = 0.07 by Fisher’s exact test). Therefore, although annual transmission potentials were similar during each of the 2 years preceding bed-net distribution, that number dropped to zero for the year after bed-net distribution (Fig. 3). The proportion of anopheline mosquitoes that tested positive for W. bancrofti DNA, an indicator of the reservoir of microfilariae, was 19.4% (761 mosquitoes) before bed-net distribution and 14.9% (248 mosquitoes) after bed-net distribution (P = 0.13 by Fisher’s exact test).
Figure 2. Proportion of Anopheles punctulatus Mosquitoes Carrying Nematodes Causing Lymphatic Filariasis, before and after the Distribution of Insecticide-Treated Bed Nets in Five Villages in Papua New Guinea.
Shown are the percentage of mosquitoes that were found to be infected (solid colors) and the percentage that were found to be infective (hatched areas) on dissection before bed-net distribution (8181 mosquitoes) and after bed-net distribution (678 mosquitoes). The T bars indicate 95% confidence intervals.
Figure 3. Annual Transmission Potential for Lymphatic Filariasis in the Five Study Villages during the 26 Months before and 11 Months after Bed-Net Distribution.
Shown are the estimated numbers of infective larvae that were inoculated per person per year during the periods of July 2007 through August 2008, September 2008 through August 2009, and September 2009 through July 2010. Insecticide-treated bed nets were distributed in the villages in August 2009.
MOSQUITO-BITING RATES
The daily biting rates for anopheline mosquitoes decreased significantly after bed-net distribution, with a mean (±SE) of 61.3±4.9 bites per person per day before bed-net distribution versus 9.4±1.9 after bed-net distribution in Yauatong, 22.6±2.5 versus 7.3±2.0 in Albulum, 6.4±0.8 versus 1.5±0.4 in Peneng, 21.5±1.3 versus 1.1±0.2 in Nanaha, and 8.9±0.8 versus 1.5±0.3 in Ngahmbule (P<0.001 for all comparisons by the Mann–Whitney U test). The rates remained consistently low for an additional 2 years in Yauatong and Nanaha (Fig. 4).
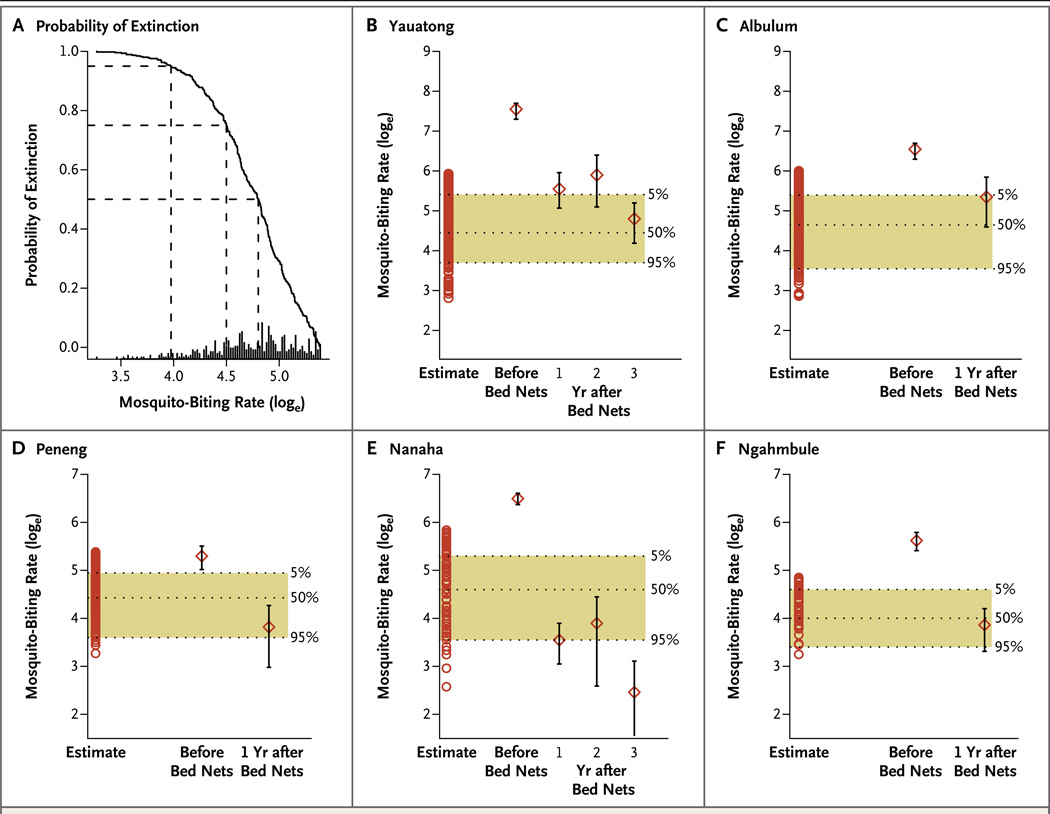
Figure 4. Probabilities of the Cessation of Transmission of Lymphatic Filariasis before and after Bed-Net Distribution on the Basis of Village-Specific Goodness of Fit with the Anopheline Transmission Model.
Panel A shows the probability of the cessation of transmission of lymphatic filariasis in the village of Peneng, according to mosquito-biting rates, which are expressed as the natural logarithm (loge) on the x axis. The horizontal dashed lines show the biting thresholds associated with cessation probabilities of 50%, 75% and 95%. The bars at the bottom of the panel indicate the frequency distribution of the modelestimated biting thresholds for Peneng. Panels B through F show changes in the mosquito-biting rate and probabilities of cessation in the five study villages before the distribution of insecticide-treated bed nets and 1 or more years after bed-net distribution. (Mosquito-biting rates were available for Yauatong and Nanaha for years 2 and 3 after the distribution.) Shown are estimates (open circles) of the most likely 500 biting thresholds calculated by goodness of fit of the model with 2008 data regarding the prevalence of microfilariae, stratified according to the age of residents in the five villages in the study. The shaded bands between dashed lines denote the range and biting threshold values associated with 5%, 50%, and 95% probabilities of transmission cessation. The diamonds indicate measured biting rates, and the I bars 95% confidence intervals.
The probability of the transmission cessation was less than 1.0% in all five villages before bed-net distribution. After bed-net distribution, the probabilities increased to 4.9%, 7.7%, 90.5%, 95.8%, and 61.5% in Yauatong, Albulum, Peneng, Nanaha, and Ngahmbule, respectively. Further reductions in biting rates by years 2 and 3 after bed-net distribution increased the probabilities to 36.8% and more than 99% in Yauatong and Nanaha, respectively. These high probabilities provide support for the empirical finding that annual transmission potentials were reduced to zero after bed-net distribution (Fig. 2).
DISCUSSION
Residents of villages in our study participated in a 5-year program of mass administration of anti-filarial drugs, with 77 to 86% of eligible residents receiving such drugs annually from 1994 through 1998.20 The prospect of the elimination of lymphatic filariasis was promising at the end of the campaign and seemed even more likely after a 2003 survey showed very few mosquitoes containing developing larvae, no mosquitoes containing infective larvae, and no children under the age of 10 years who tested positive for filarial antigen.24 However, it is clear from observations in 2008 and 2009 that transmission was still occurring, since annual transmission potentials among the study villages ranged from 5 to 325 infective larvae that were inoculated per person per year, and the prevalence of microfilariae had significantly rebounded in three villages. Human migration may have contributed to continuing transmission and increased prevalence of microfilariae after the cessation of mass treatment.31 The fact that worm breakpoints that were necessary for the cessation of transmission were not attained is probably of greater importance. In 1997, after four annual mass drug administrations, the prevalence of microfilariae in moderate- and high-transmission zones was 1% and 5%, respectively.20 However, worm breakpoints for anopheline systems are estimated to be 0.75%.11
The introduction of insecticide-treated bed nets profoundly affected the vector population and therefore the transmission of lymphatic filariasis. A similar proportion of mosquitoes tested positive for W. bancrofti DNA before and after bed-net distribution. However, significantly fewer mosquitoes contained developing worms after bed-net distribution. These findings indicate that mosquitoes were imbibing microfilaremic blood, but larval development was interrupted. The use of bed nets may reduce the transmission of vectorborne diseases by shortening the life span of mosquitoes,32 and W. bancrofti microfilariae require at least 13 days to develop into infective larvae in A. punctulatus.33 Therefore, a slight reduction in average life span could have a major effect on the number of infective larvae. In addition, after the introduction of bed nets, most mosquitoes that fed successfully probably were feeding before residents went to bed. We saw that a greater proportion of the mosquito population was biting at earlier hours after bed nets were introduced. Because of the nocturnal periodicity of microfilaremia in Papua New Guinea, earlier biters will ingest fewer microfilariae than those biting during the time of peak blood density of microfilariae (around 1:30 a.m.) (Fig. S1 in the Supplementary Appendix).
Previous studies have shown how vector control alone can be used to reduce the prevalence of microfilariae34 and to accelerate this decrease when combined with mass administration of antifilarial drugs.35,36 However, our study quantifies the effect of the most widely implemented vector-control measure, the use of insecticide-treated bed nets. Although vector control is not currently a part of the global strategy to eliminate lymphatic filariasis, universal bed-net distribution is now used widely for malaria control-and-elimination efforts in Papua New Guinea and sub-Saharan Africa. Thus, our study highlights the importance of integrating vectorborne disease interventions. However, in order for the use of bed nets to be a sustainable strategy to eliminate lymphatic filariasis, biting rates must remain below the threshold until lymphatic-dwelling adult worms in the population die. The likelihood of transmission cessation that we observed is a snapshot of a temporally dynamic transmission system. A small increase in biting rate with no change in human prevalence of microfilariae could quickly lead to a reestablishment of stable transmission.
Program managers wanting to determine transmission end points during elimination campaigns are met with the challenge of detecting human infection at progressively lower levels.37 In our study, we used data on the prevalence of microfilariae before the intervention and a model of the transmission of lymphatic filariasis to quantify the probability of transmission cessation on the basis of mosquito-biting rates alone. Annual transmission potentials dropped to zero after bed-net distribution in all villages on the basis of an absence of infective larvae in blood-seeking mosquitoes, though detection of infective mosquitoes was constrained by very low vector densities. The probability of transmission cessation was more than 50% in the two moderate-transmission villages, where the prevalence of microfilariae was 3.4% or less before bed-net distribution. A similar likelihood of transmission cessation was calculated for Peneng, where the prevalence of microfilariae in 2008 was 23.7%, but the mosquito-biting rate was similar to rates in the moderate-transmission villages after bed-net distribution.
If the use of bed nets remains high and vector populations continue to be susceptible, the use of bed nets may eliminate lymphatic filariasis in areas where the reservoir of microfilariae has first been reduced by mass drug administration, as in the populations included in this study, or where preintervention endemicity is already low, such as in the Solomon Islands.18 In high-transmission areas, the use of bed nets could work synergistically with mass drug administration by increasing the worm breakpoint to a more easily attainable level. Given the challenges of reaching 80% compliance with mass drug administration for at least 5 years,38,39 efforts to eliminate lymphatic filariasis would greatly benefit from integrated vector management.40
Supplementary Material
Acknowledgments
Funded by the U.S. Public Health Service and a grant (U19 AI065717) from the National Institutes of Health.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org
REFERENCES
- 1.Michael E, Bundy DA, Grenfell BT. Re-assessing the global prevalence and distribution of lymphatic filariasis. Parasitology. 1996;112:409–428. doi: 10.1017/s0031182000066646. [DOI] [PubMed] [Google Scholar]
- 2.Yamey G. Global alliance launches plan to eliminate lymphatic filariasis. BMJ. 2000;320:269. [PMC free article] [PubMed] [Google Scholar]
- 3.Ottesen EA. The Global Programme to Eliminate Lymphatic Filariasis. Trop Med Int Health. 2000;5:591–594. doi: 10.1046/j.1365-3156.2000.00620.x. [DOI] [PubMed] [Google Scholar]
- 4.Ottesen EA, Hooper PJ, Bradley M, Biswas G. The Global Programme to Eliminate Lymphatic Filariasis: health impact after 8 years. PLoS Negl Trop Dis. 2008;2(10):e317. doi: 10.1371/journal.pntd.0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyelem D, Biswas G, Bockarie MJ, et al. Determinants of success in national programs to eliminate lymphatic filariasis: a perspective identifying essential elements and research needs. Am J Trop Med Hyg. 2008;79:480–484. [PMC free article] [PubMed] [Google Scholar]
- 6.Gyapong M, Gyapong JO, Adjei S, Vlassoff C, Weiss M. Filariasis in northern Ghana: some cultural beliefs and practices and their implications for disease control. Soc Sci Med. 1996;43:235–242. doi: 10.1016/0277-9536(95)00365-7. [DOI] [PubMed] [Google Scholar]
- 7.Sunish IP, Rajendran R, Mani TR, Gajanana A, Reuben R, Satyanarayana K. Long-term population migration: an important aspect to be considered during mass drug administration for elimination of lymphatic filariasis. Trop Med Int Health. 2003;8:316–321. doi: 10.1046/j.1365-3156.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- 8.Global programme to eliminate lymphatic filariasis. Wkly Epidemiol Rec. 2007;82:361–380. [PubMed] [Google Scholar]
- 9.Schwab AE, Boakye DA, Kyelem D, Prichard RK. Detection of benzimidazole resistance-associated mutations in the filarial nematode Wuchereria bancrofti and evidence for selection by albendazole and ivermectin combination treatment. Am J Trop Med Hyg. 2005;73:234–238. [PubMed] [Google Scholar]
- 10.Gambhir M, Michael E. Complex ecological dynamics and eradicability of the vector borne macroparasitic disease, lymphatic filariasis. PLoS One. 2008;3(8):e2874. doi: 10.1371/journal.pone.0002874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gambhir M, Bockarie M, Tisch D, et al. Geographic and ecologic heterogeneity in elimination thresholds for the major vector-borne helminthic disease, lymphatic filariasis. BMC Biol. 2010;8:22. doi: 10.1186/1741-7007-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michael E, Malecela-Lazaro MN, Kabali C, Snow LC, Kazura JW. Mathematical models and lymphatic filariasis control: endpoints and optimal interventions. Trends Parasitol. 2006;22:226–233. doi: 10.1016/j.pt.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Southgate BA, Bryan JH. Factors affecting transmission of Wuchereria bancrofti by anopheline mosquitoes 4 Facilitation, limitation, proportionality and their epidemiological significance. Trans R Soc Trop Med Hyg. 1992;86:523–530. doi: 10.1016/0035-9203(92)90096-u. [DOI] [PubMed] [Google Scholar]
- 14.Burkot TR, Durrheim DN, Melrose WD, Speare R, Ichimori K. The argument for integrating vector control with multiple drug administration campaigns to ensure elimination of lymphatic filariasis. Filaria J. 2006;5:10. doi: 10.1186/1475-2883-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bockarie MJ, Pedersen EM, White GB, Michael E. Role of vector control in the Global Program to Eliminate Lymphatic Filariasis. Annu Rev Entomol. 2009;54:469–487. doi: 10.1146/annurev.ento.54.110807.090626. [DOI] [PubMed] [Google Scholar]
- 16.van den Berg H, Kelly-Hope LA, Lindsay SW. Malaria and lymphatic filariasis: the case for integrated vector management. Lancet Infect Dis. 2013;13:89–94. doi: 10.1016/S1473-3099(12)70148-2. [DOI] [PubMed] [Google Scholar]
- 17.Prasittisuk C. Vector-control synergies, between ‘roll back malaria’ and the Global Programme to Eliminate Lymphatic Filariasis, in South-east Asia. Ann Trop Med Parasitol. 2002;96(Suppl 2):S133–S137. doi: 10.1179/000349802125002482. [DOI] [PubMed] [Google Scholar]
- 18.Webber RH. Eradication of Wuchereria bancrofti infection through vector control. Trans R Soc Trop Med Hyg. 1979;73:722–724. doi: 10.1016/0035-9203(79)90031-2. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen EM, Mukoko DA. Impact of insecticide-treated materials on filaria transmission by the various species of vector mosquito in Africa. Ann Trop Med Parasitol. 2002;96(Suppl 2):S91–S95. doi: 10.1179/000349802125002437. [DOI] [PubMed] [Google Scholar]
- 20.Bockarie MJ, Tisch DJ, Kastens W, et al. Mass treatment to eliminate filariasis in Papua New Guinea. N Engl J Med. 2002;347:1841–1848. doi: 10.1056/NEJMoa021309. [DOI] [PubMed] [Google Scholar]
- 21.National Strategic Plan of Action for PNGELF (2004–2020) Waigani: Papua New Guinea Department of Health; [Google Scholar]
- 22.Bockarie M, Kazura J, Alexander N, et al. Transmission dynamics of Wuchereria bancrofti in East Sepik Province, Papua New Guinea. Am J Trop Med Hyg. 1996;54:577–581. doi: 10.4269/ajtmh.1996.54.577. [DOI] [PubMed] [Google Scholar]
- 23.Bockarie MJ, Alexander ND, Hyun P, et al. Randomised community-based trial of annual single-dose diethylcarbamazine with or without ivermectin against Wuchereria bancrofti infection in human beings and mosquitoes. Lancet. 1998;351:162–168. doi: 10.1016/S0140-6736(97)07081-5. [DOI] [PubMed] [Google Scholar]
- 24.Kazura JW, Bockarie MJ. World Health Organization. Geneva: Report of the Scientific Working Group on Filariasis; 2005. 4C: Long term impact of mass drug administration on Bancroftian filariasis in Dreikikir, Papua New Guinea; pp. 58–61. [Google Scholar]
- 25.Belkin JN. The mosquitoes of the South Pacific (Diptera, Culicidae) Berkeley: University of California Press; 1962. [Google Scholar]
- 26.Nelson GS. Staining of filarial larvae in insects before dissection. Bull World Health Organ. 1958;19:204. [PMC free article] [PubMed] [Google Scholar]
- 27.Idem. The identification of infective filarial larvae in mosquitoes: with a note on the species found in “wild” mosquitoes on the Kenya coast. J Helminthol. 1959;33:233–256. doi: 10.1017/s0022149x00033484. [DOI] [PubMed] [Google Scholar]
- 28.Beebe NW, Saul A. Discrimination of all members of the Anopheles punctulatus complex by polymerase chain reaction-restriction fragment length poly-morphism analysis. Am J Trop Med Hyg. 1995;53:478–481. doi: 10.4269/ajtmh.1995.53.478. [DOI] [PubMed] [Google Scholar]
- 29.Henry-Halldin CN, Reimer L, Thomsen E, et al. High throughput multiplex assay for species identification of Papua New Guinea malaria vectors: members of the Anopheles punctulatus (Diptera: Culicidae) species group. Am J Trop Med Hyg. 2011;84:166–173. doi: 10.4269/ajtmh.2011.10-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farid HA, Morsy ZS, Helmy H, Ramzy RM, El Setouhy M, Weil GJ. A critical appraisal of molecular xenomonitoring as a tool for assessing progress toward elimination of lymphatic filariasis. Am J Trop Med Hyg. 2007;77:593–600. [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander ND, Bockarie MJ, Dimber ZB, Griffin L, Kazura JW, Alpers MP. Migration and dispersal of lymphatic filariasis in Papua New Guinea. Trans R Soc Trop Med Hyg. 2001;95:277–279. doi: 10.1016/s0035-9203(01)90233-0. [DOI] [PubMed] [Google Scholar]
- 32.Takken W. Do insecticide-treated bednets have an effect on malaria vectors? Trop Med Int Health. 2002;7:1022–1030. doi: 10.1046/j.1365-3156.2002.00983.x. [DOI] [PubMed] [Google Scholar]
- 33.Backhouse TC. Anopheles punctulatus as an experimental intermediate host of Wuchereria bancrofti: some preliminary observations. Trans R Soc Trop Med Hyg. 1934;27:365–370. [Google Scholar]
- 34.Bockarie MJ, Tavul L, Kastens W, Michael E, Kazura JW. Impact of untreated bednets on prevalence of Wuchereria bancrofti transmitted by Anopheles farauti in Papua New Guinea. Med Vet Entomol. 2002;16:116–119. doi: 10.1046/j.0269-283x.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 35.Maxwell CA, Mohammed K, Kisumku U, Curtis CF. Can vector control play a useful supplementary role against bancroftian filariasis? Bull World Health Organ. 1999;77:138–143. [PMC free article] [PubMed] [Google Scholar]
- 36.Sunish IP, Rajendran R, Mani TR, Munirathinam A, Dash AP, Tyagi BK. Vector control complements mass drug administration against bancroftian filariasis in Tirukoilur, India. Bull World Health Organ. 2007;85:138–145. doi: 10.2471/BLT.06.029389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gass K, Beau de Rochars MV, Boakye D, et al. A multicenter evaluation of diagnostic tools to define endpoints for programs to eliminate bancroftian filariasis. PLoS Negl Trop Dis. 2012;6(1):e1479. doi: 10.1371/journal.pntd.0001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michael E, Malecela-Lazaro MN, Simonsen PE, et al. Mathematical modelling and the control of lymphatic filariasis. Lancet Infect Dis. 2004;4:223–234. doi: 10.1016/S1473-3099(04)00973-9. [DOI] [PubMed] [Google Scholar]
- 39.Stolk WA, Swaminathan S, van Oortmarsen GJ, Das PK, Habbema JD. Prospects for elimination of bancroftian filariasis by mass drug treatment in Pondicherry, India: a simulation study. J Infect Dis. 2003;188:1371–1381. doi: 10.1086/378354. [DOI] [PubMed] [Google Scholar]
- 40.WHO position statement on integrated vector management to control malaria and lymphatic filariasis. Wkly Epidemiol Rec. 2011;86:121–127. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.