Abstract
Bungarus multicinctus venom contains several α-toxins in addition to the widely used α-bungarotoxin (Bgt 2.2). We have found that two of the α-toxins (Bgt 3.1 and 3.3) inhibit neuronal acetylcholine (AcCho) sensitivity when tested on ciliary ganglion neurons in cell culture. Over 90% of the AcCho sensitivity recorded in response to iontophoretic application of AcCho was blocked when the neurons were incubated with either of the toxins at 10-7 M for 1 hr at 37°C. The blockade could be partially reversed by incubating the neurons for 1-2 hr in medium lacking the toxins. The neurons also had a high-affinity binding site for Bgt 2.2, as indicated by binding studies with rhodamine-labeled Bgt 2.2. Concentrations of Bgt 2.2(10-7 M) that should be nearly adequate to saturate the high-affinity site, however, had no detectable effect on AcCho sensitivity of the neurons. Higher concentrations of Bgt 2.2(10-5 M) produced a partial inhibition of AcCho sensitivity, suggesting either that the neurons had two classes of binding sites for Bgt 2.2 (with the low-affinity site affecting AcCho sensitivity) or that the preparation of Bgt 2.2 contained minor components (e.g., Bgt 3.1 or 3.3) that were responsible for the blockade. The mechanisms by which Bgt 3.1 and 3.3 inhibit neuronal AcCho sensitivity remain unknown. If they bind specifically to the AcCho receptor, they will be useful agents for studying the distribution and regulation of this membrane component.
Keywords: ciliary ganglion neurons, acetylcholine receptors, rhodamine-labeled α-bungarotoxin, cell culture
Full text
PDF
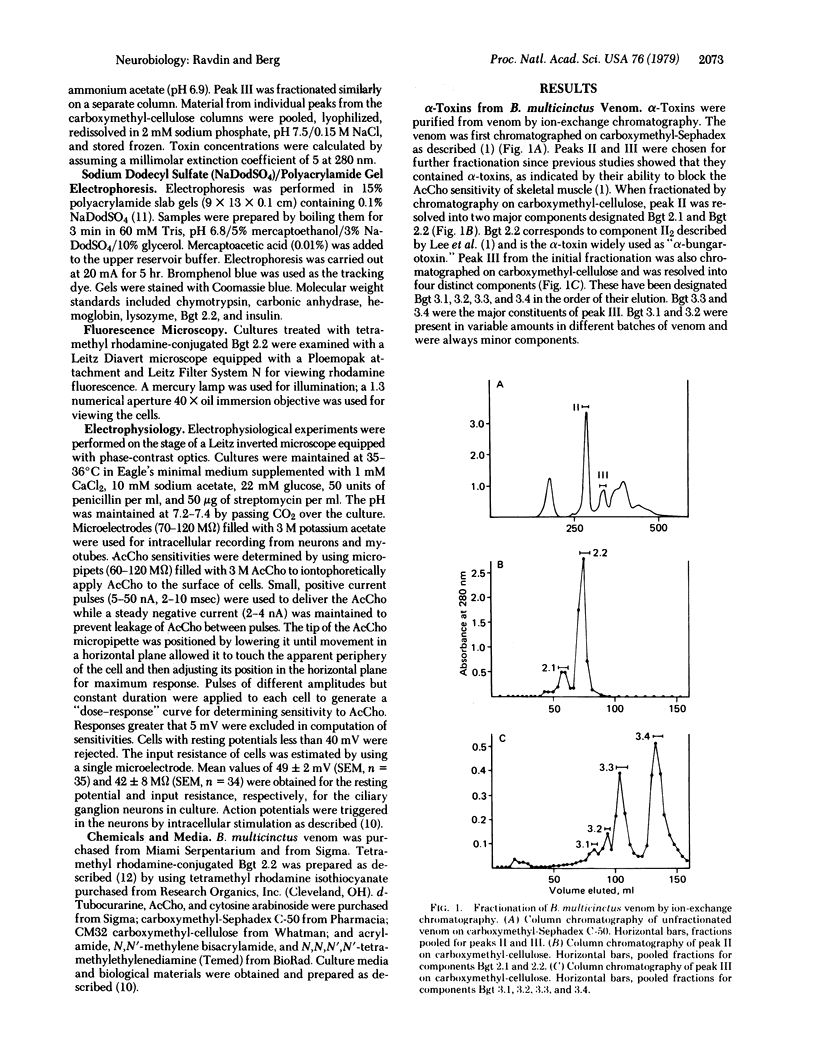

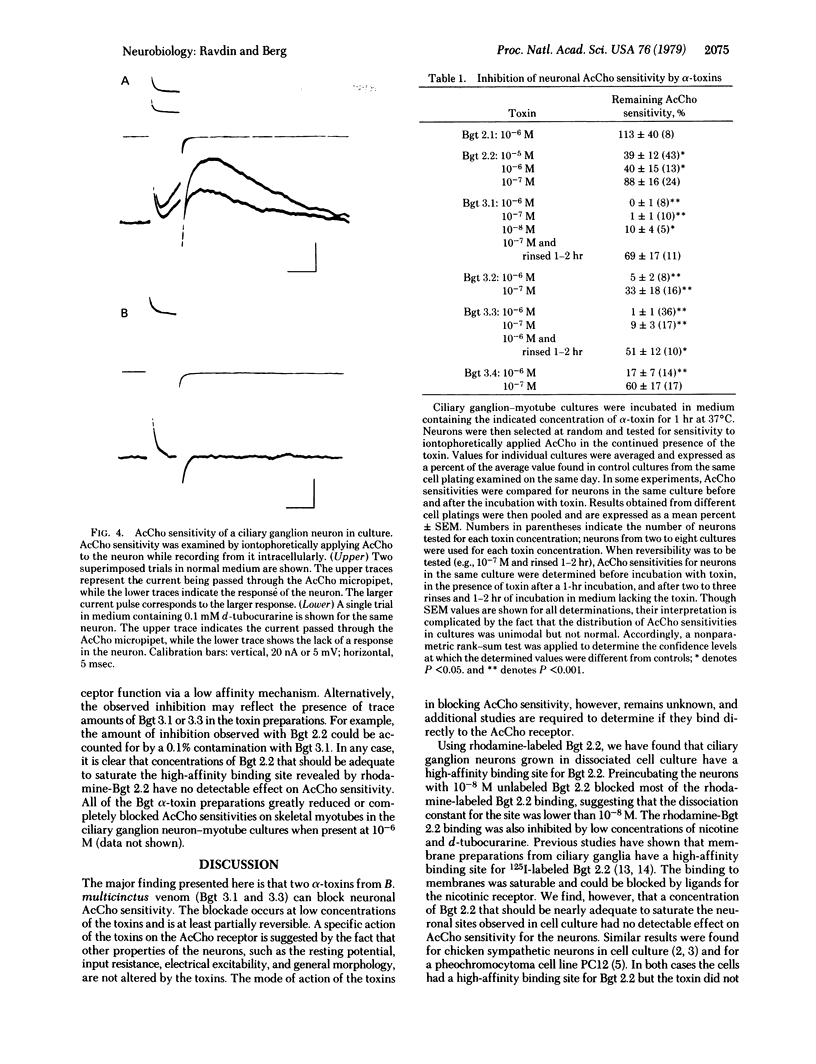

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. A., Fumagalli L. Department of Pharmacology, The School of Pharmacy, University of London, London, Great Britain. Brain Res. 1977 Jun 24;129(1):165–168. doi: 10.1016/0006-8993(77)90981-7. [DOI] [PubMed] [Google Scholar]
- Carbonetto S. T., Fambrough D. M., Muller K. J. Nonequivalence of alpha-bungarotoxin receptors and acetylcholine receptors in chick sympathetic neurons. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1016–1020. doi: 10.1073/pnas.75.2.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappinelli V. A., Giacobini E. Time course of appearance of alpha-bungarotoxin binding sites during development of chick ciliary ganglion and iris. Neurochem Res. 1978 Aug;3(4):465–478. doi: 10.1007/BF00966328. [DOI] [PubMed] [Google Scholar]
- Chiappinelli V. A., Zigmond R. E. alpha-Bungarotoxin blocks nicotinic transmission in the avian ciliary ganglion. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2999–3003. doi: 10.1073/pnas.75.6.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli L., De Renzis G., Miani N. alpha-Bungarotoxin-acetylcholine receptors in the chick ciliary ganglion: effects of deafferentation and axotomy. Brain Res. 1978 Sep 15;153(1):87–98. doi: 10.1016/0006-8993(78)91130-7. [DOI] [PubMed] [Google Scholar]
- Hanley M. R., Eterović V. A., Hawkes S. P., Hebert A. J., Bennett E. L. Neurotoxins of Bungarus multicinctus vernom. Purification and partial characterization. Biochemistry. 1977 Dec 27;16(26):5840–5849. doi: 10.1021/bi00645a031. [DOI] [PubMed] [Google Scholar]
- Kouvelas E. D., Dichter M. A., Greene L. A. Chick sympathetic neurons develop receptors for alpha-bungarotoxin in vitro, but the toxin does not block nicotinic receptors. Brain Res. 1978 Oct 6;154(1):83–93. doi: 10.1016/0006-8993(78)91053-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Synaptic transmission and cell death during normal ganglionic development. J Physiol. 1974 Sep;241(3):737–749. doi: 10.1113/jphysiol.1974.sp010681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Y., Chang S. L., Kau S. T., Luh S. H. Chromatographic separation of the venom of Bungarus multicinctus and characterization of its components. J Chromatogr. 1972 Oct 5;72(1):71–82. doi: 10.1016/0021-9673(72)80009-8. [DOI] [PubMed] [Google Scholar]
- Lentz T. L., Chester J. Localization of acetylcholine receptors in central synapses. J Cell Biol. 1977 Oct;75(1):258–267. doi: 10.1083/jcb.75.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi R., Berg D. K. Dissociated ciliary ganglion neurons in vitro: survival and synapse formation. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5171–5175. doi: 10.1073/pnas.74.11.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K. Transmitter sensitivities of some nerve and muscle cells in culture. Brain Res. 1974 Jun 14;73(1):71–88. doi: 10.1016/0006-8993(74)91008-7. [DOI] [PubMed] [Google Scholar]
- Patrick J., Stallcup B. alpha-Bungarotoxin binding and cholinergic receptor function on a rat sympathetic nerve line. J Biol Chem. 1977 Dec 10;252(23):8629–8633. [PubMed] [Google Scholar]
- Patrick J., Stallcup W. B. Immunological distinction between acetylcholine receptor and the alpha-bungarotoxin-binding component on sympathetic neurons. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4689–4692. doi: 10.1073/pnas.74.10.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin P., Axelrod D. Fluorescent tetramethyl rhodamine derivatives of alpha-bungarotoxin: preparation, separation, and characterization. Anal Biochem. 1977 Jun;80(2):585–592. doi: 10.1016/0003-2697(77)90682-0. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Maloney G. J., Ling A., Daniels M. P. Identification of synaptic acetylcholine receptor sites in retina with peroxidase-labeled alpha-bungarotoxin. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3268–3272. doi: 10.1073/pnas.74.8.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]