Abstract
Short day-length is used to programme adult diapause in the mosquito, Culex pipiens. The downstream endocrine event that halts ovarian maturation is a shutdown in juvenile hormone (JH) production, and recent evidence suggests that the insulin signalling pathway may be a key upstream player in executing this developmental arrest. Genes encoding insulin-like peptides-1, -2 and -5 were identified in C. pipiens, and we report that transcript levels of insulin-like peptides-1 and -5 were significantly lower in diapausing females than in their nondiapausing counterparts. Genes encoding both insulin-like peptides-1 and -5 were suppressed using RNA interference in mosquitoes programmed for nondiapause, and ovarian maturation was monitored. Knocking down insulin-like peptide-1 with RNAi in nondiapausing mosquitoes resulted in a cessation of ovarian development akin to diapause, and this arrest in development could be reversed with an application of JH. Knocking down insulin-like peptide-5 did not alter ovarian development. These results are consistent with a role for insulin-like peptide-1 in the signalling pathway leading from the perception of short day-lengths to the shut-down in JH production that characterizes adult diapause in C. pipiens.
Keywords: Insulin, Diapause, Juvenile hormone, dsRNAi
Introduction
Insulin signalling is emerging as an important pathway regulating growth, development and reproduction in insects (Riehle & Brown, 1999; Nijhout, 2003; Riehle et al., 2006; Wu & Brown, 2006; Brown et al., 2008). Several insulin-like peptides (ILPs) are known from insects (Riehle & Brown, 2002; Riehle et al., 2006), but thus far, unlike vertebrates, only a single insulin-like receptor has been identified (Wu & Brown, 2006, but see Brown et al., 2008).
Diapause may be amongst the key developmental pathways mediated by insulin signalling, as suggested by experiments on organisms as diverse as the nematode Caenorhabditis elegans (Lee et al., 2001), Drosophila melanogaster (Tatar et al., 2001; Williams et al., 2006) and the mosquito Culex pipiens (Sim & Denlinger, 2008). In each of these examples, a shut-down in insulin signalling is proposed to lead to developmental arrest.
In the case of C. pipiens, several lines of evidence point to insulin signalling as a component of the cascade regulating adult diapause (Sim & Denlinger, 2008). Knocking down the insulin-like receptor in long-day reared mosquitoes (i.e. those not programmed for diapause) halts ovarian development in a stage indistinguishable from diapause. This arrest in development, like the diapause of C. pipiens (Spielman, 1974; Meola & Petralia, 1980; Readio et al., 1999), can be reversed by application of a juvenile hormone (JH) analogue (Sim & Denlinger, 2008), thus suggesting that insulin signalling may be involved in the pathway leading to JH synthesis, an idea also consistent with observations in D. melanogaster (Tatar et al., 2001) and the honey bee (Corona et al., 2007). A role for insulin signalling in the diapause of C. pipiens is further supported by knock-down experiments focusing on a downstream gene, forkhead transcription factor (FOXO). When the insulin signalling pathway is activated, FOXO is normally suppressed (Lee et al., 2001; Junger et al., 2003), thus the likely scenario is that expression of FOXO is suppressed in long-day reared mosquitoes that develop without entering diapause. By contrast, when the insulin signalling pathway is not activated, the proposed situation in short-day reared mosquitoes programmed for diapause, the repression of FOXO is lifted, potentially leading to features of the diapause syndrome such as the lipid accumulation that characterizes diapause in C. pipiens. This scenario is consistent with the effect observed when diapause-programmed females are injected with double-strand RNA (dsRNA) directed against FOXO. Such females fail to accumulate the huge lipid reserves normally associated with diapause (Sim & Denlinger, 2008). Together, these results suggest a model that short days lead to a shut-down in the insulin signalling pathway and thereby release the repression of FOXO, leading to the diapause phenotype.
If this scenario is true, we would predict that levels of expression, synthesis and/or release of ILPs may preside over this developmental decision. In this study, we test the hypothesis that differences in expression of the genes encoding the ILPs affect the programming of diapause by cloning three ILPs from C. pipiens (ILP-1,-2 and -5) and evaluating expression of the genes encoding these ILPs under short-day (diapause) and long-day (nondiapause) conditions. RNA interference (RNAi) is then used to functionally evaluate the response when selected ILPs are knocked down. Our results suggest that suppression of ILP-1 is particularly important for evoking the diapause response.
Results
Culex pipiens ILP-1, -2 and -5
We identified ILPs from C. pipiens by performing Blast searches on the C. pipiens genomic database using sequences of ILPs from Aedes aegypti. The cDNA fragments of C. pipiens ILP-1 (488 bp) and ILP-2 (438 bp) shared the highest identities, 70 and 78%, respectively, with ILP-1 and ILP-2 from a closely related mosquito, Ae. aegypti. The 504-bp cDNA fragment of C. pipiens ILP-5 shared 68% identity with ILP-5 from Ae. aegypti and 82% identity to ILP-5 sequences from Anopheles gambiae. The deduced amino acid sequences of ILP-1, -2 and -5, based on a pfam search, belong to an insulin/insulin-like growth factor/relaxin family (IPR004825) with a predicted biological role in the regulation of normal glucose homeostasis, as well as other specific physiological functions including Ca. elegans dauer formation (Lee et al., 2001).
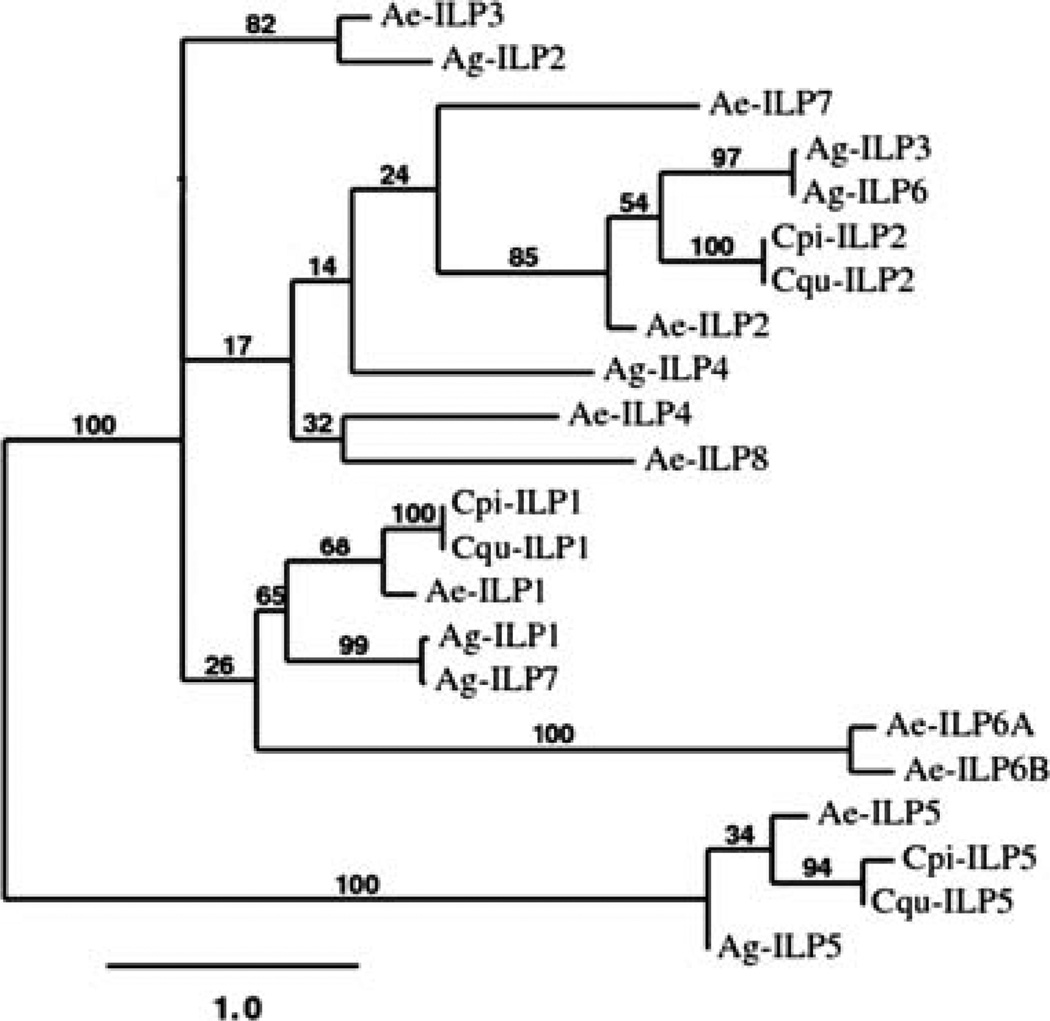
A phylogenetic analysis of known mosquito ILPs was performed to infer relationships of the Culex ILPs with those identified in An. gambiae and Ae. aegypti. All ILPs of C. pipiens (1, 2 and 5) had significant matches to the respective ILPs of Culex quinquefasciatus and Ae. aegypti (Fig. 1). The close relationship of ILP-1 and ILP-5 amongst mosquitoes was noted previously (Riehle et al., 2006). ILP-1, -2 and -5 are highly conserved between Culex species and Ae. aegypti, but these ILPs in An. gambiae are evolutionarily more distant, a result which is consistent with reported speciation patterns within the family Culicidae (Krzywinski et al., 2006).
Figure 1.
Phylogenetic tree of mosquito insulin-like peptides (ILPs) generated with the maximum likelihood method and bootstrap analysis. Bootstrap values are shown at the base of the branches and represent the percentage of times that grouping was supported. Cqu-ILPs from the Culex genome are the putative ILP operons. Cpi-ILP-1, Culex pipiens, FJ266014; Cpi-ILP-2, FJ266015; Cpi-ILP-5, FJ266016; Cqu-ILP-1, Culex quinquefasciatus, CPIJ018051; Cqu-ILP-2, CPIJ018050; Cqu-ILP-5, CPIJ001698; Ae-ILP-1, Aedes aegypti, DQ845750; Ae-ILP-2, DQ845752; Ae-ILP-3, DQ845751; Ae-ILP-4, DQ845753; Ae-ILP-5, DQ845758; Ae-ILP-6A, DQ845755; Ae-ILP-6B, DQ845756; Ae-ILP-7, DQ845757; Ae-ILP-8, DQ845754; Ag-ILP-1, Anopheles gambiae, AY324307; Ag-ILP-2, AY324308; Ag-ILP-3, AY324309; Ag-ILP-4, AY324310; Ag-ILP-5, AY324311-12 ; Ag-ILP-6, AY324313; Ag-ILP-7, AY324314-15.
Transcript levels of ILP-1, -2 and -5 in diapausing and nondiapausing females of C. pipiens
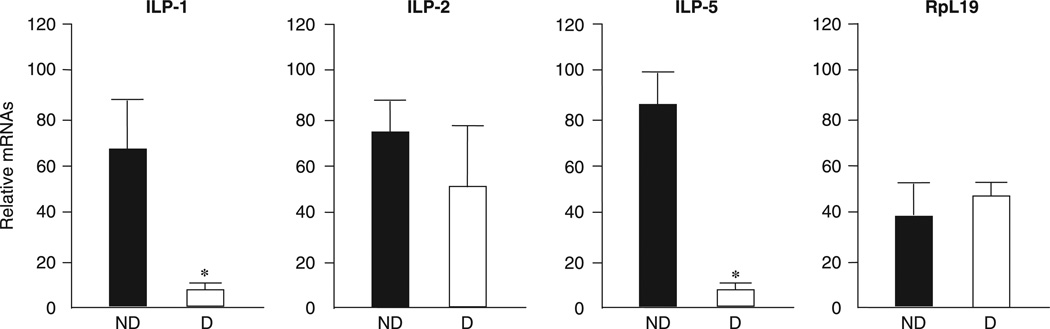
Expression patterns of the ILP-1, -2 and -5 genes in nondiapausing and diapausing C. pipiens were obtained using quantitative real-time PCR (qRT-PCR). The mRNA expression patterns of ILP-1 and -5 showed that both genes were upregulated in nondiapausing mosquitoes one week after eclosion but were down-regulated in diapausing mosquitoes reared at short day-length (Fig. 2). mRNA expression patterns of ILP-2 were similar in nondiapausing and diapausing females (Fig. 2). Reduced expression of the ILP-1 and -5 genes in mosquitoes in response to short day-length (destined to diapause) suggested the possibility that these genes may be involved in dictating the diapause phenotype.
Figure 2.
Quantitative real-time PCR showing expression of insulin-like peptide-1 (ILP-1), -2 and -5 in Culex pipiens females, one week after adult eclosion. ND (black), programmed by long day-length for nondiapause; D (white), programmed by short day-length for diapause. Both groups were maintained at 18 °C. Bars (mean ± SD) with asterisks (*) indicate significant differences at P = 0.05, t-test. Ribosomal protein large subunit 19 (RpL19) was used to confirm equal loading.
Double-strand RNA of ILP-1 halts ovarian development in nondiapausing females
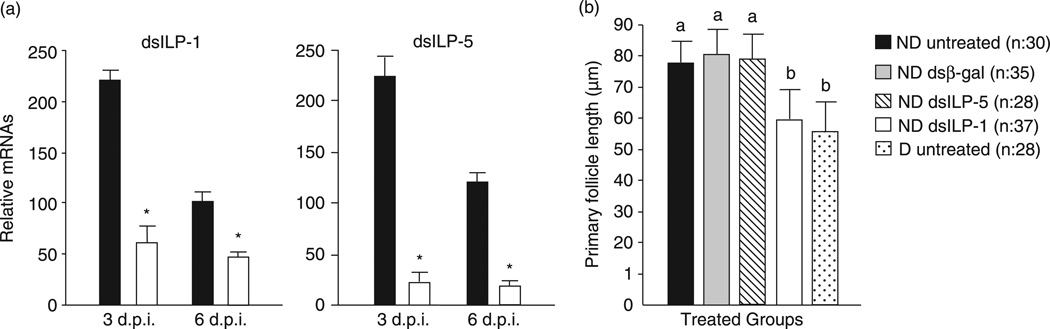
dsRNAi efficiency was first assessed by qRT-PCR. In contrast to the relatively high induction of ILP-1 and ILP-5 in double-strand RNA of β-galactosidase (dsβ-gal) injected mosquitoes, only traces of ILP-1 and ILP-5 mRNA were detected in double-strand RNA of ILP-1 (dsILP-1) and -5 injected, nondiapausing mosquitoes, using qRT-PCR and primers corresponding to the ILP-1 and ILP-5 genes (Fig. 3A), thus indicating that injection of dsILP-1 and dsILP-5 successfully inhibited induction of the ILP-1 and ILP-5 genes. Ribosomal protein L19 (RpL19), the loading control used to normalize transcript levels, showed no difference in expression three and six days postinjection (Fig. 2), thus indicating that the low expression levels observed for the ILP-1 and ILP-5 genes were related to the knock-down effect of dsRNAi rather than variation in sample loading.
Figure 3.
RNA interference efficiency and the effect of double-strand RNA of insulin-like peptide-1 (dsILP-1) and -5 on ovarian follicle length in Culex pipiens. (A) ILP-1 and ILP-5 transcript levels in females injected with dsILP-1 or ILP-5 (white bars) were compared with the double-strand RNA of β-galactosidase (dsβ-gal) controls (black bars). Expression levels were measured by quantitative real-time PCR three and six days after RNA interference injection, which was normalized using a RpL19 loading control. (B) Effects of dsILP-1 and dsILP-5 knockdowns on ovarian development of females programmed for nondiapause. Injections of dsILP-1 and dsILP-5 (~0.7 µg/♀) were made into the thorax of cold-anesthetized mosquitoes within one day after eclosion (controls, dsβ-gal), and females were dissected 10 days later. ND, programmed by long day-length for nondiapause; D, programmed by short day-length for diapause. Both groups were maintained at 18°C. d.p.i., days post injection. Bars (mean ± SD) with the same letters are not significantly different at P = 0.05, anova.
Ten days after injection, primary follicles in untreated nondiapausing females and dsβ-gal injected controls were nearly 50% larger than follicles in diapausing females reared at short day-length (Fig. 3B). By contrast, when dsILP-1 was injected into nondiapausing females, follicle length was greatly reduced and was not significantly different from that observed in diapausing females, thus this treatment mimicked the diapause response.
When ovarian status was monitored using the standard classification for diapause (Spielman & Wong, 1973), the proportion of nondiapausing females that fell into the diapause category 10 days following injection was low for untreated mosquitoes and those injected with dsβ-gal, but diapause-like status was high in nondiapausing mosquitoes injected with dsILP-1 and reached a level similar to that typically observed in diapausing mosquitoes (Table 1).
Table 1.
Proportion of Culex pipiens with diapause-type ovarian follicles after injection of double-strand RNA of insulin-like peptide-1 (dsILP-1) and application of juvenile hormone III (JH III)
| Double-strand RNA injection | n | Females with diapause- type ovarioles (%) |
|---|---|---|
| Programmed for nondiapause | ||
| Untreated control | 30 | 10.0 |
| dsβ-gal | 35 | 8.6 |
| dsILP-5 | 28 | 14.3 |
| dsILP-1 | 37 | 83.8* |
| Programmed for diapause | ||
| Untreated control | 24 | 92.9 |
| dsILP-1 + acetone | 24 | 87.5 |
| dsILP-1 + 5 ng JH III | 20 | 75.0* |
| dsILP-1 + 50 ng JH III | 23 | 39.1* |
| dsILP-1 + 500 ng JH III | 21 | 0.0* |
dsβ-gal, double-strand RNA of β-galactosidase.
Asterisks indicate significant difference from untreated controls
(χ2-goodness of fit test at P < 0.05 and d.f. = 1).
JH rescues the halt in ovarian development caused by dsILP-1
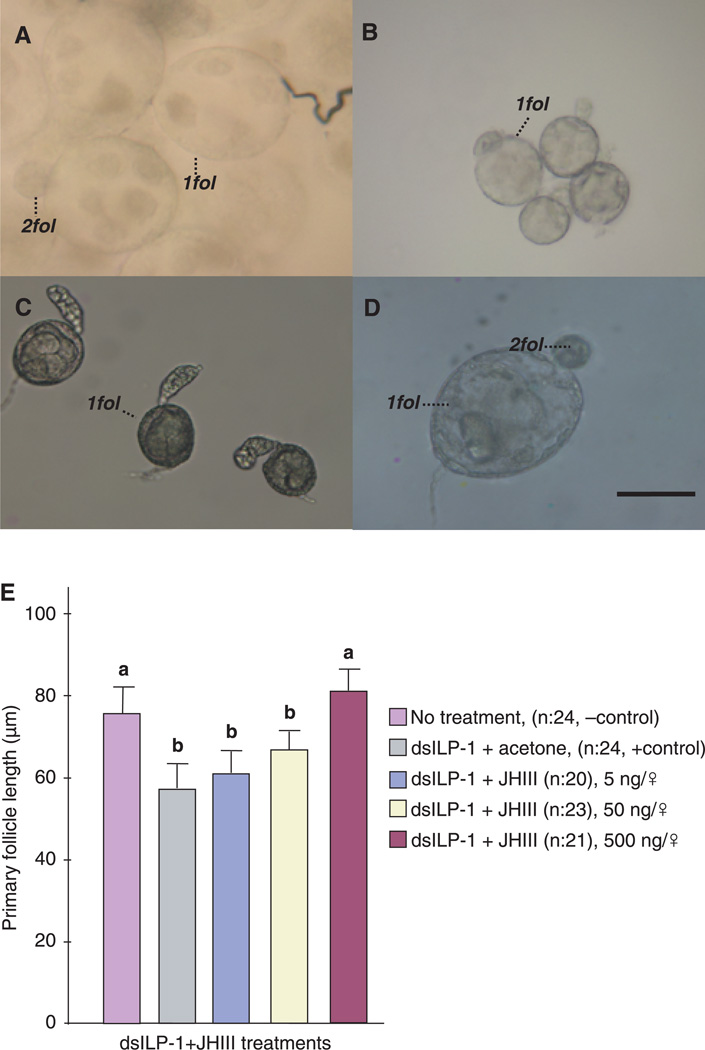
As demonstrated above, ovaries of dsILP-1 injected nondiapausing females halted development in a state simulating diapause. This was evident not only in differences of follicle length but also by distinctions in oocyte morphology. By day 10, the primary follicles in untreated nondiapausing females were robust, and secondary follicles had already formed (Fig. 4A), whereas ovarian development was halted in untreated diapausing females (Fig. 4B). Primary follicles in dsILP-1 injected nondiapausing females were arrested in a state indistinguishable from diapause (Fig. 4C). This arrest, however, could be rescued in dsILP-1 mosquitoes with a topical application of JH III (Fig. 4D). Primary follicle length in dsILP-1 injected mosquitoes increased in direct proportion to the concentration of JH III applied (Fig. 4E). In addition, JH dramatically decreased the incidence of diapause in the dsILP-1 injected mosquitoes in a dose-dependent manner (Table 1).
Figure 4.
Juvenile hormone (JH) rescue of ovarian development in insulin-like peptide-1 (ILP-1) knocked-down mosquitoes that were programmed by long day-length for nondiapause. (A) Primary (1fol) and secondary follicles (2fol) from wild-type nondiapausing (ND) females, prepared 10 days after eclosion (B) Primary follicles from diapausing (D) females, prepared 10 days after eclosion, showing the cessation of ovarian development. (C) Primary follicles from double-strand RNA of ILP-1 (dsILP-1) + acetone ND treated females dissected 10 days after eclosion. These follicles were arrested in Christopher’s stage I, similar to the ovarian arrest observed in diapause. (D) Primary follicles from dsILP-1 ND treated females that received a 500 ng topical application of JH III in 0.5 µl acetone, showing nondiapause characteristics, including secondary follicles. Scale bar = 50 µM. (E) Mean ± SD length of ovarian follicles in dsILP-1 injected ND females that subsequently received graded doses of JH III. Each n = 21–24 females. Bars with the same letters are not significantly different at P = 0.05, ANOVA.
Discussion
In our previous study of C. pipiens (Sim & Denlinger, 2008), we knocked down expression of the insulin receptor in nondiapausing females and simulated a diapause-like state. This study builds on our previous work on insulin signalling in C. pipiens by testing the hypothesis that the shut-down in insulin signalling during diapause may be reflected in differences in expression of one or more of the genes encoding ILPs. Genes encoding ILP-1, ILP-3 and ILP-5 were identified, and both ILP-1 and ILP-5 were expressed at significantly lower levels in diapausing than nondiapausing females. Although both ILP-1 and ILP-5 were expressed at lower levels during diapause, knocking down ILP-5 expression using RNAi had no effect in arresting ovarian development. By contrast, dsILP-1 arrested ovarian development in the previtellogenic (diapause-like) stage in mosquitoes programmed by long day-length for continuous (nondiapausing) development, and we thus propose that the shut-down in insulin signalling during diapause may be the consequence of a shut-down in the synthesis of ILP-1. Although one often thinks of neuropeptides exerting their influence through regulation of their release rather than synthesis, our results presented here suggest that regulation of synthesis is important in this case, as is also true for diapause hormone and prothoracicotropic hormone in the regulation of pupal diapause in species of the Heliothis/ Helicoverpa complex (Xu & Denlinger, 2003; Xu et al., 2003) and in Manduca sexta (Xu & Denlinger, 2004).
Although we detected only three ILPs in females of C. pipiens one week after adult eclosion, additional ILPs are likely to exist in different developmental stages or in adult males of C. pipiens, as suggested by previous mosquito ILP expression studies (Krieger et al., 2004; Riehle et al., 2006). It is also possible that certain ILPs may have evolved faster than the three ILPs identified in this study, thus making them difficult to identify using the current cloning strategy based on ILP sequences from Ae. aegypti. The phylogenetic tree of mosquito ILPs that we have produced differs from the tree previously presented by Riehle et al. (2006). The previous tree suggested that Aedes ILP-2 is phylogenetically most closely related to An. gambiae ILP-2 (Riehle et al., 2006), but our phylogenetic tree suggests that Ae. aegypti ILP-2 is more closely related to An. gambiae ILP-3/6 and C. pipiens ILP-2. This discrepancy is most likely to be a result of the updated sequence information that is now available for Ae. aegypti and An. gambiae. This issue may be further resolved with progress in annotating the Culex genome.
ILPs in humans and other mammals are categorized as insulins, insulin-like growth factors and relaxins, based on structural domains and functions, and distinct receptors are involved with these different neuropeptides (De Meyts & Whittaker, 2002). Although it was thought that insects possessed only a single insulin receptor (Wu & Brown, 2006), a recent study with Ae. aegypti suggests this may not be the case: ILP-3 binds with high affinity to insulin receptor, but other ILP family members may use a relaxinrelated G-protein coupled receptor (Brown et al., 2008). Such a divergence in receptors probably suggests functional differences in ILPs of insects, a possibility that is supported by the distinct differences we have observed in ILP gene expression and in the different results we observed when we knocked down different ILPs in C. pipiens.
In our previous work with C. pipiens, we demonstrated that a JH analogue, methoprene, could be used to counter the developmental arrest induced by dsRNAi of the insulin receptor in nondiapausing females (Sim & Denlinger, 2008). A shut-down in JH synthesis is well known to be one of the final steps leading to diapause in C. pipiens (Spielman, 1974; Readio et al., 1999), as well as in other insects that enter diapause as adults (Denlinger et al., 2004). In this study, we demonstrate that JH can also rescue the shut-down in development caused by knocking down expression of the gene encoding ILP-1. The current experiments used the authentic mosquito hormone, JH-III, rather than the JH analogue used previously, but the result was the same, demonstrating a dose-dependent rescue of the developmental arrest. A higher dose of JH-III was needed to elicit the effect than when the JH analogue was used, a result that is consistent with the known higher potency of methoprene than the natural hormone (Wilson, 2004). We thus suspect that insulin signalling is involved in the regulatory cascade leading to JH synthesis. A similar link between insulin signalling and JH synthesis has also been proposed for D. melanogaster (Tatar et al., 2001) and the honey bee (Corona et al., 2007). There is evidence for ILP localization in the corpora allata, the endocrine site of JH synthesis, in females of An. gambiae (Krieger et al., 2004), but exactly how insulin signalling regulates JH synthesis remains to be resolved in all of these cases.
We have also proposed that the shut-down of insulin signalling during diapause in C. pipiens activates the downstream FOXO, thus leading to the conspicuous hypertrophy of the fat body that is associated with the advent of diapause (Sim & Denlinger, 2008). Knocking down expression of FOXO in diapausing females with RNAi blocked the dramatic fat accumulation normally associated with diapause. Based on our current experiments we propose that the halt in ILP-1 synthesis, initiated by the short-day programming of diapause, leads to the translocation of the FOXO protein into the nucleus where it initiates transcription of downstream genes ultimately involved in fat accumulation and possibly other physiological responses associated with diapause.
Many components of the pathway leading from perception of short day-length to the halt in JH synthesis that results in diapause remain to be resolved, but our current working hypothesis is that insulin signalling is a vital intermediate in this pathway. Might the ILP-containing cells noted in the protocerebrum and optic lobes of Ae. aegypti (Riehle et al., 2006) and other flies (Cao & Brown, 2001) be the target or site for integration of the photoperiodic signal? The clock operating system resides in distinct lateral neurones of the central brain (Ewer et al., 1992; Renn et al., 1999; Goto et al., 2009), but whether there are direct connections between these two potential components is not yet known.
A similar shut-down in insulin signalling appears to regulate the adult diapause of D. melanogaster (Williams et al., 2006) and dauer formation in the nematode Ca. elegans (Lee et al., 2001), thus suggesting some commonality to these diverse forms of arrested development that is based on insulin signalling. Yet, an interesting dichotomy exists. Although we propose a model that links a shut-down in insulin signalling to the expression of diapause and associated lipid accumulation, insulin signalling has been linked to sequestration of nutrient reserves following a bloodmeal in nondiapausing females of Ae. aegypti (Roy et al., 2007; Brown et al., 2008). Is this a diapause/nondiapause distinction, perhaps related to nectar vs. blood feeding? Are different ILPs involved? Answers to these questions are not yet clear, but the common reliance on insulin signalling for so many diverse physiological responses (Riehle & Brown, 1999; Luckhart & Riehle, 2007; Roy et al., 2007) perhaps suggests that insulin signalling is used in very different ways by stages as distinct as diapause and nondiapause.
Experimental procedures
Insect rearing
The stock colony of C. pipiens (Buckeye Strain) was reared at 25 °C and 75% relative humidity (RH) under a 15 h light : 9 h dark (L : D) photoperiod, as previously described (Robich & Denlinger, 2005). When larvae reached the second instar, rearing containers were placed under one of two environmental conditions: nondiapausing (ND) adults were generated by rearing at 18 °C, 75% RH and 15 : 9 L : D. To induce diapause (D), mosquitoes were reared at 18 °C, 75% RH and 9:15 L : D. To confirm diapause status, primary follicle and germarium lengths were measured, and the stage of ovarian development was determined according to described methods (Christophers, 1911).
Identification and bioinformatic analysis of Culex insulin sequences
To retrieve sequences of C. pipiens ILPs, sequences of Ae. aegypti ILPs were utilized in discontinuous Mega–Blast searches on trace archives of genome data from the NCBI database (http://www.ncbi.nlm.nih.gov/blast/tracemb.shtml), and identities of the retrieved ILPs were confirmed by blasting against the C. quinquefasciatus genome database (http://cpipiens.vectorbase.org/Tools/BLAST/). cDNAs containing ILP coding regions were amplified by using the following primers (5′–3′): ILP-1, GCGTGGTGTTCATACTGTGC and TCGGCACAGTACTGCTTGAG; ILP-2, CTGGCGCTTAC-GATCCTC and GCATTCACCGTCTTGCAGTA; ILP-5, GCCATCT TGCTGGTCCAC and GTACCGGTTGATCCGCTTGT. Protein domains were identified by searching the pfam database (http://pfam.sanger.ac.uk/). The evolutionary history was inferred using the maximum likelihood (ML) method (Guindon & Gascuel, 2003; Guindon et al., 2005). The bootstrap consensus tree inferred from 500 replicates was taken to represent the evolutionary history of the mosquito ILPs analysed (Fig. 1). The percentage of replicate trees in which the associated ILPs clustered together in the bootstrap test (500 replicates) is shown next to the branches. The tree was drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree.
Transcript levels in diapausing and nondiapausing mosquitoes using qRT-PCR
To compare transcript levels of ILP-1 (FJ266014), -2 (FJ266015) and -5 (FJ266016) in early diapause and in nondiapausing mosquitoes, total RNA samples were extracted with Trizol (Invitrogen, Carlsbad, CA, USA) from three batches of 15 adult female mosquitoes, 7–10 days after eclosion. To remove genomic DNA contamination, RNA samples were treated with 1.0 µl DNase I following the manufacturer’s instructions (50–375 units/µl; Invitrogen). For reverse transcription, 5 µg total RNA was reverse transcribed with Superscript III RNase H-reverse transcriptase (Invitrogen). qRT-PCR was performed using an iQ5 real-time PCR detection system (Bio-Rad, Hercules, CA, USA), as described previously (Sim et al., 2005, 2007). Briefly, standard curves were generated for each transcript tested using tenfold serial dilutions of plasmids which included each ILP fragment, ranging from 100 to 0.01 pg per reaction. All reactions were performed in triplicate in a total volume of 20 µl containing 10 µl of SYBR Green PCR Master Mix and 300 nmol of each primer at the following conditions: 95 °C for 10 min followed by 45 cycles of denaturation at 95 °C for 15 s, annealing at 55 °C for 30 s and extension at 72 °C for 30 s. Ribosomal protein L19 (RpL19) (FJ266017) was used as an internal control. Sequences of gene-specific qRT-PCR primer sets are listed accordingly (5′–3′): qILP-1, CTACTG TGGC AAGGTGCTGA and CCATC-GACTTCTTCCTCCAC; qILP-2, ATCATCGAGTCC ACCGAGTT and GACTACCCGGTTGACGACTG; qILP-5, GATCCGAACATGGA GGTCAC and CAGTACATGTGCCGGATGAG; qRpL19, CGCT-TTGTTTGATCGT GTGT and CCAATCCAGGAGTGCTTTTG. Statistical significance of differences in the expression of individual genes was determined by using a Student’s t-test between the relative transcript values derived from the diapause and nondiapause mosquitoes across three replicates for each gene, and a P-value less than 0.05 was considered to be a significant transcript level change.
dsRNA preparation and injection into adult female mosquitoes
dsRNA for C. pipiens ILP-1 (dsILP-1), ILP-5 (dsILP-5) and dsβ-gal genes were prepared using the MEGAscript T7 transcription kit (Ambion, Foster City, CA, USA), as previously described (Sim et al., 2007), and T7 primers are listed accordingly (5′–3′): dsILP-1, TAATACGACTCACTATAGGGGCGTGGTGTTCATA CTGTGC and TAATACGACTCACTATAGGGTCGGCACAGTACTGCTTGAG; dsILP-5, TAATACGACTCACTATAGGGGCCATCTTGCTGGTCCAC and TAATACG ACTCACTATAGGGGTACCGGTTGATCCGCTTGT; dsβ-gal, TAATACGACTCACT ATAGGGGTCGCCAGCGGCACCG-CGCCTTTC and TAATACGACTCACTATAGG GCCGGTAGCC-AGCGCGGATCATCGG. Each PCR-derived fragment was sequenced and blasted against the C. quinquefasciatus genome database (http://cpipiens.vectorbase. org/Tools/BLAST/) to validate redundancy of the sequence and to confirm unique sequences. In knock-down experiments with ND mosquitoes, ~0.5 µl dsRNA of Culex ILP-1, ILP-5 (1.5 µg/µl) or ~0.5 µl dsβ-gal (1.5 µg/µl) were injected into the thorax of cold-anaesthetized females using a microinjector (Tritech Research, Los Angeles, CA, USA). Thus, ND females were injected with ~0.7 µg of dsILP-1 and dsILP-5.
RNAi efficiency evaluation using qRT-PCR
qRT-PCR of the dsRNA injected mosquitoes was carried out as above and as previously described (Sim & Denlinger, 2008). To evaluate RNAi efficiency, primers were used to amplify endogenous ILP-1 and ILP-5. Ribosomal protein large subunit 19 (RpL19) from C. pipiens was used as a loading control.
Follicle assay following dsILP-1 and dsILP-5
ND females, within a day after adult eclosion, were injected in the thorax with dsILP-1, dsILP-5 or dsβ-gal (control). Each treated cohort was kept in a 8 cm diameter × 12 cm cage. Cotton soaked in a 10% sucrose solution was provided 1 h after the dsRNA injection. Cages were placed at 18 °C, 75% RH, 15 : 9 h L : D, and ovaries were assessed 10 days after injection. Ovaries were dissected in a drop of saline solution, disrupted with a needle and examined at 200 and 400-fold magnifications (Zeiss Axioskop, Thornwood, NY, USA). Mean follicle length for each female was calculated from measurements of 10 follicles, and data were collected from 20–37 individuals. An anova test was used to distinguish differences in follicle sizes amongst dsRNA and control groups.
JH treatment
JH III (Sigma-Aldrich, St Louis, MO, USA) was used to evaluate the mosquito’s response to JH. ND females, within a day after adult eclosion, were injected with dsILP-1 and then topically treated the same day with serial dilutions of JH III (5, 50, 500 ng/♀) diluted in 0.5 µl acetone. Ovaries were dissected and measured as described above. An anova test was used to distinguish differences in follicle sizes.
Acknowledgements
This work was supported in part by National Institutes of Health-National Institute of Allergy and Infectious Disease Grant R01 AI058279.
Footnotes
Refereces
- Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, et al. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti . Proc Natl Acad Sci USA. 2008;105:5716–5721. doi: 10.1073/pnas.0800478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Brown MR. Localization of an insulin-like peptide in brains of two flies. Cell Tissue Res. 2001;304:317–321. doi: 10.1007/s004410100367. [DOI] [PubMed] [Google Scholar]
- Christophers S. The development of the egg follicle in Anophelines. Paludism. 1911;1:73–88. [Google Scholar]
- Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, Hughes KA, et al. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc Natl Acad Sci USA. 2007;104:7128–7133. doi: 10.1073/pnas.0701909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyts P, Whittaker J. Structural biology of insulin and IGF1 receptors: implications for drug design. Nat Rev Drug Discov. 2002;1:769–783. doi: 10.1038/nrd917. [DOI] [PubMed] [Google Scholar]
- Denlinger D, Yocum G, Rinehart J. Hormonal control of diapause. In: Gilbert L, Iatrou K, Gill S, editors. Comprehensive Molecular Insect Science. Amsterdam: Elsevier; 2004. pp. 615–650. [Google Scholar]
- Ewer J, Frisch B, Hamblen-Coyle MJ, Rosbash M, Hall JC. Expression of the period clock gene within different cell types in the brain of Drosophila adults and mosaic analysis of these cells’ influence on circadian behavioral rhythms. J Neurosci. 1992;12:3321–3349. doi: 10.1523/JNEUROSCI.12-09-03321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto SG, Shiga S, Numata H. Photoperiodism in insects: perception of light and the role of clock genes. In: Nelson RJ, Denlinger DL, Somers DE, editors. Photoperiodism: The Biological Calendar. Oxford: Oxford University Press; 2009. in press. [Google Scholar]
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Guindon S, Lethiec F, Duroux P, Gascuel O. PHYML Online - a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res. 2005;33:W557–W559. doi: 10.1093/nar/gki352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger M, Rintelen F, Stocker H, Wasserman J, Vegh M, Radimerski T, et al. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger MJ, Jahan N, Riehle MA, Cao C, Brown MR. Molecular characterization of insulin-like peptide genes and their expression in the African malaria mosquito, Anopheles gambiae . Insect Mol Biol. 2004;13:305–315. doi: 10.1111/j.0962-1075.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- Krzywinski J, Grushko OG, Besansky NJ. Analysis of the complete mitochondrial DNA from Anopheles funestus: an improved dipteran mitochondrial genome annotation and a temporal dimension of mosquito evolution. Mol Phylogenet Evol. 2006;39:417–423. doi: 10.1016/j.ympev.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Lee R, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11:1950–1957. doi: 10.1016/s0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- Luckhart S, Riehle MA. The insulin signaling cascade from nematodes to mammals: insights into innate immunity of Anopheles mosquitoes to malaria parasite infection. Dev Comp Immunol. 2007;31:647–656. doi: 10.1016/j.dci.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meola R, Petralia R. Juvenile hormone induction of biting behavior in Culex mosquitoes. Science. 1980;209:1548–1550. doi: 10.1126/science.209.4464.1548. [DOI] [PubMed] [Google Scholar]
- Nijhout H. The control of body size in insects. Dev Biol. 2003;261:1–9. doi: 10.1016/s0012-1606(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Readio J, Chen M, Meola R. Juvenile hormone biosynthesis in diapausing and nondiapausing Culex pipiens (Diptera: Culicidae) J Med Entomol. 1999;36:355–360. doi: 10.1093/jmedent/36.3.355. [DOI] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila . Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Riehle M, Brown M. Insulin stimulates ecdysteroid production through a conserved signaling cascade in the mosquito Aedes aegypti . Insect Biochem Mol Biol. 1999;29:855–860. doi: 10.1016/s0965-1748(99)00084-3. [DOI] [PubMed] [Google Scholar]
- Riehle M, Brown M. Insulin receptor expression during development and a reproductive cycle in the ovary of the mosquito Aedes aegypti . Cell Tissue Res. 2002;308:409–420. doi: 10.1007/s00441-002-0561-8. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Fan Y, Cao C, Brown MR. Molecular characterization of insulin-like peptides in the yellow fever mosquito, Aedes aegypti: expression, cellular localization, and phylogeny. Peptides. 2006;27:2547–2560. doi: 10.1016/j.peptides.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Robich R, Denlinger D. Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony. Proc Natl Acad Sci USA. 2005;102:15912–15917. doi: 10.1073/pnas.0507958102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SG, Hansen IA, Raikhel AS. Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti . Insect Biochem Mol Biol. 2007;37:1317–1326. doi: 10.1016/j.ibmb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Denlinger DL. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc Natl Acad Sci USA. 2008;105:6777–6781. doi: 10.1073/pnas.0802067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Hong Y, Tsetsarkin K, Vanlandingham D, Higgs S, Collins F. Anopheles gambiae heat shock protein cognate 70B impedes o’nyong-nyong virus replication. BMC Genomics. 2007;8:231. doi: 10.1186/1471-2164-8-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Hong Y, Vanlandingham D, Harker B, Christophides G, Kafatos F, et al. Modulation of Anopheles gambiae gene expression in response to o’nyong-nyong virus infection. Insect Mol Biol. 2005;14:475–481. doi: 10.1111/j.1365-2583.2005.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman A. Effect of synthetic juvenile hormone on ovarian diapause of Culex pipiens mosquitoes. J Med Entomol. 1974;11:223–225. doi: 10.1093/jmedent/11.2.223. [DOI] [PubMed] [Google Scholar]
- Spielman A, Wong J. Environmental control of ovarian diapause in Culex pipiens . Ann Entomol Soc Am. 1973;66:905–907. [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu M, Yin C, Garofalo R. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Williams K, Busto M, Suster M, So A, Ben-Shahar Y, Leevers S, et al. Natural variation in Drosophila melanogaster diapause due to the insulin-regulated PI3-kinase. Proc Natl Acad Sci USA. 2006;103:15911–15915. doi: 10.1073/pnas.0604592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TG. The molecular site of action of juvenile hormone and juvenile hormone insecticides during metamorphosis: how these compounds kill insects. J Insect Physiol. 2004;50:111–121. doi: 10.1016/j.jinsphys.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Wu Q, Brown M. Signaling and function of insulin-like peptides in insects. Annu Rev Entomol. 2006;51:1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- Xu WH, Denlinger DL. Molecular characterization of prothoracicotropic hormone and diapause hormone in Heliothis virescens during diapause, and a new role for diapause hormone. Insect Mol Biol. 2003;12:509–516. doi: 10.1046/j.1365-2583.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- Xu WH, Denlinger DL. Identification of a cDNA encoding DH, PBAN and other FXPRL neuropeptides from the tobacco hornworm, Manduca sexta, and expression associated with pupal diapause. Peptides. 2004;25:1099–1106. doi: 10.1016/j.peptides.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Xu WH, Rinehart JP, Denlinger DL. Structural characterization and expression analysis of prothoracicotropic hormone in the corn earworm, Helicoverpa zea . Peptides. 2003;24:1319–1325. doi: 10.1016/j.peptides.2003.08.001. [DOI] [PubMed] [Google Scholar]