Abstract
Objective
Endothelial function, as measured by non-invasive techniques, is known to vary widely within populations. Our study was designed to test the hypothesis that this variation is determined in large part by a person’s habitual dietary intake of flavonoids.
Methods
This was an analytical study examining the relationship between endothelial function and dietary flavonoids in 19 healthy older adults (mean age 72 years). The study took place in the inpatient Clinical Research Center of the Brigham and Women’s Hospital. Habitual flavonoid intake was assessed via a focused food frequency questionnaire. Endothelial function, measured as the reactive hyperemia response to one dose of flavonoid-rich cocoa, was recorded with a plethysmograpic device via peripheral arterial tonometry (PAT).
Results
Background flavonoid intake and the reactive hyperemia (RH)-PAT response were significantly correlated (r=0.7, p=0.001); subjects with higher habitual flavonoid intake showed a significantly greater RH-PAT response than did lower consumers. PAT response to cocoa was also significantly correlated with simultaneous flavanol concentration in the blood (r=0.5, p=0.03).
Conclusion
Individual variation in endothelial function among healthy older people, measured as PAT response to flavonoid-rich cocoa, is highly dependent upon usual daily flavonoid consumption. These data raise the possibility that the consumption of fruits and vegetables dictates basal endothelial function, likely related to their flavonoid content and influence on nitric oxide.
Keywords: flavonoids, diet, endothelial function, aging
INTRODUCTION
Endothelial function, as measured by non-invasive techniques, is known to vary widely within populations [1;2]. This study was designed to test the hypothesis that this variation is determined in large part by a person’s habitual dietary intake of flavonoids.
Most experts advocate a substantial intake of fruits and vegetables as an important part of a healthful diet. Remarkably, the precise mechanism involved in their protective effects remains obscure. One possibility is their rich content of flavonoids, a large group of phytochemicals. Possible health benefits of flavonoids elucidated from in vitro studies include scavenging of superoxide anions, singlet oxygen, and lipid peroxy-radicals [3]; and inhibiting atherogenicity and cytotoxicity of low-density lipoproteins [4]. More recently, we and others have shown that flavonoids activate nitric oxide (NO) [5]. These in vitro findings are supported by observational studies suggesting that flavonoids reduce cardiovascular risk [6–8].
We undertook a prospective quantification of flavonoid intake in healthy older adults by focused food frequency questionnaire, and measured the baseline blood concentration of the subset of flavonoids called flavanols in blood. We then analyzed intake in relation to the vascular response to flavonoid-rich cocoa.
MATERIALS AND METHODS
Study Population
Nineteen healthy older people were studied (mean age 71.7 ± 6.8; Table). Subjects had normal routine laboratories, blood pressure and mean cholesterol and were mildly overweight. None smoked. The study was approved by the Brigham and Women’s Hospital’s institutional review committee, and informed consent was obtained prior to study. Vascular responses of some of these subjects to flavanol-rich cocoa have been previously reported [9;10].
Table.
Subject Characteristics (n=19)
| Males | 8 |
| Caucasians | 18 |
| Age (yrs) | 72 ± 7 |
| SBP (mm Hg) | 119 ± 11 |
| DBP (mm Hg) | 70 ± 8 |
| BMI (kg/m2) | 25.6 ± 5 |
| Cholesterol (mg/dL) | 189±41 |
| Median flavonoid intake (mg/day) | 347 |
PAT Technique
Each subject underwent repeated measurements of peripheral arterial tonometry (PAT) using a plethysmographic device on the fingertip (PAT 1000RD; Itamar Medical, Inc., Caesarea, Israel) designed to reflect only pulsatile arterial volume changes. Readings were taken twice, at baseline and following five minutes of radial artery occlusion at 30 mm Hg above systolic blood pressure. Flow-mediated vasodilation, measured as reactive hyperemia (RH-PAT), was assessed one minute post release. The procedure has been described in detail [5]. A single technician, blinded to subject, study day, time and treatment calculated pulsatile volume, employing area under the curve.
Protocols
On the study day, after an overnight fast, baseline blood pressure, basal PAT and RH-PAT readings were taken on the metabolic ward. Temperature was controlled between 24 and 25°C. Baseline blood was drawn for flavanol concentration. Each subject then drank a cocoa dose (one packet of Cocoapro ™, Mars Inc., Hackettstown, NJ, mixed in one cup of water) in order to measure the highest PAT and RH-PAT response acutely. The cocoa contained 451 mg flavanols per packet as measured by high-performance liquid chromatography, including 84 mg epicatechin, 28 mg catechin, and 339 mg flavanol oligomers; also 19 mg caffeine, 204 mg theo bromine and 119 calories. Measures were taken 2, 4, 6 and 8 hours after cocoa ingestion; peak (RH) PAT was marked at the time of highest absolute (RH) PAT reading; (RH) PAT response was the difference between peak and baseline.
Analyses
A focused food frequency questionnaire was based on the United States Department of Agriculture Databases for the Flavonoid Content of Selected Foods [11], and the Proanthocyanidin Content of Selected Foods [12]. Twenty-two foods highest in flavonoid content and readily available in the American diet were chosen. The flavonoid content for each food was normalized to typical serving size. Because flavanol monomers are quantified in both the flavonoid and the proanthocyanidin databases, the average value was used, similar to methods from Mink et al [13]. On the study day one technician administered the questionnaire; subjects based responses upon consumption during an average week. Total flavanol concentration in the blood was measured by reversed-phase HPLC and included the monomers catechin and epicatechin, as well as their glucuronide and methyl metabolites.
Subjects were divided into low, medium, and high habitual flavonoid consumption according to their weekly intake. Eight subjects were low consumers (<2000 mg/week), six were medium consumers (2000–4500 mg/week), and five were high consumers (>4500 mg/week). One subject in the high intake group and two in the low intake group were taking statins. None was taking any anti-hypertensive medication. In the high intake group there was one moderate exerciser (three or more 30- minute aerobic sessions/week); in the low intake group there were two. Data are described with means and standard deviations. An analysis of variance focused contrast was used to test for trends across the ordered groups. The Pearson correlation coefficient was used to assess correlations. SAS version 9.2 was used.
RESULTS
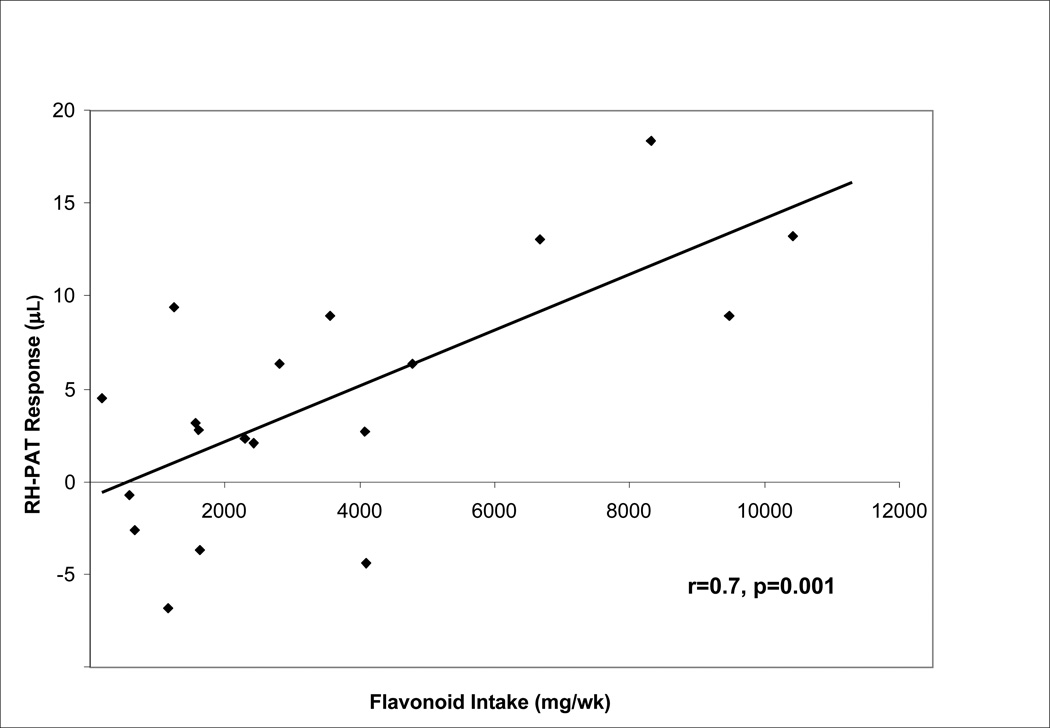
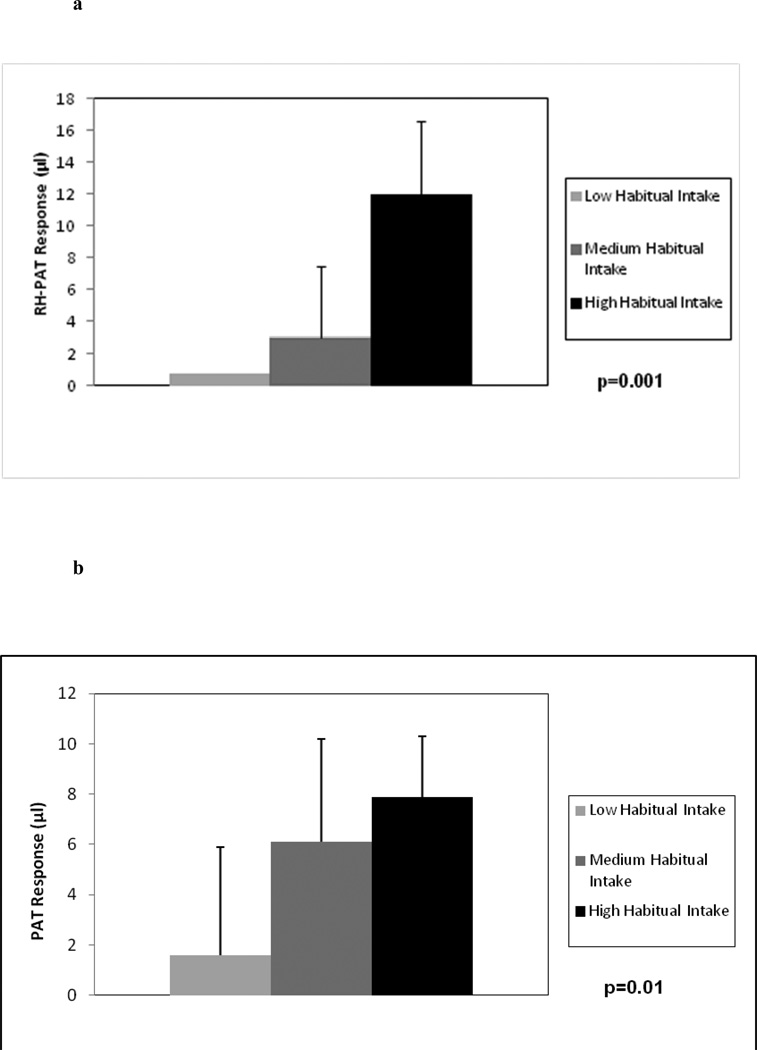
Median flavonoid intake among all subjects was 2428 mg/week (lower quartile 1242 mg; upper quartile 4789 mg). There was a highly significant correlation between habitual flavonoid intake and endothelial function as measured by the RH-PAT response to an acute dose of cocoa at baseline (r=0.7; p=0.001; Figure 1). Likewise habitual flavonoid intake was significantly correlated to the basal pulse wave PAT response prior to hyperemia (r=0.56, p=0.01). When analyzed categorically (Figure 2), there was a significantly increasing trend across the low, medium and high habitual flavonoid intake groups for RH-PAT response (p=0.001) and for PAT response (p=0.01). The main analyses were rerun excluding three subjects taking statins and correlations were virtually identical.
Figure 1.
There was a highly significant correlation between habitual flavonoid intake and endothelial function as measured by the continuous reactive-hyperemia peripheral arterial tonometry (RH- PAT) response to an acute dose of cocoa (r=0.7, p=0.001).
Figure 2.
PAT responsiveness according to habitual flavonoid consumers at baseline, after initial cup of flavanol-rich cocoa. There was a significantly increasing trend across the low, medium and high habitual flavonoid intake groups for RH-PAT response (p=0.001; Figure 2a) and for PAT response (p=0.01; Figure 2b).
Total flavanol concentration in the blood was measured fasting, in the morning before baseline PAT response to cocoa was measured. The correlation between PAT response and flavanol concentration was positive and significant (r=0.49, p=0.03). Likewise, RH-PAT response with hyperemic stimulus was also positively correlated with total blood flavanol concentration (r=0.44, p=0.06).
DISCUSSION
This study demonstrated that spontaneous dietary flavonoid intake was associated with superior endothelial function in healthy older people, assessed as the magnitude of the vascular response to a cup of flavonoid-rich cocoa. Evidence was multi-layered. There was a significant correlation between peripheral vascular response and dietary flavonoids as assessed by questionnaire. There was also a correlation between vascular response and flavanols as measured in the blood. Our data indicate that basal endothelial function is largely a reflection of background dietary flavonoids. This study was prompted by the well-described substantial variation in the peripheral vasodilator response to acute dosing of flavonoid-rich cocoa, which cannot be attributed solely to imprecision in measurements [1;2].
A major role for NO in the vasodilator response to flavonoid-rich cocoa is supported by multiple lines of investigation. We previously demonstrated that a NO inhibitor, L-NAME (NG-Nitro-L-arginine-methylester-HCl), reversed completely the vasodilator response to cocoa [5]. Multiple laboratories have since confirmed that observation [14]. Although we used flavonoid-rich cocoa as a convenient source of flavonoid, others have performed similar studies with grape juice, red wine, and tea [4;15–17].
The fact that purified epicatechin mimics the response to cocoa indicates at least one responsible chemical moiety for the vascular effects of cocoa [18]. The monomeric flavanols catechin and epicatechin, as well as their isomers, are absorbed and present in circulation for up to six hours [18]. In addition, however, while larger oligomeric procyanidins are not absorbed and not present in human plasma, many metabolites of flavanols are [19]. Because we saw relationships between endothelial function and flavonoid intake even in the fasting state, our data support the potential importance of these metabolites.
Limitations
The study was small, making it impossible to control for comorbidities that might contribute to endothelial dysfunction and for lifestyle behaviors that might be beneficial. However, most existing conditions likely to result in endothelial dysfunction were excluded, including diabetes mellitus, hypertension, and smoking; also, there was not a disproportionate number of exercisers in the high intake group. Further, substantial correlations emerged despite the small sample size. Dietary recall is imperfect, and the questionnaire may not be completely precise. However, data suggest that older people exhibit a substantial reduction in food variety and food choices, so a diet consumed in one week is likely to be typical of habitual intake over a longer period of time [20]. Despite the small study size, the flavonoid distribution of these subjects generally reflected the distribution reported by Mink et al in a much larger cohort [13].
CONCLUSION
Our data indicate that flavonoid intake is likely a major determinant of endothelial function. If correct, this study may begin to shed light on practical preventive measures against vascular heart disease, stroke, and diabetes mellitus—among the most common causes of morbidity and mortality in the western world.
Acknowledgments
Dr. Fisher’s research was sponsored by RO1HL89570. The project described was also supported by Grant Number 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center, from the National Center for Research Resources, and M01-02635 supported GCRC resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Cocoa was supplied by Mars Inc. Flavanol assays were run by Dr. Carl Keen at the University of California, Davis.
Abbreviations
- PAT
peripheral arterial tonometry
- RH
reactive hyperemia
- USDA
United States Department of Agriculture
- NO
nitric oxide
REFERENCES
- 1.Donald AE, Halcox JP, Charakida M, Storry C, Wallace SM, Cole TJ, Friberg P, Deanfield JE. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am Coll Cardiol. 2008;51:1959–1964. doi: 10.1016/j.jacc.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 2.Ghiadoni L, Versari D, Giannarelli C, Faita F, Taddei S. Non-invasive diagnostic tools for investigating endothelial dysfunction. Curr Pharm Des. 2008;14:3715–3722. doi: 10.2174/138161208786898761. [DOI] [PubMed] [Google Scholar]
- 3.Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem. 2000;48:3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- 4.Stein JH, Keevil JG, Wiebe DA, Aeschlimann S, Folts JD. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation. 1999;100:1050–1055. doi: 10.1161/01.cir.100.10.1050. [DOI] [PubMed] [Google Scholar]
- 5.Fisher ND, Hughes M, Gerhard-Herman M, Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens. 2003;21:2281–2286. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Sesso HD, Gaziano JM, Buring JE, Hennekens CH. Coffee and tea intake and the risk of myocardial infarction. Am J Epidemiol. 1999;149:162–167. doi: 10.1093/oxfordjournals.aje.a009782. [DOI] [PubMed] [Google Scholar]
- 7.Mukamal KJ, Maclure M, Muller JE, Sherwood JB, Mittleman MA. Tea consumption and mortality after acute myocardial infarction. Circulation. 2002;105:2476–2481. doi: 10.1161/01.cir.0000017201.88994.f7. [DOI] [PubMed] [Google Scholar]
- 8.Geleijnse JM, Launer LJ, Van der Kuip DA, Hofman A, Witteman JC. Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam Study. Am J Clin Nutr. 2002;75:880–886. doi: 10.1093/ajcn/75.5.880. [DOI] [PubMed] [Google Scholar]
- 9.Fisher ND, Hollenberg NK. Aging and vascular responses to flavanol-rich cocoa. J Hypertens. 2006;24:1575–1580. doi: 10.1097/01.hjh.0000239293.40507.2a. [DOI] [PubMed] [Google Scholar]
- 10.Sorond FA, Lipsitz LA, Hollenberg NK, Fisher NDL. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatric Disease and Treatment. 2008 in press. [PMC free article] [PubMed] [Google Scholar]
- 11.USDA Database for the Flavonoid Content of Selected Foods, Release 2.1. Internet. 2007 Ref Type: Electronic Citation. [Google Scholar]
- 12.USDA Database for the Proanthocyanidin Content of Selected Foods. Internet. 2004 Ref Type: Electronic Citation. [Google Scholar]
- 13.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR., Jr Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 14.Heiss C, Kleinbongard P, Dejam A, Perre S, Schroeter H, Sies H, Kelm M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol. 2005;46:1276–1283. doi: 10.1016/j.jacc.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 15.Diebolt M, Bucher B, Andriantsitohaina R. Wine polyphenols decrease blood pressure, improve NO vasodilatation, and induce gene expression. Hypertension. 2001;38:159–165. doi: 10.1161/01.hyp.38.2.159. [DOI] [PubMed] [Google Scholar]
- 16.Leikert JF, Rathel TR, Wohlfart P, Cheynier V, Vollmar AM, Dirsch VM. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Circulation. 2002;106:1614–1617. doi: 10.1161/01.cir.0000034445.31543.43. [DOI] [PubMed] [Google Scholar]
- 17.Duffy SJ, Keaney JF, Jr, Holbrook M, Gokce N, Swerdloff PL, Frei B, Vita JA. Short- and Long-Term Black Tea Consumption Reverses Endothelial Dysfunction in Patients with Coronary Artery Disease. Circulation. 2001;104:151–156. doi: 10.1161/01.cir.104.2.151. [DOI] [PubMed] [Google Scholar]
- 18.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stalmach A, Troufflard S, Serafini M, Crozier A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol Nutr Food Res. 2009;53(Suppl 1):S44–S53. doi: 10.1002/mnfr.200800169. [DOI] [PubMed] [Google Scholar]
- 20.Westenhoefer J. Age and gender dependent profile of food choice. Forum Nutr. 2005:44–51. doi: 10.1159/000083753. [DOI] [PubMed] [Google Scholar]