Abstract
The Kuna Indians who reside in an archipelago on the Caribbean Coast of Panama have very low blood pressure levels, live longer than other Panamanians, and have a reduced frequency of myocardial infarction, stroke, diabetes mellitus, and cancer -- at least on their death certificates. One outstanding feature of their diet includes a very high intake of flavanol-rich cocoa. Flavonoids in cocoa activate nitric oxide synthesis in healthy humans. The possibility that the high flavanol intake protects the Kuna against high blood pressure, ischemic heart disease, stroke, diabetes mellitus, and cancer is sufficiently intriguing and sufficiently important that large, randomized controlled clinical trials should be pursued.
Keywords: Flavanols, nitric oxide, cocoa
Introduction
(1) Blood Pressure and the Kuna Indians of Panama
About twenty years ago, the Brigham and Women’s Hospital Hypertension group was one of five across the United States chosen to study the genetics of hypertension by the NIH. During that first decade, it became increasingly apparent that the inheritance of hypertension is polygenic. Thus, any gene polymorphism identified was likely to influence only a small percentage of the total hypertension problem. For that reason, we decided to ask a novel question. Clearly there are “bad genes” that promote hypertension. Might there not also be “good genes”, which protect against hypertension? The strategy that we adopted for identifying protective genes involved finding a geographically-isolated, ethnically homogeneous group of individuals -- ideally the product of a small founder colony -- who had lived in isolation for generations, and thus had inbred. A founder colony is a small group of individuals who move from the main population to an isolated location, which facilitates inbreeding. Somewhere, we hoped, a small founder colony had included many individuals who carried these protective genes. Such a population would have a number of easily identified features. Hypertension would be uncommon and perhaps nonexistent. Blood pressure rises with age, and we hypothesized that blood pressure might not rise with age in a genetically-protected community.
Such communities are not uncommon (1): Indeed, several dozen communities worldwide show these features. Among them were the Kuna Indians, whom Kean had described over sixty years ago (2). Kean was in the U.S. Army stationed in the Canal zone in Panama, and many Kuna Indians worked in the canal zone. Kean was interested in the fact that their blood pressure was low and remained low even when they got old.
The Kuna Indians’ home on offshore islands on the Caribbean Coast of Panama has probably been their home for centuries, since the time of the Spanish Conquistadors (3). The isthmus of Panama must have been one of the worst places on earth for an indigenous people to live. Spain became the first world power when the tons of gold and silver stolen from the Inca Indians in what is now Chile and Peru made its way to Spain. Chile and Peru are on the Pacific side of the Americas. Spain is on the Atlantic side. How was the gold moved from the Pacific to the Atlantic side? It was not carried by the Conquistadors: The death rate among those pressed into service by the Spanish was very high. A number of Kuna made their way through the dense Darien jungle to the San Blas Islands in the Caribbean Sea. Not only were the Spanish not there, an additional dividend was that the islands are rocky, windswept, and dry. Thus, there are no mosquitoes. As their major health problems on the mainland were malaria, yellow fever, and dengue, and each has the mosquito as a major vector, these islands must have seemed like paradise. To make matters even better, a barrier reef separates most of the San Blas archipelago from the ocean, and thus the islands were inaccessible to Spanish Galleons.
In the early 1990’s we began collaboration with members of the Panamanian Ministry of Health who were involved in delivering care to the Kuna. Most of our collaborators are Kuna. We confirmed very quickly in several hundred Kuna volunteers who lived in their isolated indigenous home that hypertension was extremely uncommon, even among older individuals (4). This absence of hypertension did not reflect a low salt diet: data obtained from 24-hour recalls and 24-hour urine collections indicated that the intake of sodium and chloride exceeded most western populations (4). The data on blood pressure were so striking that we began to entertain the possibility that we were studying a Mendelianism dominant, a rare occurrence as a major player in a complex disease like hypertension.
If they really were genetically protected, when they moved to an urban environment they would remain normotensive. Their genes obviously had to travel with them. We were disappointed to learn that immigrants to an urban environment enjoyed all the benefits of modern western urban life. Hypertension had become common, and blood pressure now rose significantly with age. They were not protected by genes. The protective factor or factors are environmental. A major investment in studying environmental factors was initially unattractive. What probably played the largest role in our decision to continue was the actual magnitude of the difference in blood pressure between older indigenous Kuna and the “usual” blood pressure in most developed countries. In several hundred Kuna over the age of sixty years, the average systolic blood pressure was less than 110 mm Hg, and the average diastolic blood pressure was less than 70 mm Hg (4). While we know a large number of potential contributors to blood pressure from the diet, including intake of sodium, potassium, magnesium, calcium, soluble fiber, Omega-3 fatty acids, alcohol, protein, and calories, these individually have small effects. These individually have small effects, and even if one added them up, they would not likely account for a blood pressure below 110/70 mm Hg.
We examined a number of environmental candidates. One was stress, under the organizing principle that life in the San Blas was akin to living in paradise. Thus, we began to record measures of stress (5). The responsible factor did not appear to be stress. Another possibility is pollution, as there are no factories, no motorcars, and few engines in the San Blas islands. As in most urban settings, these sources of pollution are abundant in urban Panama. Unfortunately, there is no simple solution to quantifying these exposures and identifying responsible pollutants. This remains an area of potential future investigation.
(2) Diet
As diet is the major portion of our interaction with the environment under most circumstances, and is known to influence blood pressure (6); it seemed to be an obvious step in our efforts to identify putative factors. Thus, we undertook the task of measuring dietary intakes of individuals living their traditional lifestyle in the San Blas Islands and comparing estimates with those collected from Kuna who had emigrated to the mainland.
We employed both standard dietary assessment instruments and developed new instruments tailored to the Kuna (7, 8). We recognized the importance of involving our Kuna colleagues in all aspects of the study. One was a registered dietitian who is in charge of nutrition oversight in the San Blas. The comprehensive dietary assessment involved several steps. We were interested in assessing absolute dietary intakes as well as long-term, “usual” dietary intake. To achieve the former, we collected 24-hour recalls from both island and mainland-dwelling Kuna (4). The 24-hour recalls, supplemented with national dietary data and advice from our Kuna colleagues, helped us develop a Kuna-specific food frequency questionnaire to assess usual dietary patterns. Biospecimens and aliquots of Kuna foods and beverages complimented the self-reported intakes. Trained Kuna data collectors visited the homes of Kuna participants. Working with the “Jefe” (woman of the house), study staff collected samples of prepared foods, stews, and beverages and interviewed adult members of each family using a 24-hour recall and the Kuna-specific food frequency questionnaire (FFQ). Participants were also instructed on how to collect a 24-hour urine sample. With these tools in hand, we examined the diet of island-dwelling and mainland-dwelling Kuna.
Most aspects of the diet of the island-dwelling and mainland-urbanized Kuna were similar (8). Neither consumed substantial amounts of dairy products or green-leafy vegetables, two components of an overall diet pattern shown to lower blood pressure in U.S. study participants (9). The most striking difference in their diet, by far, was what they drank. The islands are dry, and water must be fetched by boat from a mainland river every day. As the water supply is somewhat uncertain, under most circumstances, the Kuna boil the water before they drink it. As there is no refrigeration, the drinking water is tepid: thus most Kuna, if not all, end up drinking considerable quantities of cocoa every day. Sugar intake is higher on the island as it is an ingredient in the beverages. They begin drinking cocoa when they are weaned and seem to stop drinking cocoa only when they die. From our surveys collected over time, we have consistently observed that the Kuna drink more than five cups of cocoa daily (7, 8). Kuna cocoa sources (home-grown and Columbian cocoa powder) were shown to be high in certain flavonoids, especially the flavanols and procyanidins (7, 8). We estimate that the Kuna consume approximately 1,880 mg per day. The several glasses of cocoa consumed daily by island-dwelling Kuna leads to what is probably the highest flavanol intake of any community on earth.
A second more modest difference in Kuna living in the islands and Kuna living in the mainland is the source of protein. On the islands, the source of protein is largely fish. On the mainland, the source is much more likely to be chicken than fish. Could omega-3 fatty acids contribute to the blood pressure difference? This seems unlikely to be important as the protein intake of the island-dwelling Kuna is extremely low (4). Even high doses of omega-3 fatty acids have only a modest effect on blood pressure.
(3) Chocolate, Cocoa, and Blood Pressure
Nothing could be simpler than feeding people chocolate and measuring their blood pressure. Despite that simplicity, there are a number of relevant variables. First, what is being administered exactly -- chocolate or cocoa? They are different. The Kuna partake in cocoa, not by highly-refined and derived confectionary product, chocolate, so commonly consumed in the developed world. What was the flavanol content of the chocolate and what dose is employed? Most commercially-available products do not have quantification of the flavanols in their label. Next, who are the subjects? Are they young or old, hypertension, or normotensive? For how long was cocoa or chocolate administration carried out? And, equally important, what is the control? Our studies employed flavanol-poor cocoa while others administered sham chewing or white chocolate to the control arm. These studies are disadvantaged by the necessary lack of double-blind administration and the implications of the placebo effect. Perhaps because of the many differences in study design, the effect of cocoa on blood pressure remains unproven. There are data to indicate that the onset of flavanol response to cocoa is gradual (10). We were not surprised to find no blood pressure influence of a high dose of unambiguously flavanol-rich cocoa in normotensive healthy subjects studied for less than a week (10). An identical comment applies to a recent report (11). In contrast, in healthy elderly subjects with hypertension, both cocoa and chocolate induced a fall in both systolic and diastolic blood pressure (12–14). Blood pressure also fell in overweight subjects given either dark chocolate or cocoa (15). Perhaps the most dramatic results were reported by Taubert, et al who demonstrated a significant blood pressure fall with as little as one square of chocolate a day in subjects with high-normal or mild hypertension (13). Recent meta analyses have confirmed a significant blood pressure fall (16, 17). In the even larger study in 470 elderly male men, cocoa intake was inversely related to blood pressure (18). Despite the absence of very large randomized controlled trials, the hints provided that Kuna appear to have been confirmed in a wide variety of clinical investigations.
How are we to use the information? Although it is possible that chocolate might become part of the medical model and be prescribed the way drugs are, that seems unlikely to us. A more likely candidate is the patient with “pre-hypertension” who is at risk of hypertension in the fairly near future and for whom drug treatment is not yet considered. In such patients a prudent diet is often recommended. We believe that it is reasonable to consider flavanol-rich chocolate or cocoa to be part of such a prudent diet (19).
(4) Nitric Oxide and Cardiovascular Health
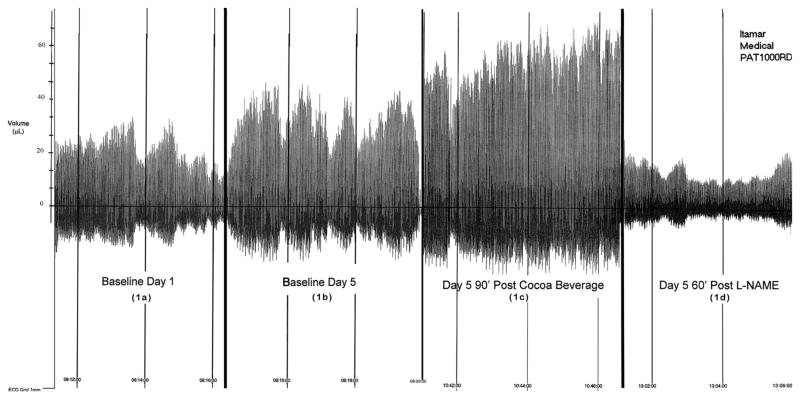
Of special importance was the discovery that the flavanols activated nitric oxide synthase in humans. Karim, et al (20) documented an influence of cocoa flavanols on endothelial function in the rabbit aorta. We extended that observation to the blood supply of the extremities in healthy humans (10). Flavanols induced vasodilation, which increased gradually to become striking after several days of cocoa ingestion. The cocoa “dose” employed was that calculated from our studies in the Kuna to provide about 900 mg of epicatechin per day. As the vasodilator response was reversed completely by arginine analogs that block nitric oxide synthesis, such as L-NAME, nitric oxide was the major, if not the sole, determinant of the vasodilator response (Figure 1). As flavanol-poor cocoa did not induce the vasodilator response, flavanols were the major determinant of the response (10).
Figure 1.
Vascular responses to cocoa and NG-nitro-L-arginine methyl ester (LNAME). (a) 5 minutes of baseline assessment in a volunteer. The baseline demonstrated normal variability in pulse wave amplitude. (b) After 4 days of ingestion of flavanol-rich cocoa by this volunteer, there was a clear increase in pulse wave amplitude measured in the morning more than 12 hours after the last dose of cocoa. (c) On that day, exposure to an additional 230 ml dose of flavanol-rich cocoa led to a further increase in pulse wave amplitude 90 minutes later. (d) After ingestion of cocoa on day 5, the nitric oxide synthesis inhibitor L-NAME had a dramatic effect in reversing dilatation.
*Reproduced from Fisher NDL, et al, J Hypertension 2003; 21:2281–6 (reference 10).
These studies were confirmed and extended in an elegant study by Schroeter, et al (21) who showed that purified epicatechin mimicked the effect of flavanol-rich cocoa in the same individuals, and showed a clear parallelism between nitric oxide metabolites, flavonoids, and the vasodilator response. They also confirmed the striking effect of arginine analogs on the response. They went on to examine the levels of circulating nitric oxide species and the levels of circulating flavanol metabolites. Statistical analysis identified both epicatechin (Figure 2) and its metabolite – epicatechin 7-O-glucuronide --as an important predictors of the vascular effects after flavanol-rich cocoa ingestion. Finally, there is a statistically significant correlation between the chronic conception of a cocoa flavanol-rich diet and augmented urinary excretion of NO metabolites: As the samples we collected from Kuna living either on the islands or the mainland, this observation closed the logic loop. These observations demonstrate that in humans the ingestion of the flavanol epicatechin is at least in part linked to the vascular effects observed after the consumption of flavanol-rich cocoa (21).
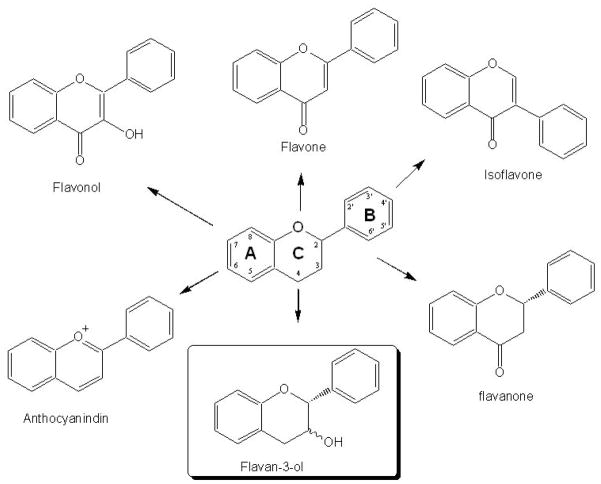
Figure 2.
The generic 3 ring (A, B, and C) carbon backbone structures (C6-C3-C6) of the major flavonoid subclasses found in foods (flavone, isoflavone, flavonol, anthocyanidin, flavanone, and flavanol) are depicted. The flavanol generic structure is highlighted.
*Reproduced from Robbins RJ, et al, J Cardiovascular Pharmacology 2006: 47 (Suppl 2):S110–S118 (reference 41).
Nitric oxide is a messenger molecule that performs diverse biologic functions, appears to occur in virtually every tissue, and impairment of nitric oxide bioactivity has been documented in a wide variety of diseases (22). Nitric oxide has been suggested to play a role in diseases of blood vessels, the heart, brain, lungs, liver, kidney, stomach, the immune system, musculoskeletal, and reproductive systems. The evidence in most cases has been rather slim involving measures of either nitric oxide synthetic activity or the effects of blockers. The discovery that flavanol-rich cocoa can stimulate nitric oxide synthesis provides a new and powerful approach to assessing the contribution of the system to disease and perhaps to treatment.
(5) Flavanols and Cardiovascular Disease Risk
The potential role of flavonoids in prevention of cardiovascular disease (CVD) and other diseases has been reviewed often (23–25). Several potential mechanisms for a protective effect of flavonoids, in particular the flavanols, include antioxidant, anti-inflammation, anti-platelet aggregation, and nitric oxide-mediated vascular changes (26).
Epidemiological evidence for an association between flavonoid intake, food sources, and cardiovascular health continues to grow. Over twenty observational studies, including ecological (27), cross-sectional (28), case-control (29, 30), and prospective cohort studies (27; 31–33) examined these relationships. Although the majority support a protective role, some studies have been negative, or even reversed (31–33).
A number of epidemiologic studies have specifically examined the relationship between flavan-3-ols or their food sources and CVD outcomes (24, 28, 29). Cross-sectional and hospital-based case-control studies suggest inverse associations between these compounds and various CVD outcomes, but have study design limitations. Mennon, et al (28) cross-sectionally evaluated the association between risk of CVD and specific flavonoid-rich foods. Statistically significant inverse associations were reported for both tea and chocolate. In a hospital-based case-control study in Greece, Lagiou, et al (29) reported a significant, 24% lower risk of CHD with a 21 mg increase in reported flavan-3-ol intake, primarily from wine and tea. In contrast, using a similar study design in Italy, Tavani et al (30) did not observe a linear trend in risk of acute myocardial infarction (AMI) with catechin and epicatechin intake, although multivariate-adjusted odds ratios were significantly inverse for intermediate intake levels. In this study, the mean intake of epicatechins and catechins was 55.6 mg. Although the potential for recall and selection biases limit the interpretation of case-control studies, it is doubtful that patients were aware of the flavanol (wine/tea/chocolate)-heart disease hypothesis.
Prospective studies have examined these relationships (33–35). Arts and colleagues (35) observed a significant inverse relationship between fatal ischemic heart disease, but not other CVD outcomes, among elderly Dutch men with highest vs. lowest catechin intakes who were followed for 10 years. They also found a non-statistically significant lower risk of CVD in the Iowa Women’s Health Study among women with a high intake of catechin-based compounds (24). Pears and wine were associated with significantly lower risk, but tea was not. Chocolate was inversely related with risk, but this finding was not statistically significant. “Chocolate” in this study included a variety of desserts that may not be concentrated in flavan-3-ols, and which may contain other less healthful ingredients. More recently, Buijsee et al (37) found that elderly European men in the highest tertile of cocoa intake had a 50% lower risk of CVD and total mortality, after controlling for several dietary and non-dietary CVD risk factors.
At the time of this review, only one prospective cohort study (33) had reported on the relationship between flavonoids and CVD mortality using the most recent USDA tables (US Department of Agriculture 34–36). After controlling for important confounders, including physical activity, obesity and smoking, Mink et al (33) found that anthocyanidins (most concentrated in berries, grapes, red wine, and some leafy and root vegetables) and flavanones (most concentrated in citrus fruits) were significantly inversely related to mortality from CVD, particularly coronary heart disease (CHD). No flavonoids were significantly inversely related to death from stroke. Neither flavan-3-ols nor proanthocyanidins were related to CVD risk or fatal stroke, fatal CHD or total mortality after multivariate adjustment. Median intakes across quintiles examined ranged from 4.2–182 mg/day for flavanols and 61.9 to 524 mg for proanthocyanidins, lower than typical intakes estimated in the island-dwelling Kuna. Chocolate consumption was minimally and non-significantly related to CVD and stroke mortality.
Several sources of between-study heterogeneity and measurement error likely contribute to inconsistencies in the observational study of flavonoids and health outcomes. Comprehensive nutrient databases with analytic values for flavonoid compounds have only recently become available (38–40), and more work is still needed to understand the effects of food processing on flavonoid content of foods and how to integrate this information into databases. The flavanol content in foods is highly variable, depending on processing and several other factors, and appropriate analytic methods are necessary for determining these levels with accuracy (41). Second, the range of flavan-3-ol intake varies considerably depending on the population. Intakes of monomeric flavan-3-ols in the U.S., Netherlands and Germany range from 11 (Germany) to 121 (U.S.) mg/day, and average proanthocyanidin intakes in the U.S. are estimated at 58 mg/day (41). Third, whether the intake levels estimated from FFQs or other dietary instruments capture the full range of intakes that may be important for cardiovascular disease prevention (or whether they correlate with true, higher levels associated with prevention) is likewise unknown. For example, FFQs used to assess usual dietary intakes in many epidemiological studies may not list items concentrated in specific flavonoids, such as dark chocolate, cocoa, berries or types of tea. It is also not clear whether dose response relationships between flavonoid intakes and CVD risk are linear across the entire range of consumption levels, or whether certain thresholds must be exceeded to observe an association. Finally, because foods high in flavanols usually contain other biologically active compounds and nutrients, it is difficult to identify the responsible factor/s with absolute certainty in observational studies.
(6) The Kuna, Nitric Oxide, and Patterns of Disease, and Longevity
The observation that flavanol-rich cocoa increased nitric oxide activity led to new questions. Do island-dwelling Kuna show more evidence of nitric oxide bioactivity than mainland dwellers? Does nitric oxide system activation in indigenous Kuna lead to a longer life or change the expression of disease (42)?
To ascertain whether or not the indigenous Kuna showed activation of nitric oxide bioactivity, we collected fresh urine samples from Kuna living in indigenous islands and who were heavy cocoa drinkers and compared them with urine samples drawn from Kuna who had migrated to the mainland and had a much lower intake of flavanol-rich cocoa (17). The samples were frozen on dry ice immediately and stored in that state until assay. Urinary levels of flavanol metabolites, expressed as epicatechin equivalents, were more than six times higher in island dwellers than in mainland inhabitants and urinary nitrate plus nitrite was two times higher in island dwellers than in mainland inhabitants.
Studies on natural history are inherently expensive, and so answering the next two questions – Did the island-dwelling Kuna live longer, and did they show a different pattern of disease – is not straightforward. Is it possible to have a general election without there being a census? We were in Panama during a general election several years ago, which prompted us to realize that there must be information from a census available (37) Indeed, there had been a census. From the age of 55 years on, there were twice as many island-dwelling Kuna, both men and women, than their age-matched equivalents on the Panamanian mainland: There was a large and very consistent greater proportion of older Kuna on the islands. Thus, the Kuna clearly are living longer.
Next we examined cause-specific mortality rates in Panama and specifically among the Kuna (38). Panamanian citizens cannot be legally buried without a death certificate. We used death certificates over five years to address the issue of patterns of death. We had identified four major causes of death that are thought to be sensitive to nitric oxide bioactivity, ischemic heart disease, stroke, diabetes mellitus, and cancer (21). On the mainland over those five years, cardiovascular disease was the leading cause of death (83.4 ± 0.7 age adjusted deaths per 100,000). Cancer stood second (68.4 ± 1.6). The cardiovascular deaths were made up almost equally between stroke and ischemic heart disease. Among island-dwelling Kuna both cardiovascular disease (9.2 ± 3.1) and cancer (4.4 ± 4.4) respectively were much lower. Similarly, deaths due to diabetes mellitus were much more common in the mainland (24.1 ± 0.7) than in island dwellers in the San Blas (6.6 ± 1.9). We cannot prove that the relationship between flavanol intake and lower cardiovascular disease and cancer mortality is causal or exclusively due to cocoa, but the data support the hypothesis that the very high flavanol intake and sustained nitric oxide activation protect the Kuna from common causes of morbidity and mortality.
These common chronic diseases are extraordinarily important. An alternative to death certificates as the primary source of information is required. We are in search of funds for that next step.
Conclusion
Observational studies cannot provide unambiguous evidence on the value of a specific food. However, available evidence suggests that the contribution of flavonoids to cardiovascular health and possibly to diabetes mellitus and cancer merits further pursuit. Clearly, a randomized, controlled clinical trial would be an important next step.
Acknowledgments
Grants and Other Support: M&M Mars, Inc.
We would like to acknowledge the helpful discussions that we have had with colleagues, including Malti Kelm, M.D., Helmet Sies, M.D., Hagen Schroeter, Ph.D., Catherine Kwik-Uribe, Ph.D., and Harold Schmitz, Ph.D.
Footnotes
Conflict of Interest: None
References
- 1.James GD, Baker PT. Human population biology and hypertension. Evolutionary and ecological aspects of blood pressure. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Management. Raven Press, Ltd; NY: 1995. pp. 115–27. [Google Scholar]
- 2.Kean BH. The blood pressure of the Kuna Indians. Am J Trop Med Hyg. 1944;24:341–43. [Google Scholar]
- 3.Howe J. Contemporary Village Politics in Panama. University of Texas Press; Austin, TX: 1986. The Kuna Gathering; pp. 9–11. [Google Scholar]
- 4.Hollenberg NK, Martinez G, McCullough M, Meinking T, Passan D, Preston M, Rivera A, Taplin D, Vicaria-Clement M. Aging, acculturation, salt intake, and hypertension in the Kuna of Panama. Hypertension. 1997;29:171–76. doi: 10.1161/01.hyp.29.1.171. [DOI] [PubMed] [Google Scholar]
- 5.Hollenberg NK, Mohres E, Meinking T, Preston M, Crespo B, Rivera A, Jackson L, Martinez G, Loken W. Stress and blood pressure in Kuna Amerinds. J Hypertension. 2005;7:714–20. doi: 10.1111/j.1524-6175.2005.04717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCullough M, Lin PH. Nutrition, diet, and hypertension. In: Coulston AM, Rock CL, Monsen ER, editors. Nutrition in the prevention and treatment of disease. Academic Press; San Diego, CA: 2001. [Google Scholar]
- 7.Chevaux KA, Jackson L, Villar ME, Mundt JA, Commisso JF, Adamson GE, McCullough ML, Schmitz HH, Hollenberg NK. Proximate, mineral, and procyanidin content of certain foods and beverages consumed by the Kuna Amerinds of Panama. J Food Comp Anal. 2001;14:553–63. [Google Scholar]
- 8.McCullough ML, Chevaux K, Jackson L, Preston M, Martinez G, Schmitz HH, Coletti C, Campos H, Hollenberg NK. Hypertension, the Kuna, and the epidemiology of flavanols. J Cardiovasc Pharmacol Suppl. 2006;47:103–39. doi: 10.1097/00005344-200606001-00003. [DOI] [PubMed] [Google Scholar]
- 9.Appel L, Moore T, Obarzanek E, Vollmer W, Svetkey L, Sacks F, Bray G, Vogt T, Cutler J, Windhauser M, Lin P, Karanja N, Simons-Morton D, McCullough M, Swain J, Steele P, Evans M, Miller E, Harsha D. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–24. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 10.Fisher NDL, Hughes M, Gerhard-Herman M, Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertension. 2003;21(12):1–6. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Engler MB, Engler MM, Chen CY, Malloy MJ, Browne A, Chiu EY, Kwak HK, Milbury P, Paul SM, Blumberg J, Mietus-Snyder ML. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr. 2004;23 (3):197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- 12.Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, Desideri G, Blumberg JB, Ferri C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46(2):398–405. doi: 10.1161/01.HYP.0000174990.46027.70. [DOI] [PubMed] [Google Scholar]
- 13.Taubert D, Berkels R, Roesen R, Klaus W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA. 2003;290 (8):1029–30. doi: 10.1001/jama.290.8.1029. [DOI] [PubMed] [Google Scholar]
- 14.Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, Blumberg JB, Ferri C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr. 2008;138(9):58–63. doi: 10.1093/jn/138.9.1671. [DOI] [PubMed] [Google Scholar]
- 15.Faridi Z, Nijike VY, Dutta S, Ali A, Katz DL. Acute dark chocolate and cocoa ingestionand endothelial function: a randomized controlled crossover trial. Am J Clin Nutr. 2008;88(1):58–63. doi: 10.1093/ajcn/88.1.58. [DOI] [PubMed] [Google Scholar]
- 16.Taubert D, Roesen R, Schomig E. Effect of cocoa and tea intake on blood pressure: a meta analysis. Arch Intern Med. 2007;167(7):626–34. doi: 10.1001/archinte.167.7.626. [DOI] [PubMed] [Google Scholar]
- 17.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich roods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 18.Buijsse B, Feskens EJ, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Arch Intern Med. 2006;166 (4):411–17. doi: 10.1001/archinte.166.4.411. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan NM. Treating prehypertension: A review of the evidence. Current Hypertension Reports. 2008;10:326–29. doi: 10.1007/s11906-008-0060-8. [DOI] [PubMed] [Google Scholar]
- 20.Karim M, McCormick K, Kappagoda CT. Effects of cocoa extracts on endothelium-dependent relaxation. J Nutr Suppl. 2000;130(8):2105–8. doi: 10.1093/jn/130.8.2105S. [DOI] [PubMed] [Google Scholar]
- 21.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proceedings of the National Academy of Sciences. 2006;103:1024–9. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollenberg NK. Organ systems dependent on nitric oxide and the potential for nitric oxide-targeted therapies in related diseases. J Clin Hypertension Suppl. 2006;4:63–73. doi: 10.1111/j.1524-6175.2006.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kris-Etherton PM, Keen CL. Evidence that antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr Opin Lipid. 2002;13:14–49. doi: 10.1097/00041433-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Arts ICW, Hollman PCH. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr Suppl. 2005;81:317–25. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 25.Erdman JWJ, Balentine D, Arab L, Beecher G, Dwyer JT, Folts J, Harnly J, Hollman P, Keen CL, Mazza G, Messina M, Scalbert A, Vita J, Williamson G, Burrowes J. J Nutr Suppl; Flavonoids and heart health: proceedings of the ILSI North America Flavonoids Workshop; May 31–June 1, 2005; Washington, D.C. 2007. pp. 718–37. [DOI] [PubMed] [Google Scholar]
- 26.Keen CL, Holt RR, Oteiza PI, Fraga CG, Schmitz HH. Cocoa antioxidants and cardiovascular health. Am J Clin Nutr Suppl. 2005;81:298–303. doi: 10.1093/ajcn/81.1.298S. [DOI] [PubMed] [Google Scholar]
- 27.Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, Pekkarinen M, Simic BS, Toshnima H, Feskens EJM, Hollman PCH, Katan MB. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995;155:381–6. [PubMed] [Google Scholar]
- 28.Mennen LI, Sapinho D, DeBree A, Arnault N, Bertrais S, Galan P, Hercberg S. Consumption of foods rich in flavonoids is related to a decreased cardiovascular risk in apparently healthy French women. J Nutr. 2004;134:923–6. doi: 10.1093/jn/134.4.923. [DOI] [PubMed] [Google Scholar]
- 29.Lagiou P, Samoli E, Lagiou A, Tzonou A, Kalandidi A, Peterson J, Dwyer J, Trichopoulos D. Intake of specific flavonoid classes and coronary heart disease—acase control study in Greece. Eur J Clin Nutr. 2004;58:1643–8. doi: 10.1038/sj.ejcn.1602022. [DOI] [PubMed] [Google Scholar]
- 30.Tavani A, Spertini L, Bosetti C, Parpinel M, Gnagnarella P, Bravi F, Peterson J, Dwyer J, Lagiou P, Negri E, La Vecchia C. Intake of specific flavonoids and risk of acute myocardial infarction in Italy. Public Health Nutr. 2006;9:369–74. doi: 10.1079/phn2006859. [DOI] [PubMed] [Google Scholar]
- 31.Rimm EB, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Relation between intake of flavonoids and risk for coronary heart disease in male health professionals. Ann Intern Med. 1996;125:384–9. doi: 10.7326/0003-4819-125-5-199609010-00005. [DOI] [PubMed] [Google Scholar]
- 32.Lin J, Rexrode KM, Hu F, Albert CM, Chae CU, Rimm EB, Stampfer MJ, Manson JE. Dietary intakes of flavonols and flavones and coronary hgeart disease in US women. Am J Epidemiol. 2007;165:1305–13. doi: 10.1093/aje/kwm016. [DOI] [PubMed] [Google Scholar]
- 33.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong C, Nettleton JA, Jacobs DRJ. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 34.Hertog MG, Sweetnam PM, Fehily AM, Elwood PC, Kromhout D. Antioxidant flavonols and ischemic heart disease in a Welsh population of men: the Caerphilly Study. Am J Clin Nutr. 1997;65:1489–94. doi: 10.1093/ajcn/65.5.1489. [DOI] [PubMed] [Google Scholar]
- 35.Arts ICW, Hollman PCH, Feskens EJM, Bas Bueno De Mesquita H, Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am J Clin Nutr. 2001;74:227–32. doi: 10.1093/ajcn/74.2.227. [DOI] [PubMed] [Google Scholar]
- 36.Arts ICW, Jacobs DRJ, Harnack LJ, Gross M, Folsom AR. Dietary catechins in relation to coronary heart disease death among postmenopausal women. Epidemiology. 2001;12:668–75. doi: 10.1097/00001648-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Buijsse B, Feskens EJ, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality. Arch Intern Med. 2006;166:411–7. doi: 10.1001/archinte.166.4.411. [DOI] [PubMed] [Google Scholar]
- 38.US Department of Agriculture. USDA—Iowa State University database on the isoflavone content of foods. 1999. [Google Scholar]
- 39.US Department of Agriculture. USDA database for the flavonoid content of selected foods. 2003. [Google Scholar]
- 40.US Department of Agriculture. USDA database for the proanthocyanidin content of selected foods. 2004. [Google Scholar]
- 41.Robbins RJ, Kwik-Uribe C, Hammerstone JF, Schmitz HH. Analysis of flavanols in foods: What methods are required to enable meaningful health recommendations? J Cardiovasc Pharmacol Suppl. 2006;47:110–18. doi: 10.1097/00005344-200606001-00004. [DOI] [PubMed] [Google Scholar]
- 42.Bayard V, Chamorro F, Motta J, Hollenberg NK. Does flavanol intake influence mortality from nitric oxide-dependent processes? Ischemic heart disease, stroke, diabetes mellitus, and cancer in Panama. Int J Med. 2007;4(1):53–8. doi: 10.7150/ijms.4.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sacks F, Obarzanek E, Windhauser M, Svetkey L, Vollmer W, McCullough M, Karanja N, Lin P, Proschan M, Appel L, Bray G, Vogt T, Moore T. Rationale and design of the dietary approaches to stop hypertension trial (DASH): A multicenter controlled feeding study of dietary patterns to lower blood pressure. Am J Epidemiol. 1995;5(2):108–18. doi: 10.1016/1047-2797(94)00055-x. [DOI] [PubMed] [Google Scholar]