Summary
Laboratory (LTLS) and clinical (CTLS) tumour lysis syndrome (TLS) are frequent complications in newly diagnosed children with advanced mature B cell non-Hodgkin lymphoma (B-NHL). Rasburicase, compared to allopurinol, results in more rapid reduction of uric acid in paediatric patients at risk for TLS. However, the safety and efficacy of rasburicase for the treatment or or prevention of TLS has not been prospectively evaluated. Children with newly diagnosed stage III–IV, bone marrow+ and/or central nervous system+ mature B-NHL received hydration and rasburicase prior to cytoreductive therapy. Rasburicase was safe and well-tolerated and there were no grade III–IV toxicities probably or directly related to rasburicase. Patients with an initial lactate dehydrogenase ≥2x upper limit of normal had a significantly elevated uric acid level (P=0.005), increased incidence of TLS (p-0.005) and lower glomerular filtration rate (GFR; p<0.001). Following rasburicase, there was only a 9% and 5% incidence of LTLS and CTLS, respectively. Furthermore, there was a significant improvement in estimated GFR from Day 0 to Day 7 following rasburicase (p=0.0007) and only 1.3% of patients required new onset renal assisted support after rasburicase administration. A TLS strategy incorporating rasburicase prior to cytoreductive chemotherapy proved safe and effective in preventing new onset renal failure and was associated with a significant improvement in GFR.
Keywords: Rasburicase, Tumour lysis syndrome, Burkitt lymphoma, Diffuse large B-cell lymphoma, Paediatric
Introduction
Mature B cell non-Hodgkin lymphoma (B-NHL) in children and adolescents often presents with advanced stage disease, Burkitt histology, rapid cell proliferation, and a significant degree of spontaneous apoptosis (Miles, et al 2012). Tumour lysis syndrome (TLS) is a metabolic oncological emergency resulting from tumour cell breakdown characterized by hyperuricaemia, hyperkalaemia, hyperphosphataemia, and hypocalcaemia that can result in renal insufficiency/failure, cardiac arrhythmias, neurological dysfunction and even death (Hande and Garrow 1993, Hochberg and Cairo 2008). TLS has been reclassified into laboratory (LTLS) and clinical (CTLS) tumour lysis syndrome (Cairo and Bishop 2004). Children with Burkitt histology and advanced disease (Stage IV and Burkitt leukaemia) have a very high incidence of TLS, with up to 25% of patients who undergo induction chemotherapy requiring haemodialysis support (Bowman, et al 1996). Prior to the availability of non-recombinant urate oxidase in France and Italy, the standard of care for the prevention or treatment of TLS was allopurinol (Cairo and Bishop 2004, Hochberg and Cairo 2008). Allopurinol is a competitive inhibitor of xanthine oxidase, which blocks the formation of uric acid (UA) but has no effect on pre-formed UA. However, urate oxidase catabolizes UA to allantoin, a much more soluble molecule that is more readily excreted in the urine and does not require alkalization (Cairo and Bishop 2004, Hande and Garrow 1993, Hochberg and Cairo 2008). In the French-American-British/Lymphome Malins de Burkitt (FAB/LMB) 96 childhood and adolescent mature B-NHL trial, there was a difference in TLS prophylaxis and treatment between national cooperative groups, with European use of non-recombinant urate oxidase (Societe Francaise d’Oncologie Pediatrique [SFOP]) versus the American use of allopurinol (Children’s Cancer Group [CCG]) for TLS prophylaxis and treatment. Children and adolescents receiving the non-recombinant urate oxidase had significantly less renal insufficiency, TLS, and requirement for dialysis (Cairo, et al 2007).
In 2001, a recombinant urate oxidase (rasburicase), was found to be significantly better than allopurinol by reducing UA within 4 h and mean area-under-the-curve (AUC) exposure to UA and thereby resulting in improved renal function in children and adolescents with a wide variety of rapidly proliferative haematological malignancies (Goldman, et al 2001). However, only a few children and adolescents with advanced mature B-NHL were studied in this cohort of patients as well as a few with reported acute TLS at diagnosis (Goldman, et al 2001). Furthermore, there have been no prospective studies to date investigating the efficacy of rasburicase in the prevention and treatment of newly diagnosed LTLS or CTLS nor the grade of TLS in this ultra high-risk group of children and adolescents with advanced mature B-NHL (Cairo and Bishop 2004). The present study prospectively investigated the safety and efficacy of rasburicase in the prevention and treatment of LTLS and CTLS and the risk factors associated with LTLS and CTLS in children and adolescents with advanced stage mature B-NHL uniformly treated with chemoimmunotherapy.
Methods
General
This study, Children’s Oncology Group (COG) ANHL01P1, was a pilot trial that investigated the safety of rituximab (R) addition to the FAB/LMB 96 B4 backbone in children and adolescents with intermediate risk (stage III/IV) and advanced stage (bone marrow [BM] and/or central nervous system [CNS] involvement) B-NHL (Cairo, et al 2010a). Disease outcomes have been reported elsewhere (Cairo, et al 2010a, Goldman, et al 2013). Here, we report the results of the second primary objective, to assess the safety and efficacy of the addition of R to COP (cyclophosphamide, vincristine, prednisone ) (COP-R) reduction chemotherapy as well as to determine the incidence of LTLS, CTLS and grade of TLS prior to and following COP-R. The trial was open to all COG centres between June 2004 and June 2009, and was approved by each respective institutional review board. Patients were risk-stratified as either group B (stage III/IV) or C (BM and/or CNS involvement) as previously described (Cairo, et al 2007, Patte, et al 2007) and staged according to St. Jude classification (Murphy 1980). Parents or patients over 18 years provided informed consent. There were no study amendments regarding rasburicase.
Eligibility
Patients <30 years of age with newly diagnosed, CD20 positive B-NHL as classified by World Health Organization criteria (Swerdlow et al 2008) (including diffuse large B-cell lymphoma [DLBCL] and primary mediastinal B-cell lymphoma [PMBL]), Burkitt lymphoma, and high grade B-cell lymphoma were eligible. Slides were reviewed by central pathology. St. Jude staging and FAB risk group were determined as previously described (Cairo, et al 2007, Goldman, et al 2013). Only intermediate risk lymphoma (FAB Group B) with St. Jude stages III/IV and high risk Group C patients (BM ± CNS disease) were eligible for this study. UA, electrolytes, renal function, bilateral BM aspirate and diagnostic lumbar puncture were required prior to study entry.
Treatment
Chemotherapy
The chemotherapy backbone (Table I) was similar to that reported in the FAB/LMB 96 study (Cairo, et al 2007, Cairo, et al 2010a, Goldman, et al 2013, Patte, et al 2007). Group B and C therapy both consisted of an initial cytoreduction with COP, intrathecal methotrexate and hydrocortisone (age-based) for Group B patients, and intrathecal methotrexate, hydrocortisone, and cytarabine for Group C patients. Rasburicase was only given during the initial cytoreductive phase. The study was registered with clinicaltrials.gov (NCT00057811).
Table I.
COP-R Schema used in this study
| Day | −3 | −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|---|---|
| Vincristine (1 mg/m2) | • | |||||||||
| Prednis(ol)one (60 mg/m2/day) | • • | • • | • • | • • | • • | • • | • • | |||
| Cyclophosphamide (300 mg/m2) | • | |||||||||
| Rasburicase**(0.2 mg/m2) | • ** | • ** | • ** | • | *** | *** | *** | *** | *** | |
| IT MTX/HC | • |
Prereduction (days −3, −2, −1): Patients with Hyperuricaemia (serum uric acid ≥475.8 μmol/l) and clinical suspicion of advanced B-cell NHL and/or B-ALL
A minimum of 1 dose rasburicase on Day 0(0°) and every 24 h for up to a maximum of 5 doses.
IT MTX/HC: intrathecal methotrexate and hydrocortisone
Rasburicase
Rasburicase (generously supplied by Sanofi, Bridgewater, NJ) was administered at a standard dose of 0.2 mg/kg intravenously diluted in 50 ml normal saline over 30 min. Patients with a history consistent with glucose-6-phosphate dehydrogenase deficiency were ineligible to receive rasburicase. Patients with hyperuricaemia (serum UA ≥ 475.8 μmol/l) and clinical suspicion of advanced B-cell NHL were eligible to receive 1–3 optional doses of rasburicase (Days −3, −2, −1) at the discretion of the treating physician prior to pathological and staging completion. All patients received at least a single dose of rasburicase prior to the start of treatment (Day 0). Optional doses were allowed on Day +1, +2, +3, +4, if needed. COP cytoreduction was required to begin within 4–24 h of Day 0 rasburicase administration. Hydration was initiated either at presentation or enrollment with 5% dextrose and one-quarter normal saline fluids, infused at 125 ml/m2/hwithout alkalinization (Cairo, et al 2010b, Coiffier, et al 2008).
Chemistry evaluation and definition of TLS
Baseline (before rasburicase) and subsequent daily UA, phosphorus, calcium, potassium and creatinine levels were required with documentation of renal support (i.e. dialysis and/or haemofiltration). Plasma UA levels and serum creatinine levels were required at the following time points: 0 h prior to Day −3, −2 or −1 dose of rasburicase (if applicable); 0 h (prior to 1st dose of rasburicase) on Day 0 therapy; 4 h (after 1st dose of rasburicase); 12 h (after 1st dose of rasburicase); 24 h (after 1st dose or prior to 2nd dose of rasburicase if needed); 36 h (after 1st dose or 12 h after 2nd dose of rasburicase if needed) and 48 h (after 1st dose or prior to 3rd dose of rasburicase if needed). All UA specimens were placed immediately on ice and tested within 4 h from collection. Baseline samples were collected for anti-rasburicase antibodies and only tested if patients developed a hypersensitivity reaction. TLS was classified and graded according to the Cairo-Bishop criteria (Cairo and Bishop 2004). Grade 0 = no TLS, grade 1 = LTLS only, grade 2–5 was LTLS plus clinical manifestations. Glomerular filtration rate (GFR) normalized to body surface area was estimated for each patient using the paediatric Schwartz formula as previously described (Schwartz, et al 1987).
Toxicity grading
Adverse events were documented using the NCI Common Terminology Criteria for Adverse Events v. 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf) and were deemed to be unrelated, unlikely, possible, probable, or definite in relation to therapy. Toxicity grading was as follows: 0 = No adverse event or within normal limits, 1 = mild adverse event, 2 = moderate adverse event, 3 = severe and undesirable adverse event, 4 = life-threatening or disabling adverse event, 5 = death related to adverse event. Adverse events were reported using the NCI’s Adverse Event Expedited Reporting System (NCI, Bethesda, MD, USA).
Statistical analysis
The second primary objective of COG ANHL01P1 was to assess the toxicity of the addition of rasburicase in combination with COP-R reduction chemotherapy. We also sought to determine the incidence of TLS, renal complications, and the use of assisted renal support (dialysis, haemofiltration), during the COP-R reduction phase. Toxicity was assessed after rasburicase administration until day 7 of reduction therapy. Where indicated, analysis was performed between: treatment groups, treatment groups subdivided by initial lactate dehydrogenase (LDH) level (<or ≥2x the upper limit of normal [ULN] for age and institution), initial LDH regardless of treatment group, and the presence or absence of hyperuricaemia (UA ≥475.8 μmol/l). All statistical comparisons were performed using Prism software (GraphPad) using either the 2-tailed unpaired t-test or the 2-sided Fisher’s exact test where indicated. P values of less than 0.05 were considered significant and actual values are indicated in each figure.
Results
Patient demographics
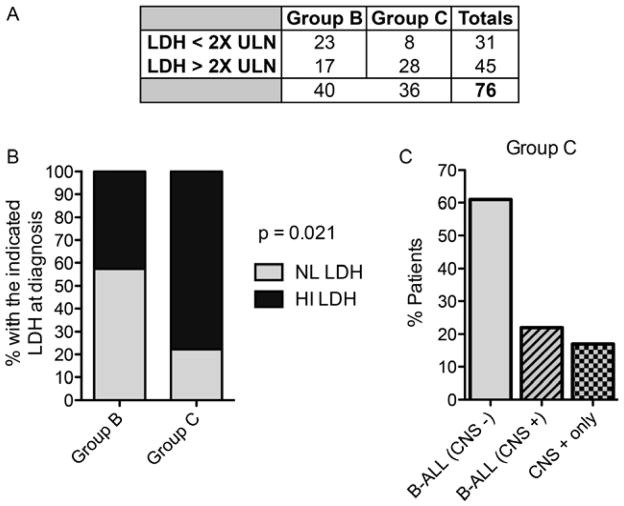
Seventy-six of the 85 patients entered on study had data evaluable for analysis. Twenty-three patients received Group B therapy with LDH < 2x ULN (“NL LDH”) and 17 with LDH ≥ 2x ULN (“HI LDH”); 8 were treated with Group C therapy with NL LDH, and 28 with HI LDH (Table II and Fig 1A). HI LDH levels occurred in 44% of patients in Group B vs. 75% in Group C (Fig 1B, p=0.021). Group C included 22 patients with CNS-negative Burkitt leukaemia, 8 with CNS-positive Burkitt leukaemia, and 6 with Burkitt lymphoma with CNS involvement (Fig 1C).
Table II.
Patient Characteristics
| Total | ||
|---|---|---|
|
| ||
| N | 76 | |
|
| ||
| Group, n (%) | Group B | 40 (53%) |
| Group C | 36 (47%) | |
|
| ||
| Gender, n (%) | Female | 15 (20%) |
| Male | 61 (80%) | |
|
| ||
| Age (years) | Mean, SD (range) | 10,1, 5.2 (1.1–23.1) |
|
| ||
| Age (years), n (%) | < 4 years | 9 (12%) |
| 4–15 years | 51 (67%) | |
| > 15 years | 16 (21%) | |
|
| ||
| Pathological diagnosis, n (%) | Burkitt lymphoma | 59 (78%) |
| DLBCL | 11 (14%) | |
| PMBL | 2 (3%) | |
| NOS | 4 (6%) | |
|
| ||
| St. Jude Stage, n (%) | III | 36 (47%) |
| IV | 40 (53%) | |
|
| ||
| LDH at reduction phase, n (%) | <2X ULN | 28 (38%) |
| ≥2X ULN | 45 (62%) | |
SD, standard deviation; DLBCL, diffuse large B-cell lymphoma; PMBL, primary mediastinal B-cell lymphoma; NOS, not otherwise specified; LDH, lactate dehydrogenase; UNL, upper limit of normal.
Figure 1. Patient characteristics.
A. Numbers of patients in each treatment group stratified by normal lactate dehydrogenase (NL LDH; < 2x upper limit of normal [ULN]) or high LDH (HI LDH; > 2x ULN) initial LDH levels. B. Incidence of increased LDH by treatment group. C. Incidence of Group C treatment group stratified by bone marrow only [B-ALL (CNS−)], central nervous system (CNS) only and combined [B-ALL (CNS+)].
Administration and safety of rasburicase
All patients received at least one dose of rasburicase prior to COP reduction and 37/77 received optional additional doses either before or after COP for a total of 143 doses (mean 1.5 doses/patient, range 1–6). Toxicities deemed to be possibly related to rasburicase included grade 1–3 nausea, vomiting, diarrhoea, headache, and anorexia. All episodes of hypersensitivity during cytoreduction were reviewed three reactions were identified: 1) a grade 3 allergic reaction on day 6 that was attributed to intravenous contrast material; 2) a grade 1 reaction on day 2 of unclear cause; 3) a grade 2 allergy noted on day 0. There were no toxicities definitely or probably attributable to rasburicase. There were no deaths during the reduction phase.
Incidence and development of LTLS and CTLS and grading of TLS
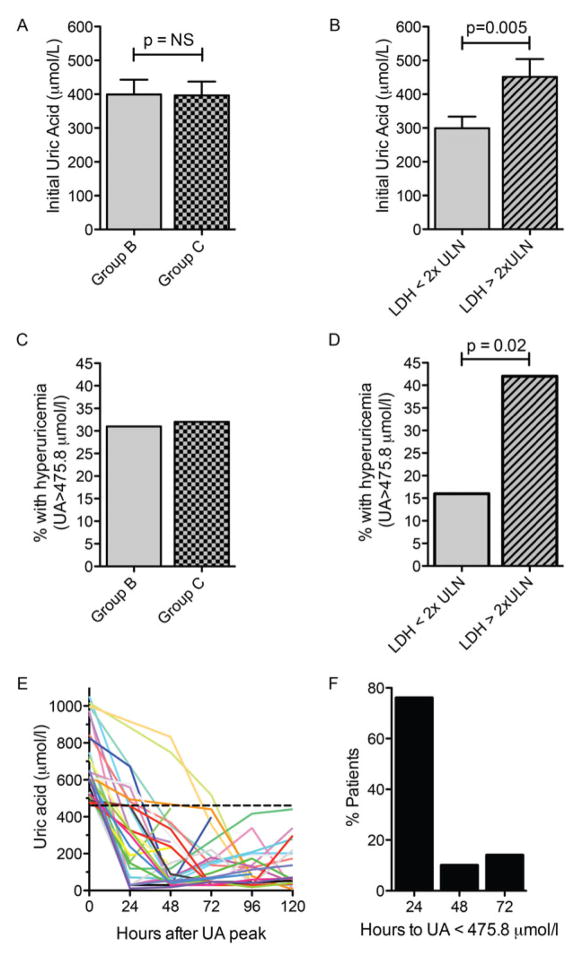
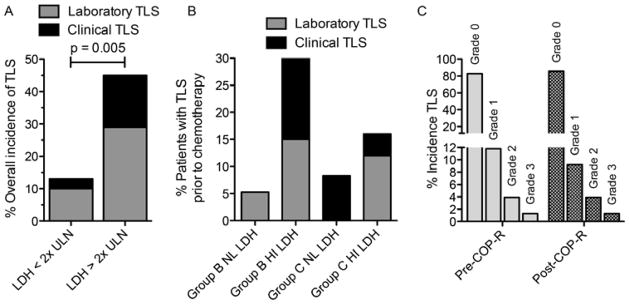
Hyperuricaemia (>475.8 μmol/l) was common, even prior to starting COP cytoreduction with no differences between Group B versus Group C patients, though there was a higher incidence of hyperuricaemia in patients with elevated LDH (p=0.005) (Fig 2A – D). Mean initial UA levels for Group B and NL LDH, Group C and NL LDH, Group B and HI LDH, Group C and HI LDH were 303±196, 291±196, 488±303, and 446±250 (μmol/l ± standard deviation) (p=0.02), respectively. There were 29 discrete episodes of hyperuricaemia in 25 patients. Peak daily UA levels dropped below 475.8 μmol/l within 1 day of receiving rasburicase in 25/29 (86%), and within 72 h for all (100%) (Fig 2E, F). The overall incidence of TLS was 32%, with an incidence of 21% LTLS and 11% of CTLS. There was a strong association between the development of TLS and elevated initial LDH, with 13% of those with NL LDH versus 45% of those with HI LDH developing TLS (Fig 3A; p = 0.005). The overall incidence of spontaneous TLS prior to COP-R was 17% (12% LTLS + 5% CTLS). The incidence of spontaneous TLS was much higher in those patients with high LDH (20%; 13% LTLS + 7% CTLS) compared with patients with normal LDH (6%; all LTLS) (Fig 3B). According to the Cairo-Bishop classification of TLS (Cairo & Bishop 2004), there were 12% of patients with grade 1 (LTLS only), 4% grade 2, and 1% grade 3 prior to COP-R. Most CTLS cases were defined by renal impairment (RI), however one patient had a seizure secondary to severe hypocalcemia (Fig 3C). The incidence of new onset TLS after COP-R was only 14% (9% LTLS + 5% CTLS; resulting in 9% with grade 1, 4% grade 2, and 1% grade 3 TLS). New onset hyperuricaemia following COP-R was uncommon.
Figure 2. Incidence and magnitude of hyperuricaemia.
A. Mean ± standard error of the mean (SEM) uric acid (UA) levels at initial measurement in each treatment group. B. Mean ± SEM initial UA values and associated initial lactate dehydrogenase (LDH) values. LDH <2x upper limit of normal (ULN) vs. LDH ≥2x ULN, p = 0.005 (unpaired t test). C. Incidence of hyperuricaemia in each treatment group. D. Incidence of hyperuricaemia associated with initial LDH values. LDH <2x ULN vs. LDH ≥2x ULN, p = 0.02 (Fisher’s exact test). E. Time course of UA level decline for all 29 episodes of hyperuricaemia in 25 patients. Time 0 represents the time of peak UA. The dashed line represents a level of 475.8 μmol/l – the cut-off point for hyperuricaemia. F. Percentage of episodes of hyperuricaemia that resolve within 24, 48, or 72 h after rasburicase administration.
Figure 3. Incidence of tumour lysis syndrome in all analysis groups.
A. Incidence of laboratory tumour lysis syndrome (LTLS) and clinical tumour lysis syndrome (CTLS) associated with initial lactate dehydrogenase (LDH) values. LDH <2x upper limit of normal (ULN)vs. LDH ≥2x ULN, p = 0.005 (Fisher’s exact test). B. Incidence LTLS or CTLS by treatment group and LDH values. C. Incidence of LTLS, or CTLS at any time prior to the initiation or following chemotherapy (COP-R; cyclophosphamide, vincristine, prednisone, rituximab).
Management of renal impairment
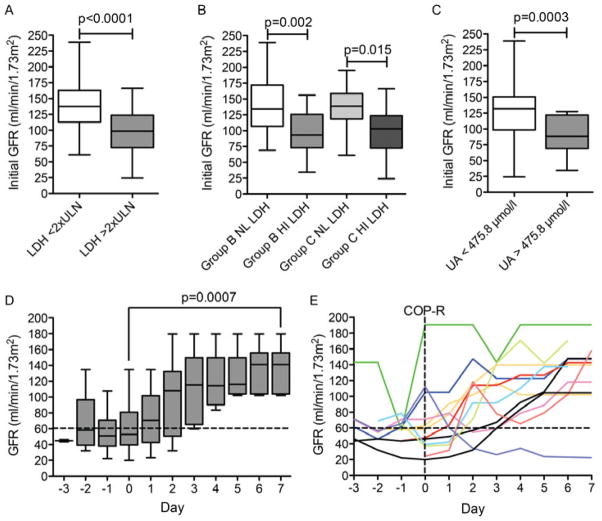
A total of 15 patients (20%) experienced RI (estimated GFR <60 ml/min/1.73m2) during the reduction phase (5 Group B, 10 Group C). Eleven patients (15%) experienced RI prior to COP-R, with only 4 patients (5%) developing RI after rasburicase, none of whom had hyperuricaemia. There was a highly significant association of decreased estimated GFR with an elevated LDH, mean initial GFR with elevated LDH (across all treatment groups) of 99 vs. 138 ml/min/1.73m2 for normal LDH (p<0.0001; Fig 4A, B). There was an inverse association between UA and GFR, with a mean initial GFR of 90 vs. 127 ml/min/1.73m2 for those with elevated vs. normal UA, respectively (p=0.0003; Fig 4C).
Figure 4. Incidence and time course of renal impairment.
A. Mean estimated glomerular filtration rate (GFR) at initial presentation in those with normal or elevated lactate dehydrogenase (LDH). The horizontal line represents the mean, the box represents the 25th–75th percentile, and the bars represent the minimum and maximum values. LDH <2x upper limit of normal (ULN) vs. LDH ≥2x ULN, p < 0.0001 (unpaired t test). B. Mean estimated GFR at initial presentation in all analysis groups. P value calculated with the unpaired t test. Group B LDH <2x ULN vs. LDH ≥2x ULN, p < 0.002 (unpaired t test). Group C LDH <2x ULN vs. LDH ≥2x ULN, p < 0.015 (unpaired t test). C. Mean estimated GFR at initial presentation in those with normal or elevated uric acid (UA). UA <475.8 μmol/l vs. ≥475.8 μmol/l p = 0.0003. D. Mean estimated GFR for patients with initial GFR < 60 ml/min/1.73m2 over the observation period. The box and whiskers are defined as in (a). The dashed line represents a GFR of 60 ml/min/1.73m2. P = 0.0007 (unpaired t test) comparing day −1 to day 7. E. Daily estimated GFR for individual patients with renal impairment any time before COP-R (cyclophosphamide, vincristine, prednisone, rituximab). The dashed line indicates 60 ml/min/1.73m2.
In patients with RI, we observed a steady improvement in estimated GFR by day 7 of COP-R, from an average GFR of 55 ml/min/1.73m2 on day −1 up to an average of 136 ml/min/1.73m2 on day 7 following COP-R (p=0.0007; Figs 4D,E). Only one patient had persistent RI by day 7. Assisted renal support (ARS) was required in six patients (8%), all of whom had high LDH at diagnosis. Five of the six patients required ARS prior to COP-R, with only one requiring new ARS after COP-R. One patient (group B therapy) required the initiation of haemodialysis following COP-R due to severe hyperphosphataemia with accompanying decreased GFR, but without hyperuricaemia. Overall, four patients required continuous veno-venous haemofiltration and two required haemodialysis but five out of the six were off ARS by day 7 of COP-R.
Discussion
This study demonstrated that rasburicase was safe and effective in children and adolescents with newly diagnosed advanced mature B-NHL at risk for TLS. Following rasburicase administration, 80% and 100% of patients with hyperuricaemia had a reduction in UA levels to below 475.8 μmol/l within 24 h and 72 h, respectively. Consequently, in the patient cohort of children with advanced mature B-NHL, there was only a 9% and 5% incidence of LTLS and CTLS, respectively. Most importantly, rasburicase administration was associated with a significant improvement in the estimated GFR between day 0 and day 7 in patients with pre-existing RI (GFR 55 ml/min/1.73m2 vs. 136 ml/min/1.73m2 [p<0.007]).
Prior to intensive induction therapy, FAB-based therapy has historically employed a low-dose cytoreductive (prophase) to specifically control metabolic derangements, particularly in the development of TLS (Cairo, et al 2007). The availability of agents, such as Rasburicase, that are more effective than allopurinol in rapidly lowering UA levels with an acceptable toxicity profile in paediatric patients at risk of TLS are of particular interest because of the impact that they may have on shortening treatment by removal of the prophase cycle as well as decreasing the overall incidence of TLS (Goldman, et al 2001). The COG ANHL01P1 trial provided a study with uniform chemotherapy and supportive care schema that allowed us to clearly demonstrate the safety of recombinant urate oxidase (rasburicase) during the COP cytoreduction phase and reduced need for new onset renal support in 99% of these high-risk patients. Original reports using Aspergillus-derived non-recombinant urate oxidase in patients at risk of TLS, demonstrated a 5–10% incidence of severe allergic reactions (bronchospasm and urticarial) requiring exclusion of patients with severe atopy and/or asthma (Goldman, et al 2001, Pui, et al 2001, Pui, et al 1997). In the current study we had no reports of significant allergic reactions or grade 3–4 toxicity probably or directly attributed to rasburicase.
Our study also provides prospective data on the incidence and timing of hyperuricaemia and TLS in children and adolescents with advanced mature B-NHL treated with a cytoreductive therapy regimen. We observed a uniformly striking incidence of spontaneous hyperuricaemia, LTLS (12%) and CTLS (5%) prior to cytoreductive therapy. There was a significant association of an elevated UA and spontaneous TLS in patients with an elevated LDH. Rasburicase caused rapid reduction in UA levels, and new onset hyperuricaemia was uncommon following cytoreduction therapy. In our cohort, the overall incidence of any TLS was 32%, with an 11% incidence of CTLS.
Direct comparison of our data with prior studies is difficult due to differences in definitions, data collection, and reporting. One analysis of TLS in high grade NHL showed an overall incidence of 42% with 6% clinically significant TLS, but this analysis was done without uniform consensus on the classification, grading or management of TLS (Hande and Garrow 1993). More recently, Cairo et al. (2007) reported a rate of 18% TLS in children and adolescents with BM ± CNS mature B-NHL treated with Group C FAB/LMB therapy (which is identical to the chemotherapy backbone used in the current study) following allopurinol prophylaxis and treatment versus 11% in the current study with rasburicase (Cairo, et al 2007). The lack of a more dramatic difference in TLS from the former study versus the current study may be due to adoption of the Cairo-Bishop criteria that differentiate laboratory versus clinical TLS in this trial (only patients with CTLS were reported in the FAB/LMB trial). However, FAB/LMB patients enrolled in France (SFOP) were routinely treated with non-recombinant urate oxidase while those enrolled in the US (CCG) and United Kingdom Children’s Cancer Study Group (UKCCSG) did not have access to this agent, with significant differences in TLS, RI, and need for ARS (Cairo, et al 2007). The reported TLS rate (26%) in the CCG (no rasburicase) cohort of the earlier trial that only utilized allopurinol prophylaxis and treatment, supports the efficacy of rasburicase in preventing CTLS as demonstrated in this current trial. Historical comparisons of patients with advanced stage Burkitt lymphoma/leukaemia at high risk of TLS receiving treatment prior to the availability of urate oxidase versus those who received it, showed that among 78 patients with TLS, a decrease in CTLS and anuria from 9.2% to 6.2% after introduction of urate oxidase, was even more pronounced (15.4% vs 3.8%, p=0.03) in the B-ALL patients (Wossmann, et al 2003).
Goldman et al. (2001) previously demonstrated that rasburicase is rapidly effective in lowering UA in children and adolescents with B-NHL with baseline hyperuricaemia, with 86% and 100% of patients achieving normal UA levels by 1 and 3 days post-treatment, respectively, and is effective in preventing (i.e. prophylaxis) new onset hyperuricaemia in children and adolescents with normal baseline UA. The results in the study are identical, with 86% and 100% achieving normal UA levels after 24 and 72 h of rasburicase, respectively. The results in this study, which were performed at 200 institutions, confirm the initial pilot study by Goldman et al. (2001). Rasburicase leads to conversion of UA to allantoin, which does not require urine or systemic alkalinization for excretion. This may be advantageous because high urine pH promotes kidney precipitation of xanthine and calcium/phosphorus crystals (Jeha 2001). Phosphorus and potassium cellular release and levels are not affected by rasburicase, making it critical to monitor and provide vigorous hydration (approximately 3 l/m2/d), to promote excretion (Coiffier, et al 2008). In fact, 4 (5%) patients in this current study developed RI after rasburicase administration and none of them were associated with hyperuricaemia. Therefore, various methods of potassium and phosphorus binding, electrocardiogram monitoring, and early institution of dialysis is critically necessary to avoid and/or treat renal failure, arrhythmia or neurological symptoms despite normalization of UA levels (Cairo, et al 2010b, Coiffier, et al 2008).
This study demonstrates the strong association between elevated LDH at diagnosis and the incidence of TLS and hyperuricaemia. Those with an initial LDH greater than twice the institutional ULN were over 3 times more likely to develop TLS, and 5 times more likely to develop CTLS. Although the groups are too small for statistical analysis, only 1/28 (4%) patients with normal LDH in our cohort had any form of TLS, compared with 7/43 (16%) with elevated LDH, suggesting that LDH is important in predicting high risk for TLS, as previously suggested in a high-risk cohort of patients with Burkitt lymphoma/leukaemia (Seidemann, et al 1998, Wossmann, et al 2003). It is also important to note that 16% of patients with normal LDH levels at diagnosis experienced hyperuricaemia, underscoring the importance of prophylaxis.
Rasburicase is effective at reducing UA, and preventing hyperuricaemia following chemotherapy. However, it is important to recognize that the high rate of spontaneous TLS in this population makes it impossible to entirely “prevent” TLS and there is a baseline rate of TLS and RI in children with advanced mature B-NHL not impacted by prophylactic measures. This underscores the importance of urgent medical attention and laboratory evaluation in children and adolescents with suspected advanced mature B-NHL. We also note that not all patients with RI had hyperuricaemia, suggesting that rasburicase cannot completely prevent this CTLS morbidity. Limitations of our study include the relatively small patient cohort of different B-NHL subtypes precluding intergroup comparisons that were powered to detect only large changes in TLS rates. In summary, we report the first prospective study of the use of rasburicase in children with advanced mature B-NHL and the effect of rasburicase in the incidence of LTLS, CTLS and grade of TLS prior to and after cytotoxic therapy. We demonstrated that children with advanced mature B-NHL at diagnosis with elevated LDH levels have a significant risk of hyperuricaemia, TLS and RI. Approximately 17% of patients present with spontaneous TLS at diagnosis prior to prophylaxis and/or treatment of TLS. Rasburicase was effective at normalizing 86% and 100% of the UA levels in patients at 24 and 72 h, respectively, and prevented new hyperuricaemia-associated RI. RI, however, can still occur in approximately 5% of patients after rasburicase and cytoreduction therapy, precipitating severe hyperkalaemia and/or hyperphosphataemia and concomitant nephrotoxicity. Rasburicase should be strongly considered for the management of children with newly diagnosed advanced mature B-NHL, especially those with an initial elevation of LDH.
Acknowledgments
The authors would like to thank Erin Morris, RN, for her expert assistance in the preparation of this manuscript and James Lynch, PhD, for his assistance in the statistical methods.
Research support was provided in part by the Division of Cancer Treatment, National Cancer Institute, and National Institutes of Health, Department of Health and Human Services (COG) (CA98543-09 and CA98413-09), and the Pediatric Cancer Research Foundation.
Presented in part at the American Society of Hematology, San Diego, December 2011.
Footnotes
Author contributions
PG: analysed the data, wrote the paper
JH: analysed the data, wrote the paper
SP: performed the research
LH: performed the research
SG: designed the research study, performed the research, analysed the data, wrote the paper
MC: designed the research study, performed the research, analysed the data, wrote the paper
Conflict of interest: MSC is a consultant for Sanofi. All other authors declare no conflicts of interest.
References
- Bowman WP, Shuster JJ, Cook B, Griffin T, Behm F, Pullen J, Link M, Head D, Carroll A, Berard C, Murphy S. Improved survival for children with B-cell acute lymphoblastic leukemia and stage IV small noncleaved-cell lymphoma: a pediatric oncology group study. J Clin Oncol. 1996;14:1252–1261. doi: 10.1200/JCO.1996.14.4.1252. [DOI] [PubMed] [Google Scholar]
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3–11. doi: 10.1111/j.1365-2141.2004.05094.x. [DOI] [PubMed] [Google Scholar]
- Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton CR, Michon J, Weston C, Perkins SL, Raphael M, McCarthy K, Patte C. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109:2736–2743. doi: 10.1182/blood-2006-07-036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo MS, Lynch J, Harrison L, Perkins S, Shiramizu B, Gross TG, Sanger WG, Goldman S. Safety, kinetics and outcome following rituximab (R) in combination with FAB chemotherapy in children and adolescents (C+A) with stage III/IV (Group B) and BM+/CNS+ (Group C) mature B-NHL: A Children’s Oncology Group Report. Journal of Clinical Oncology. 2010a;28:9545. [Google Scholar]
- Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010b;149:578–586. doi: 10.1111/j.1365-2141.2010.08143.x. [DOI] [PubMed] [Google Scholar]
- Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26:2767–2778. doi: 10.1200/JCO.2007.15.0177. [DOI] [PubMed] [Google Scholar]
- Goldman S, Smith L, Anderson JR, Perkins S, Harrison L, Geyer MB, Gross TG, Weinstein H, Bergeron S, Shiramizu B, Sanger W, Barth M, Zhi J, Cairo MS. Rituximab and FAB/LMB 96 chemotherapy in children with Stage III/IV B-cell non-Hodgkin lymphoma: a Children’s Oncology Group report. Leukemia. 2013;27:1174–1177. doi: 10.1038/leu.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SC, Holcenberg JS, Finklestein JZ, Hutchinson R, Kreissman S, Johnson FL, Tou C, Harvey E, Morris E, Cairo MS. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood. 2001;97:2998–3003. doi: 10.1182/blood.v97.10.2998. [DOI] [PubMed] [Google Scholar]
- Hande KR, Garrow GC. Acute tumor lysis syndrome in patients with high-grade non-Hodgkin’s lymphoma. Am J Med. 1993;94:133–139. doi: 10.1016/0002-9343(93)90174-n. [DOI] [PubMed] [Google Scholar]
- Hochberg J, Cairo M. Tumor lysis syndrome: current perspectives. Haematologica. 2008;93:9–13. doi: 10.3324/haematol.12327. [DOI] [PubMed] [Google Scholar]
- Jeha S. Tumor lysis syndrome. Semin Hematol. 2001;38:4–8. doi: 10.1016/s0037-1963(01)90037-x. [DOI] [PubMed] [Google Scholar]
- Miles RR, Arnold S, Cairo MS. Risk factors and treatment of childhood and adolescent Burkitt lymphoma/leukaemia. Br J Haematol. 2012;156:730–743. doi: 10.1111/j.1365-2141.2011.09024.x. [DOI] [PubMed] [Google Scholar]
- Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin’s lymphomas: dissimilarities from lymphomas in adults. Semin Oncol. 1980;7:332–339. [PubMed] [Google Scholar]
- Patte C, Auperin A, Gerrard M, Michon J, Pinkerton R, Sposto R, Weston C, Raphael M, Perkins SL, McCarthy K, Cairo MS. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–2780. doi: 10.1182/blood-2006-07-036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pui CH, Relling MV, Lascombes F, Harrison PL, Struxiano A, Mondesir JM, Ribeiro RC, Sandlund JT, Rivera GK, Evans WE, Mahmoud HH. Urate oxidase in prevention and treatment of hyperuricemia associated with lymphoid malignancies. Leukemia. 1997;11:1813–1816. doi: 10.1038/sj.leu.2400850. [DOI] [PubMed] [Google Scholar]
- Pui C-H, Mahmoud HH, Wiley JM, Woods GM, Leveger G, Camitta B, Hstings C, Blaney SM, Relling MV, Reaman GH. Recombinant urate oxidase for the prophylaxis or treatment of hyperuricemia in patients With leukemia or lymphoma. J Clin Oncol. 2001;19:697–704. doi: 10.1200/JCO.2001.19.3.697. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- Seidemann K, Meyer U, Jansen P, Yakisan E, Rieske K, Fuhrer M, Kremens B, Schrappe M, Reiter A. Impaired renal function and tumor lysis syndrome in pediatric patients with non-Hodgkin’s lymphoma and B-ALL. Observations from the BFM-trials. Klin Padiatr. 1998;210:279–284. doi: 10.1055/s-2008-1043892. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of tumours of haematopoietic and lymphoid tissues. IARC Press; Lyon, France: 2008. [Google Scholar]
- Wossmann W, Schrappe M, Meyer U, Zimmermann M, Reiter A. Incidence of tumor lysis syndrome in children with advanced stage Burkitt’s lymphoma/leukemia before and after introduction of prophylactic use of urate oxidase. Ann Hematol. 2003;82:160–165. doi: 10.1007/s00277-003-0608-2. [DOI] [PubMed] [Google Scholar]