Hereditary hemorrhagic telangiectasia (HHT), previously known as Osler–Weber–Rendu syndrome, is an autosomal dominant vascular disorder with a variety of clinical manifestations. Mucocutaneous telangiectasias are characteristic of the entity, producing epistaxis and gastrointestinal bleeding that often result in an iron-deficiency anemia. However, life-threatening conditions such as hemorrhage or stroke are usually attributable to the presence of arteriovenous malformations (AVMs) in the pulmonary, cerebral, or hepatic circulations. Pulmonary arteriovenous malformations (PAVMs) are direct communications between the pulmonary arteries and pulmonary veins without an interposed capillary bed; PAVMs thereby serve as a potential conduit for paradoxical embolism that can cause cerebrovascular ischemia or cerebral abscess formation. Also, because PAVMs serve as a physiologic right-to-left shunt, patients can present with high-output heart failure, dyspnea, platypnea, and/or secondary polycythemia. Finally, the friability of the abnormal vasculature often causes PAVMs to increase in size over time or hemorrhage, resulting in hemoptysis or hemothorax. The technical complexity of PAVM embolotherapy and the clinical depth needed to adequately provide long-term follow-up care for the proband and their affected family members makes PAVM management of HHT patients an especially rewarding aspect of clinical interventional radiology.
Patient Selection
Epidemiological studies suggest that approximately 90% of patients with PAVMs have underlying HHT, although conversely PAVMs are present in only 30 to 50% of the HHT population.1 2 PAVMs usually manifest by the fifth decade of life, and risk of hemorrhagic complications are greatest during pregnancy. Unfortunately, clinical signs and symptoms of PAVMs are often absent before the development of complications. Therefore, international consensus guidelines recommend screening to detect treatable PAVMs in HHT patients at their first clinical evaluation.3 After a negative initial screening evaluation, repeat screening should be considered after puberty, within 5 years preceding pregnancy, after pregnancy, and every 5 to 10 years thereafter. Transthoracic echocardiography (TTE) using agitated saline (“echobubble study”) is a commonly employed method to screen for right-to-left shunts because of its lack of ionizing radiation exposure, and yields a sensitivity of 93% and specificity of 52% compared with “gold standard” tests (chest computed tomography [CT] or pulmonary angiography) for the detection of PAVMs.1 Of note, TTE has demonstrated a higher prevalence and larger size of right-to-left shunts in patients with HHT due to mutations in the endoglin (ENG) gene (i.e., HHT type 1 patients) compared with patients with mutations in the activin A receptor-like kinase (ACVRL1) gene (i.e., HHT type 2 patients).2 The higher prevalence of PAVMs in HHT type 1 patients (85 vs. 35%) has led some to suggest that chest CT be performed as the first-line screening examination in this subgroup of patients.
Although the natural history of PAVMs is difficult to delineate, mortality or significant morbidity due to stroke or cerebral abscess occurs in approximately 23% of patients with untreated PAVMs over a mean follow-up period of 4.5 to 10 years.4 Therefore, percutaneous image-guided embolotherapy is the recommended treatment for all adults and symptomatic children with PAVMs to prevent neurologic complications and hemorrhage. Symptomatic patients with hypoxemia may be treated to improve dyspnea and exercise tolerance. Recommendations are unclear for the treatment of PAVMs in asymptomatic children younger than 12 years, that is, children without dyspnea, exercise intolerance, growth delay, cyanosis or clubbing, or prior complications. Delaying treatment until an older age is an acceptable approach in asymptomatic children (assuming adequate follow-up is pursued) because of the propensity for new PAVMs to develop, for small PAVMs to enlarge, and for treated PAVMs to reperfuse over time.5
Preprocedural Evaluation
Transcatheter embolization has been adopted as the preferred method for occlusion of PAVMs in HHT patients, supplanting surgical techniques. Traditionally, a size cut-off of ≥ 3 mm for the feeding artery was advocated based on an observation that the risk of cerebral infarction was low when the size of the feeding artery was < 3 mm,6 as well as historical difficulty catheterizing arteries smaller than 3 mm in diameter. However, more recent data in a larger patient cohort have shown no evidence of association between PAVM feeding artery diameter (or any other measure of PAVM severity) and risk of ischemic stroke or cerebral abscess.7 Given the current armamentarium of microcatheters and embolic agents, many interventional radiologists now can and will preemptively treat any lesion identified by pulmonary angiography. Often, the patient prefers early treatment rather than extended follow-up of known PAVMs.
Careful evaluation of a preprocedural contrast-enhanced CT allows for (1) determination of the number and location of PAVMs; (2) identification of the number, location, and size of supplying arteries and draining veins; (3) the presence of thrombosis; and (4) location of any upstream side branches useful for anchoring coils. “Simple PAVMs” are supplied by one or more feeding vessels that arise from a single segmental artery, while “complex PAVMs” (∼ 10%) are supplied by vessels arising from multiple segmental arteries. A subset of complex AVMs (∼ 5% of them) are termed “diffuse AVMs” because of innumerable feeding vessels, often in a lobar distribution, and are a particular challenge to treat effectively. The interpreting radiologist should also understand that a steal phenomenon may occur in which accessory feeding arteries are not opacified on contrast-enhanced CT (or catheter-based pulmonary angiography) because of rapid, high flow from the dominant feeding artery into the low resistance pulmonary venous circulation. Also, sizing measurements to guide the choice of embolic device should be confirmed during catheter-based pulmonary angiography, as CT evaluation alone may cause an unacceptable underestimation of the diameter of the feeding artery. Embolic coils should be oversized at least 2 mm compared with the feeding artery diameter. Systemic arterial supply to PAVMs via the bronchial, inferior phrenic, musculophrenic, internal mammary, or intercostal arteries is not uncommon, particularly after prior treatment.8
In patients with bilateral PAVMs, one may choose to treat one lung at a time, selecting the lung with the largest or culprit lesion first, to minimize patient discomfort and radiation dose. The durability of the embolization procedure can then be assessed angiographically when the patient returns for treatment of the contralateral lung. Alternatively, embolization of numerous bilateral PAVMs can be performed during a single session on an outpatient basis, according to patient and physician preference.9
Procedure
A single prophylactic dose of 1 to 2 g of cephazolin is given within the hour before the procedure. Proper diligence should be exercised to ensure that no air or clot is introduced into the patient's blood circulation, and air filters should be applied to all intravenous lines. Intravenous heparin is administered before and during the procedure to minimize the risk of periprocedural paradoxical embolus (70–100 IU/kg bolus followed by additional heparin doses as determined by the operator). All wire and catheter exchanges should be performed under saline irrigation to avoid the potential introduction of air emboli. Regular flushing of catheters reduces the potential for clot embolization. Blood should always be aspirated before injection of contrast or saline via the catheter. Placement of a small sidehole within 1 cm of the end of the catheter allows blood aspiration in most cases. If blood cannot be aspirated after catheter manipulation, the catheter must be retracted until blood can be aspirated.
Technique
Through a femoral venous sheath, a 5-Fr Omni Flush catheter (AngioDynamics Inc, Queensbury, NY) is navigated into the main pulmonary artery.10 Pulmonary arterial pressure measurements should be obtained and if elevated, consultation with a pulmonary specialist is warranted. After positioning the flush catheter in the left and then right pulmonary arteries, digital subtraction pulmonary arteriograms are obtained at 4 to 6 frames/s in bilateral oblique projections with an injection rate of 8 to 10 mL/s using 16 to 20 mL of contrast. The pulmonary arterial catheter is exchanged over an exchange length wire for an 80-cm-long 7-Fr guiding sheath (White LuMax sheath, Cook Medical, Bloomington, IN).11 Proper placement of the guiding sheath is critical to ensure adequate stability and control during embolization. Through the guiding sheath, a 5-Fr end-hole catheter with an added sidehole is placed to select lower lobe PAVMs, particularly in the lateral segments. For medial lower lobe, lingular, or middle lobe PAVMs, use of a Cobra 2 catheter (Cook Medical) may ease subselection through the guiding sheath. The sheath provides excellent catheter stability to deploy larger Nestor (Cook Medical) coils accurately. Selection of upper lobe PAVMs may be difficult using this stiff guiding sheath, and other combinations of sheaths and catheters can be used. In these cases, a microcatheter set can be used and the authors' preference has been to use MicroNestor coils (Cook Medical), although detachable coils can also be used according to the familiarity of the operator.
The major technical hurdle of the procedure is safely deploying an embolic device in position to occlude the lesion despite the torrentially high flow that can encourage passage into the systemic arterial circulation. Several techniques, three of which are described below, have been developed to reduce the likelihood of nontarget embolization.
Anchor Technique
To keep the coil from embolizing distally, the proximal 2 cm of a long coil (10–14 cm) can be delivered into a side branch as close as possible to the aneurysmal sac. The catheter is withdrawn into the feeding artery, and the remainder of the coil is packed into a tight mass just proximal to the side branch (Fig. 1). After initial anchor coil deployment, additional coils can be delivered safely next to the anchoring coil to achieve cross-sectional occlusion.
Figure 1.
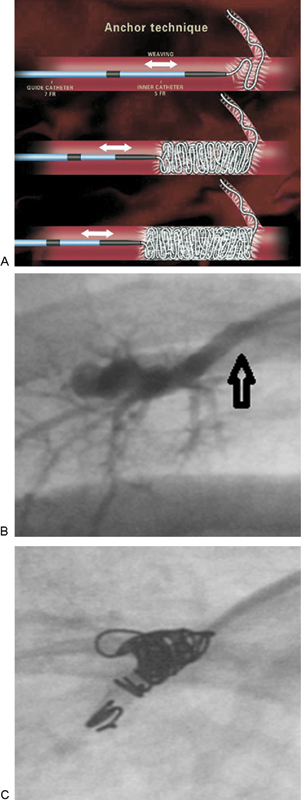
(A) Schematic demonstrating anchor coil technique to decrease the risk of distal migration of coils (reproduced with permission from White11) (B) A right lower lobe PAVM with a 3-mm feeding artery (arrow). (C) After deploying the first 2 cm of a 4-mm Nestor anchoring coil in a side branch, three additional coils were deployed safely. PAVM, pulmonary arteriovenous malformation.
Scaffold Technique
In high-flow PAVMs supplied by larger feeding vessels, one may first place a long, stainless steel coil with high radial force to create a matrix upon which to deploy smaller coils (Fig. 2). In higher risk situations, such as high-flow fistula-type PAVMs, a detachable coil can be used. Then, smaller high-radial force coils can be placed within the initial matrix. Finally, smaller and softer platinum coils (e.g., Nestor coils) can be placed to achieve complete cross-sectional occlusion.
Figure 2.
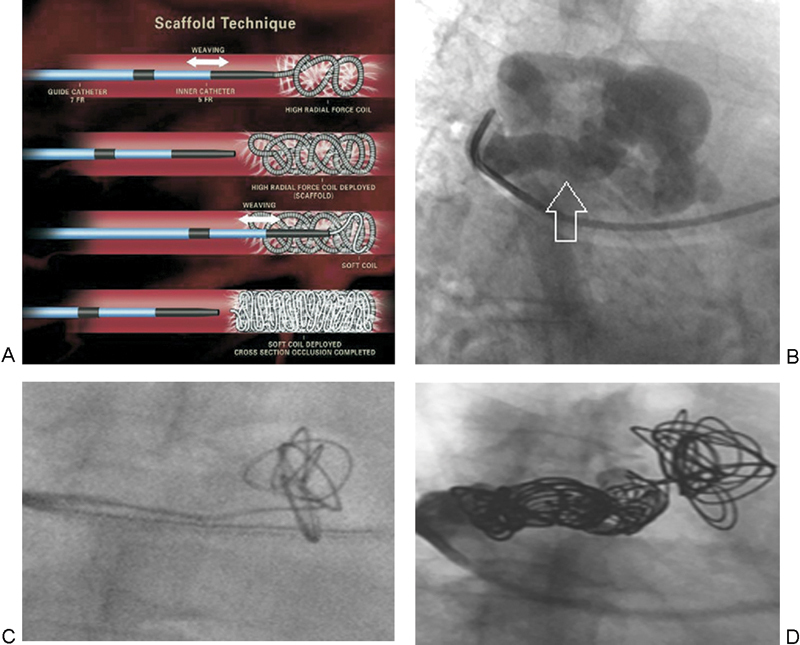
(A) Schematic demonstrating scaffold technique to decrease the risk of distal migration of coils (reproduced with permission from White11). (B) A right upper lobe PAVM with an 8-mm feeding artery (arrow). (C) A 10 mm × 26 cm stainless steel framing coil was deployed close to the aneurysm sac but within the feeding artery. (D) After deploying the framing coil, four additional coils were placed to tightly pack the matrix and achieve cross-sectional occlusion of the feeding artery. PAVM, pulmonary arteriovenous malformation.
Amplatzer Vascular Plug
The Amplatzer vascular plug (AGA Medical, Plymouth, MN) is a particularly useful tool for creating a wall in the distal feeding vessel behind which one may deploy multiple small coils (Fig. 3). The Amplatzer II device is available in sizes ranging from 4 to 16 mm, with the smaller sizes passing through a 5-Fr guiding catheter and the larger sizes requiring an 8-Fr guiding catheter. The Amplatzer Vascular Plug 4 has recently become available in the United States and can be delivered through a catheter with a 0.038 in lumen (5-Fr catheter). The manufacturer has specific sizing recommendations for each device. Perhaps the greatest advantage of using this device is the ability to retract it if sizing or positioning is not optimal. This is especially useful in light of data demonstrating that coil placement > 1 cm away from the aneurysm sac is associated with a higher rate of later recanalization.12 After deploying the initial Amplatzer plug, additional coils are usually placed behind the plug in the more proximal feeding artery to ensure complete embolization.13
Figure 3.
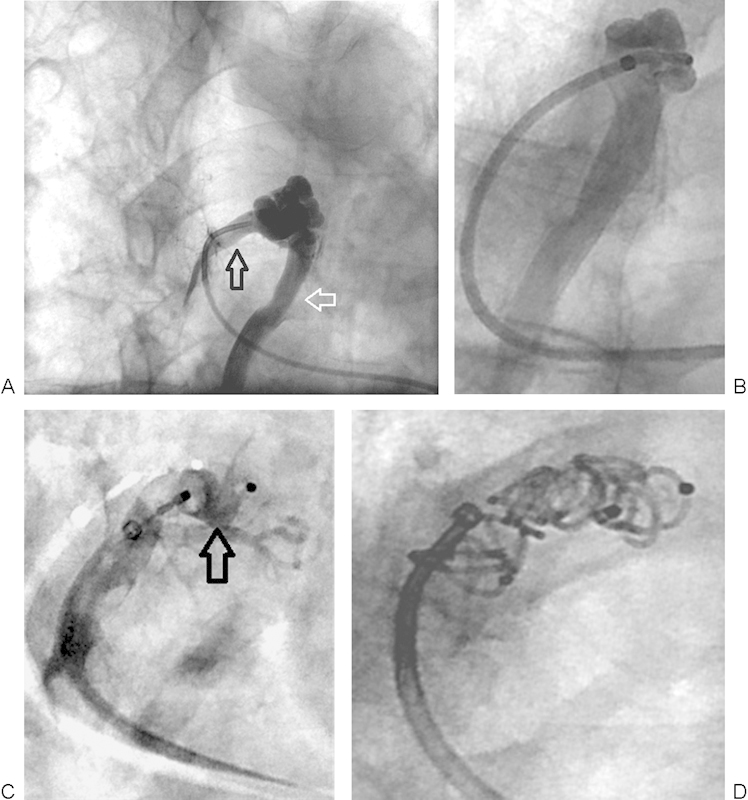
(A) Right upper lobe PAVM with 5-mm feeding artery (black arrow) and large draining vein (white arrow). (B) Successful negotiation of a 5-Fr guiding catheter into the distal feeding artery. The large draining vein is seen. (C) A 6-mm Amplatzer II vascular plug (arrow) was deployed close to the aneurysm sac but within the feeding artery. (D) After deploying the Amplatzer plug, five 5 mm × 5 cm coils were placed behind the plug to achieve cross-sectional occlusion of the feeding artery. PAVM, pulmonary arteriovenous malformation.
Postprocedural Evaluation
Immediate Complications
Self-limited pleurisy is the most common postprocedural complication, occurring in up to 10% of patients and attributable to thrombosis of the feeding artery and/or pulmonary infarction. The incidence of pleurisy is higher in patients who have large PAVMs with feeding vessels ≥ 8 mm. Pleuritic chest pain usually responds to a short course of nonsteroidal anti-inflammatory drugs (NSAIDs), although occasionally a course of prednisone is needed for pain control. However, the use of NSAIDs in HHT patients may worsen epistaxis, and this needs to be considered. More serious and uncommon immediate postprocedural complications are related to systemic arterial embolization of clot, air, or the embolic device, and occurs in < 2.3% of cases.14 Transient ischemic attacks, angina, or bradycardia are usually attributable to air embolization and are avoidable with the aforementioned precautions.
Imaging Follow-Up
Contrast echocardiography is not useful for follow-up, as it remains positive in up to 80 to 90% of patients despite successful occlusion of all angiographically visible vessels.15 This implies that patients remain at risk for endocarditis even after PAVM embolization, and should continue taking antibiotic prophylaxis for dental and other procedures that may result in transient bacteremia. If stainless steel coils were used, brain magnetic resonance imaging (MRI) (e.g., to screen for cerebral AVMs) should be deferred until 6 weeks after pulmonary embolotherapy to allow for coils to fibrose into position and minimize the chance of movement during the MRI. The potential for growth of new PAVMs and/or late recanalization of treated PAVMs necessitates follow-up imaging using contrast-enhanced chest CT 3 to 12 months following embolization and then every 5 years thereafter for the life of the patient.
Results
Successful embolization is evidenced by complete retraction of the PAVM on follow-up CT. Short-term improvement in dyspnea, arterial oxygenation, and New York Heart Association heart failure class is seen most often, and long-term reduction in risk of cerebral ischemia, cerebral abscess, hemoptysis, and hemothorax is expected.3 Reperfusion of treated PAVMs occurs in up to 15% of patients, and occurs by (1) recanalization of the embolized vessel, (2) growth of a missed or previously small accessory feeding artery, (3) bronchial or other systemic collateral flow into the feeding pulmonary artery beyond the level of the embolization, or (4) pulmonary artery to pulmonary artery collateral flow around the embolized vessel. Of these, recanalization is the most common mechanism of reperfusion. Variables associated with recanalization include embolization > 1 cm from the aneurysm sac, use of a single coil, and large feeding arteries.12 Therefore, suboptimal outcomes can be prevented by good coil packing technique, use of coils in conjunction with the Amplatzer II vascular plug,13 and embolizing as close to the fistula sac as possible, even extending into the origin of the outflow vein.16
Conclusion
Strategies for PAVM screening, characterization, and treatment have evolved significantly in recent years due to an enhanced understanding of the molecular and clinical determinants of PAVM presentation and behavior. Patients with HHT should be evaluated at least once in Centers of Excellence for HHT (HHT.org) for thorough screening and comprehensive HHT-related care; however, many HHT-related PAVMs can be treated at community hospitals thereafter. Clinical interventional radiologists play a pivotal role in caring for HHT patients and their families, and are well served by remaining up to date with the most current recommendations arising from the cumulative experience of physicians caring for this patient population.
References
- 1.Cottin V, Plauchu H, Bayle J-Y, Barthelet M, Revel D, Cordier J-F. Pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. Am J Respir Crit Care Med. 2004;169(9):994–1000. doi: 10.1164/rccm.200310-1441OC. [DOI] [PubMed] [Google Scholar]
- 2.van Gent M WF, Post M C, Snijder R J, Westermann C JJ, Plokker H WM, Mager J J. Real prevalence of pulmonary right-to-left shunt according to genotype in patients with hereditary hemorrhagic telangiectasia: a transthoracic contrast echocardiography study. Chest. 2010;138(4):833–839. doi: 10.1378/chest.09-1849. [DOI] [PubMed] [Google Scholar]
- 3.Faughnan M E, Palda V A, Garcia-Tsao G. et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet. 2011;48(2):73–87. doi: 10.1136/jmg.2009.069013. [DOI] [PubMed] [Google Scholar]
- 4.Gossage J R, Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med. 1998;158(2):643–661. doi: 10.1164/ajrccm.158.2.9711041. [DOI] [PubMed] [Google Scholar]
- 5.Faughnan M E, Thabet A, Mei-Zahav M. et al. Pulmonary arteriovenous malformations in children: outcomes of transcatheter embolotherapy. J Pediatr. 2004;145(6):826–831. doi: 10.1016/j.jpeds.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 6.Moussouttas M, Fayad P, Rosenblatt M. et al. Pulmonary arteriovenous malformations: cerebral ischemia and neurologic manifestations. Neurology. 2000;55(7):959–964. doi: 10.1212/wnl.55.7.959. [DOI] [PubMed] [Google Scholar]
- 7.Shovlin C L, Jackson J E, Bamford K B. et al. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax. 2008;63(3):259–266. doi: 10.1136/thx.2007.087452. [DOI] [PubMed] [Google Scholar]
- 8.Brillet P-Y, Dumont P, Bouaziz N. et al. Pulmonary arteriovenous malformation treated with embolotherapy: systemic collateral supply at multidetector CT angiography after 2-20-year follow-up. Radiology. 2007;242(1):267–276. doi: 10.1148/radiol.2421041571. [DOI] [PubMed] [Google Scholar]
- 9.Trerotola S O, Pyeritz R E, Bernhardt B A. Outpatient single-session pulmonary arteriovenous malformation embolization. J Vasc Interv Radiol. 2009;20(10):1287–1291. doi: 10.1016/j.jvir.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Velling T E, Brennan F J, Hall L D. Pulmonary angiography with use of the 5-F omniflush catheter: a safe and efficient procedure with a common catheter. J Vasc Interv Radiol. 2000;11(8):1005–1008. doi: 10.1016/s1051-0443(07)61330-x. [DOI] [PubMed] [Google Scholar]
- 11.White R I Jr. Pulmonary arteriovenous malformations: how do I embolize? Tech Vasc Interv Radiol. 2007;10(4):283–290. doi: 10.1053/j.tvir.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Milic A, Chan R P, Cohen J H, Faughnan M E. Reperfusion of pulmonary arteriovenous malformations after embolotherapy. J Vasc Interv Radiol. 2005;16(12):1675–1683. doi: 10.1097/01.RVI.0000182163.25493.BB. [DOI] [PubMed] [Google Scholar]
- 13.Trerotola S O, Pyeritz R E. Does use of coils in addition to Amplatzer vascular plugs prevent recanalization? AJR Am J Roentgenol. 2010;195(3):766–771. doi: 10.2214/AJR.09.3953. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson J W Hall I P Hubbard R B Fogarty A W McKeever T M Peri-Procedural Complications Associated with Transcutaneous Embolisation for Pulmonary Arteriovenous Malformations: A Systematic Review and Meta-Analysis 10th HHT Scientific Conference, Hematology Reports 3 2013;(Suppl 1):34–35
- 15.Lee W L, Graham A F, Pugash R A. et al. Contrast echocardiography remains positive after treatment of pulmonary arteriovenous malformations. Chest. 2003;123(2):351–358. doi: 10.1378/chest.123.2.351. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi S Baba Y Senokuchi T Nakajo M Efficacy of venous sac embolization for pulmonary arteriovenous malformations: comparison with feeding artery embolization J Vasc Interv Radiol 201223121566–1577., quiz 1581 [DOI] [PubMed] [Google Scholar]


