Abstract
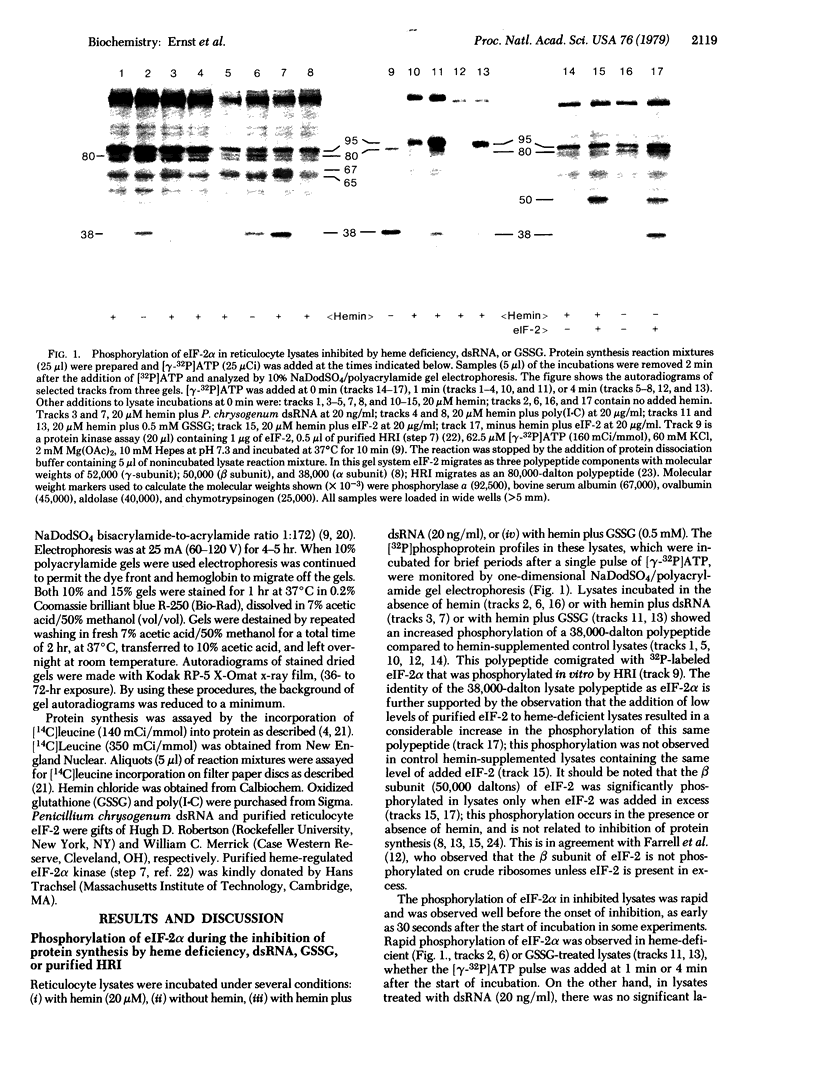
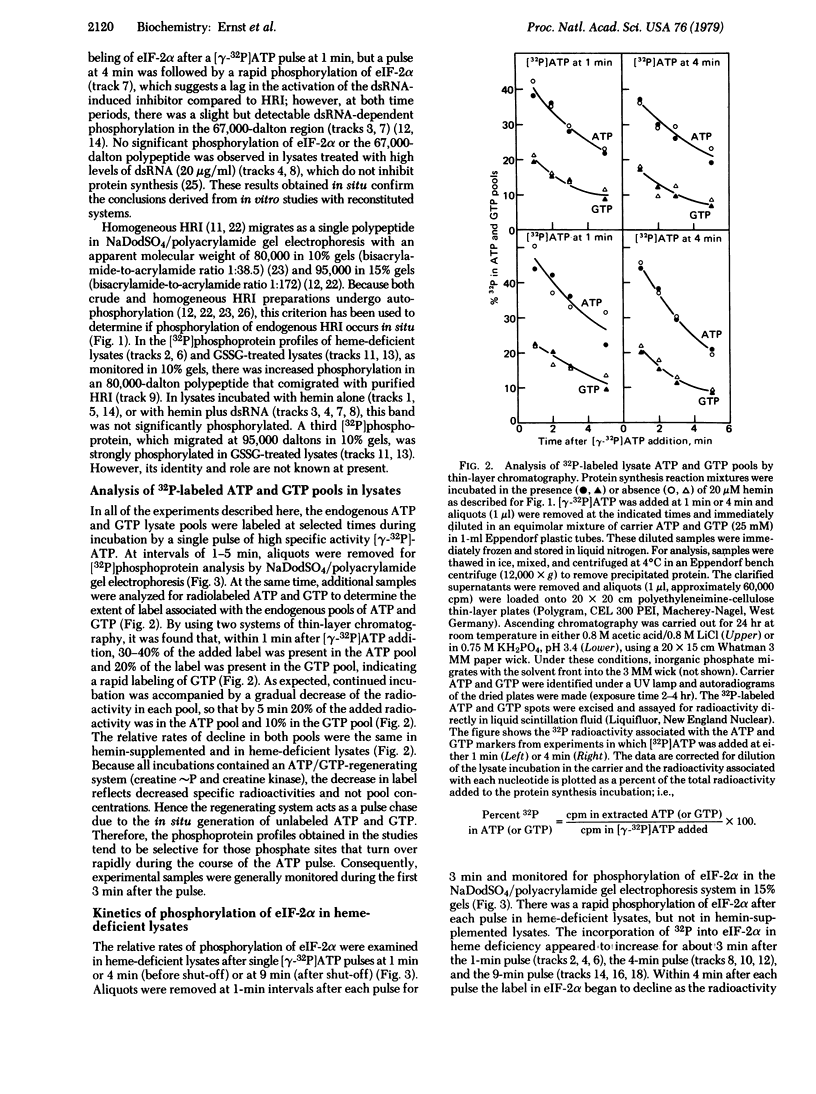
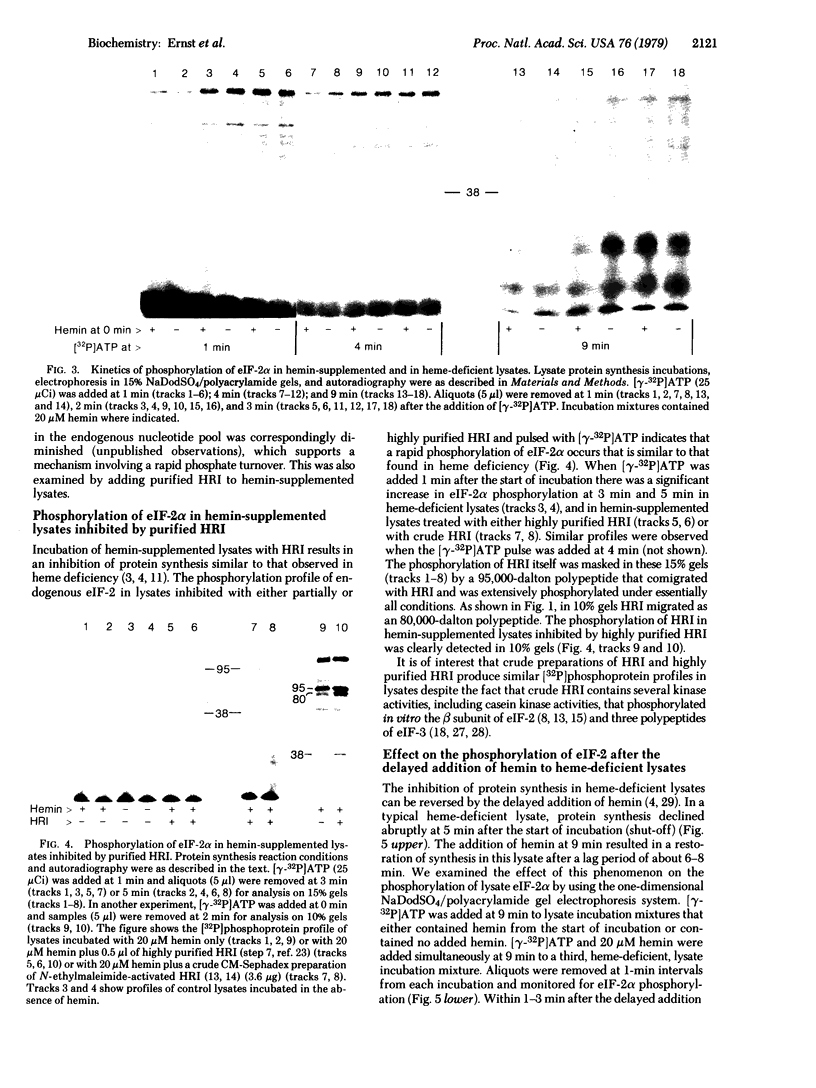
Protein synthesis initiation in reticulocyte lysates is inhibited by heme deficiency, low levels of double-stranded RNA (dsRNA), oxidized glutathione (GSSG), or the purified kinase (HRI) that acts on the α polypeptide of eukaryotic initiation factor 2 (eIF-2α). The phosphoprotein profiles produced in lysates in response to these various conditions have been monitored directly in lysates after labeling for brief periods with pulses of [γ-32P]ATP. The [32P]phosphoprotein profiles were analyzed by electrophoresis in sodium dodecyl sulfate/polyacrylamide slab gels under conditions in which the HRI and eIF-2α polypeptides were clearly distinguished. All four modes of inhibition produced a rapid phosphorylation of eIF-2α compared to control lysates, which displayed little or no phosphorylation of eIF-2α. In heme-deficient lysates, phosphorylation of eIF-2α occurred rapidly both before and after the shut-off of protein synthesis; the delayed addition of hemin to these lysates resulted in a decrease in the phosphorylation of eIF-2α and the subsequent restoration of protein synthesis. These data suggest that rapid turnover of phosphate occurs at the site(s) of eIF-2α phosphorylation. In lysates inhibited by heme deficiency, GSSG, or added HRI, the phosphorylation of eIF-2α was accompanied by the rapid in situ phosphorylation of HRI. The inhibition of initiation induced by dsRNA was accompanied by the phosphorylation of eIF-2α and a 67,000-dalton polypeptide but not HRI. These observations in situ indicate that (i) the phosphorylation of eIF-2α is the critical event in these inhibitions of protein chain initiation, and (ii) the phosphorylation of HRI is associated with its activation in heme deficiency.
Keywords: protein synthesis regulation, Met-tRNAfMet binding factor, inhibition of initiation, [32P]phosphoprotein profiles
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Herbert E., Godchaux W. Factors affecting the rate of protein synthesis in lysate systems from reticulocytes. Arch Biochem Biophys. 1968 May;125(2):671–683. doi: 10.1016/0003-9861(68)90625-5. [DOI] [PubMed] [Google Scholar]
- Adamson S. D., Herbert E., Kemp S. F. Effects of hemin and other porphyrins on protein synthesis in a reticulocyte lysate cell-free system. J Mol Biol. 1969 Jun 14;42(2):247–258. doi: 10.1016/0022-2836(69)90041-2. [DOI] [PubMed] [Google Scholar]
- Anderson W. F., Bosch L., Cohn W. E., Lodish H., Merrick W. C., Weissbach H., Wittmann H. G., Wool I. G. International symposium on protein synthesis. Summary of Fogarty Center-NIH Workshop held in Bethesda, Maryland on 18-20 October, 1976. FEBS Lett. 1977 Apr 1;76(1):1–10. doi: 10.1016/0014-5793(77)80109-9. [DOI] [PubMed] [Google Scholar]
- Benne R., Edman J., Traut R. R., Hershey J. W. Phosphorylation of eukaryotic protein synthesis initiation factors. Proc Natl Acad Sci U S A. 1978 Jan;75(1):108–112. doi: 10.1073/pnas.75.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E., Hunt T. Double-stranded poliovirus RNA inhibits initiation of protein synthesis by reticulocyte lysates. Proc Natl Acad Sci U S A. 1971 May;68(5):1075–1078. doi: 10.1073/pnas.68.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst V., Levin D. H., London I. M. Evidence that glucose 6-phosphate regulates protein synthesis initiation in reticulocyte lysates. J Biol Chem. 1978 Oct 25;253(20):7163–7172. [PubMed] [Google Scholar]
- Ernst V., Levin D. H., London I. M. Inhibition of protein synthesis initiation by oxidized glutathione: activation of a protein kinase that phosphorylates the alpha subunit of eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4110–4114. doi: 10.1073/pnas.75.9.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Hunt T., Jackson R. J. Analysis of phosphorylation of protein synthesis initiation factor eIF-2 by two-dimensional gel electrophoresis. Eur J Biochem. 1978 Sep 1;89(2):517–521. doi: 10.1111/j.1432-1033.1978.tb12556.x. [DOI] [PubMed] [Google Scholar]
- Floyd G. A., Traugh J. A. Phosphorylation of ribosomal-associated proteins in reticulocyte lysates. Methods Enzymol. 1979;60:511–521. doi: 10.1016/s0076-6879(79)60048-4. [DOI] [PubMed] [Google Scholar]
- Gross M., Mendelewski J. Additional evidence that the hemin-controlled translational repressor from rabbit reticulocytes is a protein kinase. Biochem Biophys Res Commun. 1977 Jan 24;74(2):559–569. doi: 10.1016/0006-291x(77)90340-0. [DOI] [PubMed] [Google Scholar]
- Gross M., Mendelewski J. Control of protein synthesis by hemin. An association between the formation of the hemin-controlled translational repressor and the phosphorylation of a 100 000 molecular weight protein. Biochim Biophys Acta. 1978 Oct 24;520(3):650–663. doi: 10.1016/0005-2787(78)90150-8. [DOI] [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- Hunter T., Hunt T., Jackson R. J., Robertson H. D. The characteristics of inhibition of protein synthesis by double-stranded ribonucleic acid in reticulocyte lysates. J Biol Chem. 1975 Jan 25;250(2):409–417. [PubMed] [Google Scholar]
- Issinger O. G., Benne R., Hershey J. W., Traut R. R. Phosphorylation in vitro of eukaryotic initiation factors IF-E2 and IF-E3 by protein kinases. J Biol Chem. 1976 Oct 25;251(20):6471–6474. [PubMed] [Google Scholar]
- Kosower N. S., Vanderhoff G. A., Kosower E. M. Glutathione. 8. The effects of glutathione disulfide on initiation of protein synthesis. Biochim Biophys Acta. 1972 Jul 31;272(4):623–637. [PubMed] [Google Scholar]
- Kramer G., Cimadevilla J. M., Hardesty B. Specificity of the protein kinase activity associated with the hemin-controlled repressor of rabbit reticulocyte. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3078–3082. doi: 10.1073/pnas.73.9.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin D., London I. M. Regulation of protein synthesis: activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1121–1125. doi: 10.1073/pnas.75.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D., Ranu R. S., Ernst V., London I. M. Regulation of protein synthesis in reticulocyte lysates: phosphorylation of methionyl-tRNAf binding factor by protein kinase activity of translational inhibitor isolated from hemedeficient lysates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3112–3116. doi: 10.1073/pnas.73.9.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitz M., Freedman M. L., Fisher J. M., Maxwell C. R. Translational control in hemoglobin syntheskis. Cold Spring Harb Symp Quant Biol. 1969;34:567–578. doi: 10.1101/sqb.1969.034.01.064. [DOI] [PubMed] [Google Scholar]
- Ranu R. S., London I. M., Das A., Dasgupta A., Majumdar A., Ralston R., Roy R., Gupta N. K. Regulation of protein synthesis in rabbit reticulocyte lysates by the heme-regulated protein kinase: inhibition of interaction of Met-tRNAfMet binding factor with another initiation factor in formation of Met-tRNAfMet.40S ribosomal subunit complexes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):745–749. doi: 10.1073/pnas.75.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and initial characterization of the cyclic 3':5'-AMP independent protein kinase of the heme-regulated translational inhibitor. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4349–4353. doi: 10.1073/pnas.73.12.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara S. M., Traugh J. A., Sharp S. B., Lundak T. S., Safer B., Merrick W. C. Effect of hemin on site-specific phosphorylation of eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Feb;75(2):789–793. doi: 10.1073/pnas.75.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel H., Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and characterization of heme-reversible translational inhibitor. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3654–3658. doi: 10.1073/pnas.75.8.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traugh J. A., Lundak T. S. Phosphorylation of translational initiation factor 3 (eIF-3) by cyclic AMP-regulated protein kinase. Biochem Biophys Res Commun. 1978 Jul 28;83(2):379–384. doi: 10.1016/0006-291x(78)91001-x. [DOI] [PubMed] [Google Scholar]
- Traugh J. A., Tahara S. M., Sharp S. B., Safer B., Merrick W. C. Factors involved in initiation of haemoglobin synthesis can be phosphorylated in vitro. Nature. 1976 Sep 9;263(5573):163–165. doi: 10.1038/263163a0. [DOI] [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. Stimulation of globin-chain initiation by hemin in the reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1968 Feb;59(2):582–589. doi: 10.1073/pnas.59.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]