Abstract
Firemaster® BZ-54 is a flame retardant additive and consists of a brominated benzoate (2-ethylhexyl 2,3,4,5-tetrabromobenzoate; TBB) and a brominated phthalate (bis (2-ethylhexyl) 2,3,4,5-tetrabromophthalate; TBPH). Previous research has shown that fathead minnows exposed in vivo to Firemaster® BZ-54 accumulate TBB and TBPH. This study examined the in vitro biotransformation potential of TBB and TBPH in hepatic subcellular fractions (i.e., S9, microsomes and cytosol) in the fathead minnow, common carp, mouse and snapping turtle. Metabolism was evaluated by measuring the loss of the parent TBB or TBPH and identifying potential metabolites in the sample extracts. Metabolic loss of TBPH was measured for all species, while TBB loss was observed for all species except for the snapping turtle. Several metabolites were observed in all of the incubations except for snapping turtle. Metabolites observed appeared to be derived from TBB, given their structures and lack of appearance in the snapping turtle incubations. One of these metabolites, 2,3,4,5-tetrabromomethylbenzoate has been identified for the first time in a biological system. When metabolized, TBB and TBPH loss was found in each subcellular fraction suggesting that the enzyme(s) involved are present in both soluble and membrane-bound forms. It can be concluded that a broad range of species are capable of metabolizing TBB and TBPH to various metabolites and further research should be carried out to ascertain the specific products formed from metabolism of TBB and TBPH.
Keywords: Microsomes, Cytosol, Fish, Metabolism, Flame retardant, Firemaster
1. Introduction
The phase-out of the PentaBDE commercial mixture of polybrominated diphenyl ether (PBDE) flame retardants over concerns for adverse effects to human and environmental health has led to the introduction of alternative chemical formulations for commercial use. One such product, Firemaster® 550 was advertised as a less bioaccumulative and toxic alternative to the PentaBDE formulations (Potrzebowksi and Chance, 2003). Firemaster® 550 consists of triaryl phosphate isomers, triphenyl phosphate, and Firemaster® BZ-54 (Stapleton et al., 2008). Firemaster® BZ-54 is composed of 2-ethylhexyl 2,3,4,5-tetrabromo-benzoate (TBB) and (2-ethylhexyl) 2,3,4,5-tetrabromophthalate (TBPH) in a ~2.5:1 ratio. Firemaster® 550 is designed for use as an additive in polyurethane foam found in furniture. Recently, the components of Firemaster® BZ-54 have been measured in a wide range of environmental matrices, including house dust, biosolids from a wastewater treatment facility, effluent from a textile mill, and marine mammals (Stapleton et al., 2008; LaGuardia et al., 2010; Lam et al., 2009), suggesting that it leaches from commercial products similar to PBDEs.
The majority of the available toxicity data for Firemaster® BZ-54 has been limited to short-term exposure studies focusing on aqueous exposures and traditional toxicity endpoints, such as survival. These data are accessible via the commercial material safety data sheets (MSDS) and TBPH’s evaluation as a High Production Volume Chemical (Chemtura, 2006a,b; Health and Environmental Horizons, 2004). These aqueous exposure toxicity tests are very limited and are not particularly applicable to likely environmental exposure scenarios given the poor solubility of the Firemaster® formulations in water (<1.15 × 10−5 mg L−1). Furthermore, these studies do not address the potential for organisms to be exposed through dietary consumption or to contaminated sediment. To date, there is only one toxicological study reported in the peer-reviewed scientific literature that considers the toxicological impact of Firemasters® 550 and BZ-54 on organisms through dietary exposure. In that study, fathead minnows (Pimephales promelas) exposed to Firemasters® 550 or BZ-54 accumulated and metabolized TBB and TBPH and exhibited an increase in hepatic DNA damage (Bearr et al., 2010). After the dietary exposure ceased, DNA damage returned to background levels. This reduction in DNA damage after exposure to TBB and TBPH ceased suggests three potential causes for the DNA damage during exposure: the parent compounds directly acting on the DNA, the formation of short half life genotoxic metabolites of TBB and TBPH, or the generation of reactive by-products (e.g., reactive oxygen species).
Species comparative studies have the power to identify characteristics that may be conserved among species. In contrast, they may also help determine the degree to which metabolism and formation of specific metabolites differ among species. This can help target organisms that may be at risk of metabolite-based toxic effects and evaluate the relevance of certain toxicological model species in assessing risk for human and environmental health. For instance, PBDEs are reductively debrominated in fish (Stapleton et al., 2004a,b; Roberts et al., 2011a,b); however, rodents and mammals in general appear to metabolize PBDEs via oxidative processes (e.g., cytochrome P450s), generating hydroxylated PBDEs (Stapleton et al., 2004a,b,c; Qiu et al., 2007; Noyes et al., 2010). Different metabolic pathways may result in different toxicological responses among species.
To expand upon our previous study (Bearr et al., 2010), we have broadened our scope to include other vertebrate species commonly used as toxicological models for evaluating impacts on human and environmental health. Fathead minnows (P. promelas), which are temperate freshwater fish native to North America, were used in our previous TBB and TBPH study and have been used extensively in the regulatory sphere in the United States. The common carp (Cyprinus carpio) was selected because it is a close relative of the fathead minnow and has been the organism of choice in several metabolic studies of PBDEs (Stapleton et al., 2004a,b,c; Benedict et al., 2007; Browne et al., 2009; Noyes et al., 2010; Roberts et al., 2011a, b). Mice (Mus musculus) are a traditional species used in metabolism studies for human health concerns, including brominated flame retardant exposures (Qiu et al., 2007). Snapping turtles (Chelydra serpentina serpentina) were included as they are a common reptilian model species and recent studies have shown PBDE accumulation and maternal transfer in turtle species (e.g., Stewart et al., 2011). To the best of our knowledge, there are no existing data published on the ability of reptiles to metabolize brominated flame retardants, and furthermore no information regarding the uptake and accumulation of Firemaster products (TBB or TBPH). In addition to a limited comparison between species and between subcellular fractions, a preliminary study looking at the effect of age on TBB and TBPH metabolism was evaluated for carp and mice.
The objective of this study was to determine whether potential metabolism of TBB and TBPH differs among hepatic subcellular fractions among four different model vertebrate species. Evaluating the level of metabolic activity or metabolic products for each subcellular fraction can be useful in identifying the potential enzymatic system(s) involved.
2. Materials and methods
2.1. Chemicals
Firemaster® BZ-54 was donated by Chemtura (Middlebury, CT, USA). Internal and surrogate standards (13C labeled 2,2′,3,4,5,5′-hexachlorodiphenylether (13C-CDE-141) and 4′-fluoro-2,3′,4,6-tetrabromodiphenyl ether (FBDE-69)) were purchased from Wellington Labs (Guelph, Ontario, Canada) and Chiron (Trondheim, Norway), respectively. All solvents and reagents used were high performance liquid chromatography (HPLC) grade, ACS grade or higher and were purchased from either Fisher Scientific (Pittsburgh, PA, USA), Sigma–Aldrich (St. Louis, MO, USA), or VWR (Bridgeport, NJ, USA).
2.2. Animal care and maintenance
All handling of live fish was approved by the University of Maryland Center for Environmental Sciences’ Institutional Animal Care and Use Committee (F-CBL-08-02). These test organisms were acclimated under laboratory conditions for at least two weeks. The photoperiod used for both fish species was 16:8 h light:dark and the temperature was kept at 25 ± 1 °C. Water quality parameters (i.e., pH and dissolved oxygen) were monitored daily and were within the U.S. Environmental Protection Agency guidelines for moderately hard water (U.S. EPA, 2002). Fish were fed 5% of their total body weight daily. Food was prepared at the Chesapeake Biological Laboratory with cod liver oil (CVS; Woonsocket, RI), fish feed (Hunting Creek Fisheries; Thurmont, MD), and fish gel (Aquatic Ecosystems; Apopka, FL) in a manner that was previous described (Bearr et al., 2010). Every other day, waste was removed and half of the water was renewed.
One-year-old adult male fathead minnows were received from Aquatic BioSystems (Ft. Collins, CO, USA). The population had an average weight of 3.3 ± 0.5 g and tail length of 63 ± 3 mm. Twenty-four fish were divided into three groups of eight. Carp were obtained from Hunting Creek Fisheries (Thurmont, MD, USA). An adult population of carp with an average weight of 41.4 ± 12.1 g and tail length of 160 ± 11 mm was used to prepare all three subcellular fractions. A second younger (juvenile) population of carp collected at the same time period was used to prepare an additional S9 fraction for comparison. It was not possible to determine sex and age for these individuals. This group was measured as significantly less in weight (13.9 ± 3.2 g; p < 0.05) and tail length (113 ± 7 mm; p < 0.05) in comparison. For each age cohort, three groups were designated with four fish each. After the fish were sacrificed, the livers were dissected, washed in a 100 mM phosphate buffer (pH 7.4), and immediately snap frozen in liquid nitrogen. Tissues were stored in a −80 °C freezer until isolation of the subcellular fractions.
This study also included mouse and snapping turtle hepatic tissue samples donated from other laboratories. Liver tissue samples from wild type mice were received from the University of Maryland – Baltimore (Baltimore, MD). All livers were collected from six-week-old males that weighed 17.3 ± 0.7 g. Three pools of three individuals were used. In addition, for comparison to the six-week-old males, a hepatic S9 fraction was prepared from a second population of four-month-old male mice livers with three individuals in each group. These mice weighed 26.3 ± 1.7 g, which was significantly greater than the other population (p < 0.05).
Liver tissue samples from fifteen field-collected juvenile male snapping turtles were received from the University of Maryland Center for Environmental Science, Chesapeake Biological Laboratory. The average weight of the turtles was 19.9 ± 4.7 g and length was 38 ± 4 mm. Each group of snapping turtles consisted of five individuals. Mouse and turtle livers were initially snap frozen and delivered to our laboratory on dry ice. The samples were then placed in storage at −80 °C until use.
2.3. Preparation of subcellular fractions
Hepatocyte subcellular fractions were prepared using differential centrifugation as previously described (Harada and Omura, 1980; Nilsen et al., 1998). A 100 mM phosphate buffer (containing 0.15 M KCl, 1 mM EDTA, 10 mM DTT, 20% (v/v) glycerol, pH 7.4) was used for homogenization. Resulting microsomal pellets were placed in a resuspension buffer (100 mM phosphate buffer containing 1 mM EDTA, 10 mM DTT, 20% (v/v) glycerol, pH 7.4) and pushed through a 22-gauge syringe before freezing in liquid nitrogen. Protein concentrations of all fractions were determined with a bicinchoninic acid assay (BCA) kit (Pierce, Rockford, IL; see Table 1).
Table 1.
Ethoxyresorufin-O-deethylase (EROD) and glutathione-S-transferase (GST) activities of the cell fractions used in this series of experiments.
| Species | Subcellular fraction | EROD activity (pmol mg−1 min−1) | GST activity (nmol mg−1 min−1) |
|---|---|---|---|
| Fathead Minnow | S9 | 11.0 ± 2.4 | 441.8 ± 50.9 |
| Pimephales promelas | Cytosol | 0.1 ± 0.1 | 616.5 ± 95.6 |
| Microsomes | 23.2 ± 8.1 | 279.6 ± 44.4 | |
| Common Carp Cyprinus carpio | S9 | 4.6 ± 0.8 | 176.9 ± 24.0 |
| Cytosol | 0.0 ± 0.1 | 197.7 ± 68.3 | |
| Microsomes | 7.8 ± 0.7 | 105.1 ± 36.5 | |
| Mouse Mus musculus (wild type) | S9 | 38.3 ± 9.1 | 1250.1 ± 431.8 |
| Cytosol | 0.9 ± 0.2 | 884.7 ± 174.3 | |
| Microsomes | 101.9 ± 10.7 | 439.6 ± 154.6 | |
| Snapping Turtle Chelydra serpentina serpentina | S9a | 0.7 ± 0.3 | 159.3 ± 29.4 |
Only S9 fraction available for snapping turtle incubations.
As a quality control to verify the viability of all three types of subcellular fractions, assays were conducted to measure glutathione-S-transferase (GST) enzyme activity and cytochrome P450 (CYP1A) activity. A spectrophotometric assay was used to measure GST enzyme activity with 1-chloro-2,4-dinitrobenzene (CDNB) as the substrate (Habig and Jakoby, 1981). CDNB is a non-specific substrate that is acted upon by many isoforms of GST that exist in the cytosol and microsomes. A 96-well microplate assay was performed at 25 °C (for fish and turtle samples) or 37 °C (for mouse samples). Buffer blanks incubated at both temperatures ensured that no degradation was occurring due to temperature alone.
All incubations were performed in a 100 mM Tris buffer containing 1 mM reduced glutathione (GSH, pH 7.4) and a10 μL aliquot of the subcellular fraction. After addition of 1 mM CDNB, absorbance was measured at 340 nm for 5 min. A fluorometric ethoxyresorufin-O-deethylase (EROD) assay was conducted to measure CYP1A1 activity in all fractions (Kennedy and Jones, 1994). Aliquots of the subcellular fractions were measured over a 5 min–1 h incubation period, depending on activity, to measure the conversion of 7-ethoxyresorufin to resorufin. The assay was performed at 25 °C (for fish and turtle samples) or 37 °C (for mouse samples) in a HEPES (pH 7.4) buffered solution containing 102 μM NADPH and 50 μM 7-ethoxyresorufin. Excitation and emissions wavelengths of 530 nm and 590 nm were used, respectively.
2.4. Experimental design of incubations
All incubations were performed identically with the exception of temperature. Time frames for the incubations were determined by performing pilot incubations with excess protein samples from each species. For each incubation an aliquot of microsomes, cytosol, or S9 containing 1 mg of protein was incubated for 120 min in glass test tubes containing a buffer solution and 300 ng of Firemaster BZ-54 (dissolved in 5 μL of acetone) in a total volume of 1 mL. The buffer solution consisted of 100 mM phosphate (pH 7.4), 10 mM reduced nicotinamide adenine dinucleotide phosphate (NADPH), and 10 mM dithiothreitol (DTT). Blanks that were absent of any subcellular material and heat-inactivated subcellular fractions (10 min at 100 °C) were also processed in the assay with the test samples. Comparison of the viable samples to these control samples ensured that the loss of TBB and TBPH and formation of metabolites were due to biotransformation and not an artifact of the experimental design or abiotic transformation processes. Incubations were performed in a shaker bath (140 rpm) in the dark to prevent any potential photodegradation (e.g., see Noyes et al., 2010). At the end of the incubation, ice-cold methanol was added to each test tube to stop the metabolic reactions.
2.5. Extraction, chemical analysis and preparation of standard
A metabolite standard, 2,3,4,5-tetrabromomethylbenzoate (TBMB), was synthesized internally for use in this study by heating twenty-two milligrams of 2,3,4,5-tetrabromophthalic anhydride to reflux in 10 mL of dimethylsulfoxide for 30 min. After 100 mL of water was added to the reaction mixture, the mixture was extracted with dichloromethane (DCM) (3 ×100 mL). The extracts were combined and extracted with 1 M NaOH, which was acidified with sulfuric acid and extracted with DCM. The resulting compound (2,3,4,5-tetrabromobenzoic acid) was derivatized with diazomethane.
After addition of ice-cold methanol, all incubations were spiked with an internal standard, 4′-fluoro-2,3′,4,6-tetrabromodiphenyl ether (F-BDE 69, Chiron). Three milliliters of solvent (50:50 hexane: dichloromethane) were added to each test tube. Samples were vortexed for 20 s and centrifuged for 10 min at 5000 × g (Sorvall RT 6000D, Thermo Fisher, Vernon Hills, IL, USA). This was carried out three times with the organic fraction removed and combined after each centrifugation. The extract composite was then reduced to a volume of 0.5 mL with a Zymark TurboVap II (Caliper Life Sciences, Mountain View, CA, USA). To measure F-BDE 69 recovery, the extract was spiked with 50 ng of 13C-2,2′,3,4,5,5′-chlorinated diphenyl ether (CDE 141). Laboratory blanks (DI water) were also incorporated in the extraction process for quality assurance purposes.
Extracted samples were analyzed using gas chromatography mass spectrometry operated in electron capture negative ionization mode (GC/ECNI–MS) in a manner previously reported (Stapleton et al., 2008). TBB and TBPH were quantified by monitoring molecular ion fragments (m/z 357 and 471 for TBB, and 463 and 515 for TBPH) using F-BDE 69 as an internal standard. Recovery of F-BDE 69 was 80.8 ± 9.0%. TBPH was not detected in the blanks (<1.5 ng; 3 times the signal noise of the blanks), whereas TBB was detected in laboratory blanks at 1.64 ± 0.44 ng. The experimental measurements were not blank corrected. The mean and standard error for TBB and TBPH recovery in the matrix spikes were 99.4 ± 6.1% and 85.7 ± 3.8%, respectively.
2.6. Comparison between in vivo and in vitro fathead minnow metabolism
Metabolite peaks from in vitro fathead minnow metabolism observed in the current study using S9 fractions was compared qualitatively to a previous unpublished in vivo study in which fathead minnows were exposed to Firemaster® BZ-54 in their diet for 28 days. Peaks for potential metabolites that were three times greater than the signal noise were considered statistically significant.
2.7. Statistical analysis
The statistical package Minitab (Ver. 14) was used for all statistical analyses in this study. The data sets were tested for normality and variance homogeneity. One-way analyses of variance (ANOVA) were calculated to determine significance between treatments (α < 0.05; p < 0.05). If conditions were met for significance by ANOVA, the chemical treatments were compared with the controls with a Student’s t-test to determine which treatments were significantly different from the control treatment (heat-inactivated; p < 0.05). Treatment results were also compared to determine significance differences in metabolic rates with respect to species, subcellular fractions, and compound using the same method as above.
3. Results
3.1. Cytochrome P450 and glutathione-S-transferase activity
All subcellular fractions were determined to be viable with enzyme activity comparable to baseline activities for the species under study (Table 1). CYP1A was active in all S9 and microsomal fractions tested in all four species. Since CYPs are membrane-bound in the endoplasmic reticulum and, consequently, not recovered in cytosolic fractions, CYP activity should not have been present in cytosolic fractions. Confirming the purity of the isolations, no significant CYP activity was observed in the cytosolic fractions of the fathead minnow and carp. Significant activity was observed in the mouse samples, although the CYP activity in the mouse cytosol was considered minimal (<1% of the microsomal CYP activity). GSTs were present in all three subcellular fractions in all of the organisms tested (Table 1). As expected, GST activity was higher in the S9 and cytosolic fractions.
3.2. Differences in metabolism of TBB and TBPH among species
The combined exposure of the subcellular fractions to the Firemaster BZ-54 mixture (TBB and TBPH in an approximate 3:1 ratio) resulted in a significant loss of TBB in mouse, fathead minnow, and carp (but not in turtle) in comparison to their respective heat-inactivated controls (p < 0.05). Rates of TBB metabolism were similar between subcellular fractions for the fathead minnow and the carp, but not in the mouse, which showed significantly greater metabolism in the microsomal incubations compared with cytosolic or S9 fractions (p < 0.05, Fig. 1). In S9 fractions, the metabolic rate measured in the mouse (1.76 ± 0.12 pmoles TBB mg−1 min−1) was significantly less (p < 0.05) than the S9 in the minnow (2.40 ± 0.15 pmoles TBB mg−1 min−1), or carp (2.34 ± 0.12 pmoles TBB mg−1 min−1). In the cytosol, metabolism of TBB in the minnows (2.46 ± 0.13 pmoles TBB mg−1 min−1) was significantly greater than in mice (1.67 ± 0.20 pmoles TBB mg−1 min−1; p < 0.05). Cytosolic metabolism of TBB in carp (2.13 ± 0.74 pmoles TBB mg−1 min−1) was not statistically different from either fathead minnows or mice. Microsomal metabolism of TBB was greater in mice (3.10 ± 0.47 pmoles TBB mg−1 min−1) than in carp (1.61 ± 0.63 pmoles TBB mg−1 min−1; p < 0.05) but the fathead minnow was not significantly different from the other two species (2.40 ± 0.57 pmoles TBB mg−1 min−1).
Fig. 1.
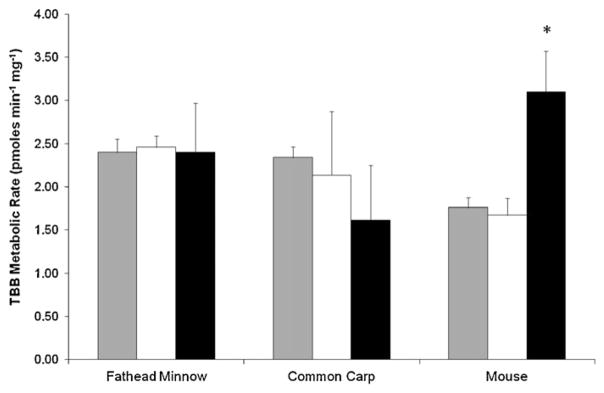
Differences in TBB metabolism between species and subcellular fractions (120 min; 1 mg protein). Gray, white and black bars signify the S9, cytosol and microsomal incubations respectively. Results presented as the mean ± SD (N = 3). Asterisk denotes significant difference from the S9 fraction for each species (t-test, p < 0.05).
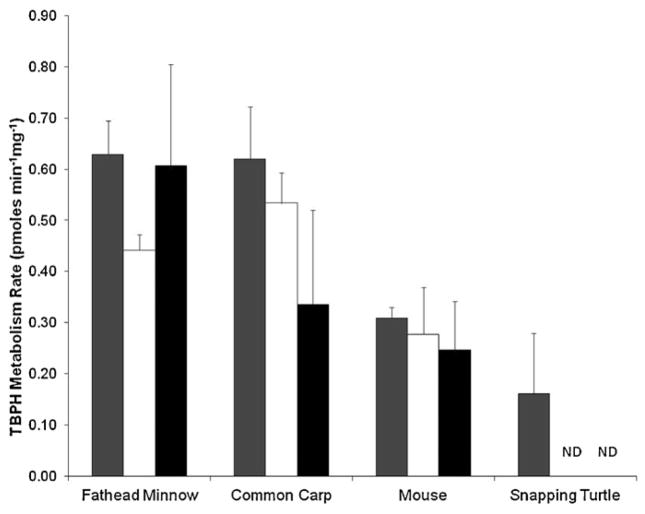
TBPH was generally metabolized at a lower rate than TBB for the fathead minnow, common carp, and mouse (Fig. 2; p < 0.05). However, whereas TBB was not metabolized in the snapping turtle, TBPH was (0.161 ± 0.118 pmoles TBPH mg−1 min−1). In fact, the loss of TBPH was significant for all species and cell fractions tested (p < 0.05). Rates of TBPH metabolism in S9 fractions were significantly lower in the turtle than those measured in the fathead minnow (0.629 ± 0.066 pmoles TBPH mg−1 min−1; p < 0.05), and carp (0.620 ± 0.103 pmoles TBPH mg−1 min−1; p < 0.05), but not the mouse (0.309 ± 0.021 pmoles TBPH mg−1 min−1). For cytosolic TBPH metabolism, mice (0.277 ± 0.92 pmoles TBPH mg−1 min−1) were significantly less (p < 0.05) than either minnow (0.441 ± 0.031 pmoles TBPH mg−1 min−1) or carp (0.534 ± 0.60 pmoles TBPH mg−1 min−1). Microsomal metabolism was greater in the minnows (0.607 ± 0.197 pmoles TBPH mg−1 min−1) than in mice (0.247 ± 0.095 pmoles TBPH mg−1 min−1; p < 0.05), but not in the carp (0.335 ± 0.186 pmoles TBPH mg−1 min−1).
Fig. 2.
Differences in TBPH metabolism between species and subcellular fractions (120 min; 1 mg protein). Gray, white and black bars signify the S9, cytosol and microsomal incubations respectively. Results presented as the mean ± SD (N = 3).
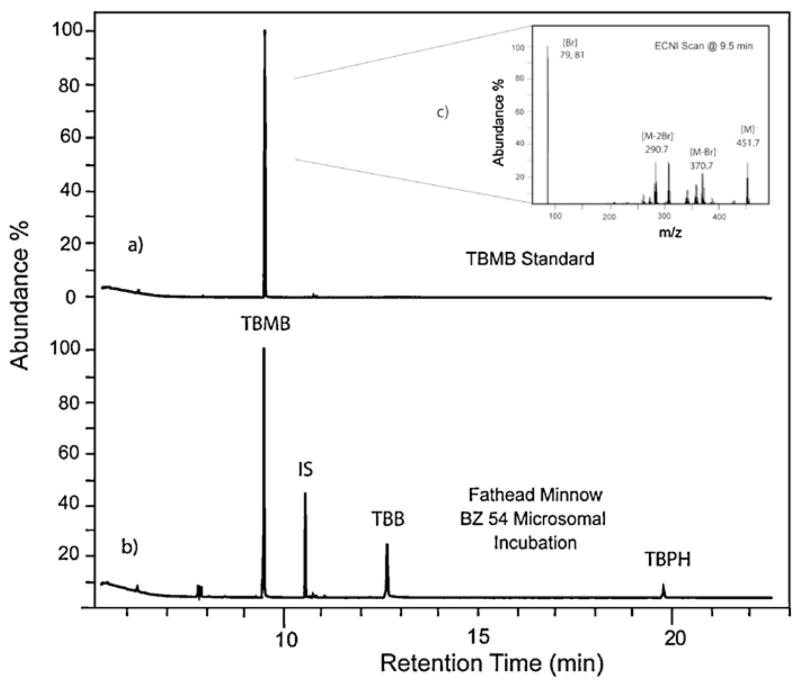
These in vitro exposures have identified a previously unknown metabolite of TBB, 2,3,4,5-tetrabromomethylbenzoate (TBMB; Fig. 4). Significant peaks (three times the background) of TBMB were observed in chromatograms from incubations using all three fathead minnow subcellular fractions with TBB, but were not significant in the carp or mouse incubations. Two other peaks were found to be significant (three times the background signal) in only the fathead minnow and were strongly associated with the microsomal fraction, but were not observed in a great enough concentration to identify their structure by full scan mass spectral analysis. To date, chemicals represented by these two peaks have not been identified.
Fig. 4.
Comparison of chromatograms of the extracted sample from a Firemaster® BZ-54 (containing TBB and TBPH) incubation with fathead minnow microsomes (a) and a sample amended with tetrabromomethylbenzoate (TBMB) standard (b) with (c) showing the detailed ECNI scan of the TBMB standard. Note: IS denotes the internal standard.
3.3. Comparison of in vivo and in vitro results
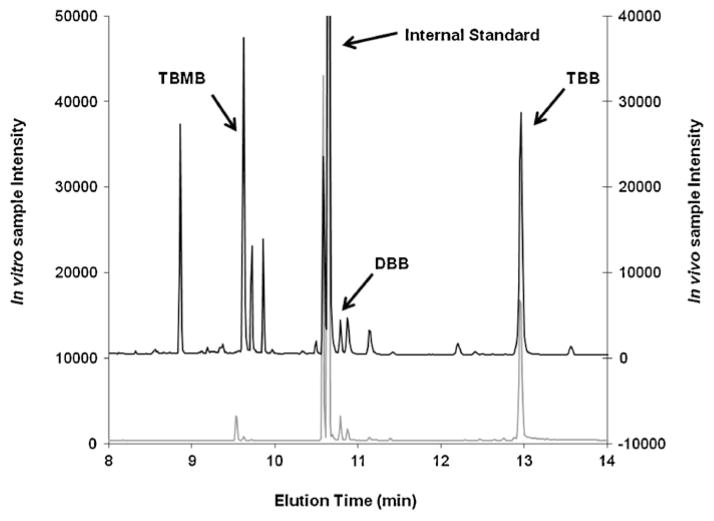
Results of the fathead minnow S9 incubations in this study were compared to an earlier in vivo study in which fathead minnows were exposed to Firemaster® BZ-54 (containing the mixture of both TBB and TBPH) in their diet. Chromatograms from each study were qualitatively assessed to determine if they shared similar peaks, which would represent similar metabolites. Several peaks for potential TBB metabolites were present in both samples (Fig. 5). Although as all incubations were conducted in the presence of both TBB and TBPH it is not definitive that these arose from TBB alone, although structurally this is suggested. Ten distinct potential metabolite peaks were observed in the in vivo study. Of those ten peaks, only one peak at 9.5 min was also observed in the in vitro sample and was identified as TBMB. It should be noted that another distinct peak was found in the in vitro sample that correlated to a significant peak in the in vivo sample, but did not meet the significance standard set by this study (three times the background noise). This peak at 10.74 min was identified as 2-ethylhexyl dibromobenzoate (DBB) in the in vivo sample.
Fig. 5.
Comparison of total ion chromatograms from an in vivo dietary study (black chromatogram above) and an in vitro hepatic S9 incubation (gray chromatogram below) for fathead minnows exposed to Firemaster® formulations. In the figure, peaks associated with 2-ethylhexyl 2,3,4,5-tetrabromobenzoate (TBB), 2,3,4,5-tetrabromomethylbenzoate (TBMB), and dibromobenzoate (DBB) are specifically noted along with the internal standard.
4. Discussion
This study is the first to assess the ability of several vertebrate species to metabolize the brominated components of the Firemaster® 550 and Firemaster® BZ-54 formulations, namely, TBB and TBPH. Except for the snapping turtle’s inability to metabolize TBB, very similar metabolic rates between minnow, carp, and mouse were observed for both TBB and TBPH. TBB was consistently metabolized to a greater extent than TBPH across all species tested in this study. The similarity found in TBB and TBPH metabolism between the fish and mouse suggests that enzyme(s) responsible for this metabolism might be similar between the two species.
Regardless of the cell fraction used in the incubations, metabolism of these compounds was relatively consistent. This observation highlights that both membrane-bound and soluble enzyme(s) are capable of metabolizing TBB and TBPH. It is possible that this metabolism and the presence of similar metabolites are driven by different groups of enzymes in each sub-fraction, but a simpler explanation would be that the enzymatic route incorporates an enzyme or enzymes present in both membrane-bound and soluble forms. If the responsible enzymes were shared, it would rule out metabolism by localized enzymes, such as cytochrome P450s; as membrane-bound enzymes, they would be unable to catalyze metabolism in the isolated cytosol. With so many metabolite peaks, the route of action may be the result of several enzyme systems as opposed to a single route dominated by a single enzyme (see Fig. 4).
This study is also the first to identify TBMB as a metabolite most likely derived from TBB and associated with both soluble and membrane-bound hepatic enzymes in the fathead minnow, carp, and mouse. TBB may be converted into TBMB in a two-step process consisting of hydrolysis and methylation (Fig. 6). The hydrolysis of the ethylhexyl group has been studied in fish and in mammals with respect to di (2-ethylhexyl) phthalate (DEHP) exposures (Barron et al., 1995; Kavlock et al., 2002). The structure of DEHP differs from TBPH in that it lacks bromines. The first intermediate product in the metabolism of DEHP, mono (2-ethylhexyl) phthalate, is structurally similar to TBB, differing by a lack of bromines and the addition of a carboxylic acid group. Lipases, a subclass of esterases, are known to initiate the metabolism of DEHP and may be involved in metabolism of these two brominated compounds as well. In fact, a recent presentation provided evidence that purified porcine esterases rapidly metabolize TBB (Roberts et al., 2011a, b). This makes lipases a rather convincing candidate for having a role in metabolizing TBB and TBPH.
Fig. 6.
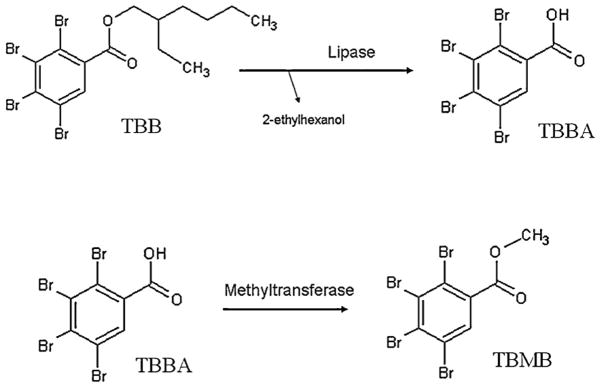
A proposed two step mechanism for metabolizing 2-ethylhexyl 2,3,4,5-tetrabromobenzoate (TBB) into 2,3,4,5-tetrabromomethylbenzoate (TBMB) with 2,3,4,5-tetrabromobenzoic acid (TBBA) as an intermediate product.
Although they are predominantly found in the pancreas, lipases are also in hepatic tissue in both soluble and membrane-bound forms (Gibbons and Wiggins, 1995; Gilham et al., 2003). The activity and substrate specificity of lipases depend on the isoenzyme in question and may help explain why the formation of TBMB was greater in cytosolic and S9 fractions than in microsomes. The cytosolic form of the enzyme may have been the main driver of TBMB production, but other forms, including membrane-bound enzymes, may also have a capacity for metabolizing TBB. It is also possible that a slower secondary pathway could also be involved and predominant in the microsomal fraction. The resulting metabolite, tetrabromobenzoic acid (TBBA), after the first step of the reaction was not identified in this study. This would occur if the methyltransferase step occurred very rapidly, preventing production and accumulation of tetrabromobenzoic acid.
The second enzyme in the proposed two-step reaction would be a methyltransferase. Similar to lipase activity, methylation is observed in both cytosolic and microsomal fractions. Consistent with this observation, methyltransferases are also present in both subcellular fractions in various forms. For instance, catechol-O-methyltransferase can be encoded at two separate transcription sites on the same gene, one site produces a soluble form and the other a membrane-bound form that is found in hepatic tissues (Weinshilboum et al., 1999). There is not a great deal of literature on the feasibility of this mechanism, but benzoic acid catechols have been shown to be acted upon by this form of methyltransferase (Graves et al., 2008). Catechols are not present in either TBB or TBPH metabolites. However, tetrabromobenzoic acid may be metabolized in the same manner with the methyltransferase acting on one hydroxyl group at a time. We could find no mention in the literature describing this in animals, but benzoic acid methyltransferase has been reported in plants (Arabidopsis thaliana; Chen et al., 2003), As such, methyltransferases are a candidate for this second step, but more research is required for any definitive conclusion.
In vitro incubations are advantageous as unlike in vivo experiments, these are a simple isolated system. Metabolites formed in vivo are often rapidly excreted or further biotransformed making many products difficult to observe. As such, it should be understood that the in vitro incubations are sub-compartment scenarios that may not fully represent metabolism in a whole organism. In vitro experiments have their limitations; they can often illuminate events that are obscured in in vivo studies. From the in vivo work, TBPH bioaccumulated in fish, but there was no evidence of metabolites. Similarly, the in vitro samples lacked any measurable metabolites. However, we observed a significant loss of TBPH in the incubations based on the initial concentration. Although this study did not identify metabolites in vitro, it appears that enzymes were present that metabolized TBPH to metabolites were not quantifiable using the techniques in this current study (i.e. polar and/or non-brominated compounds).
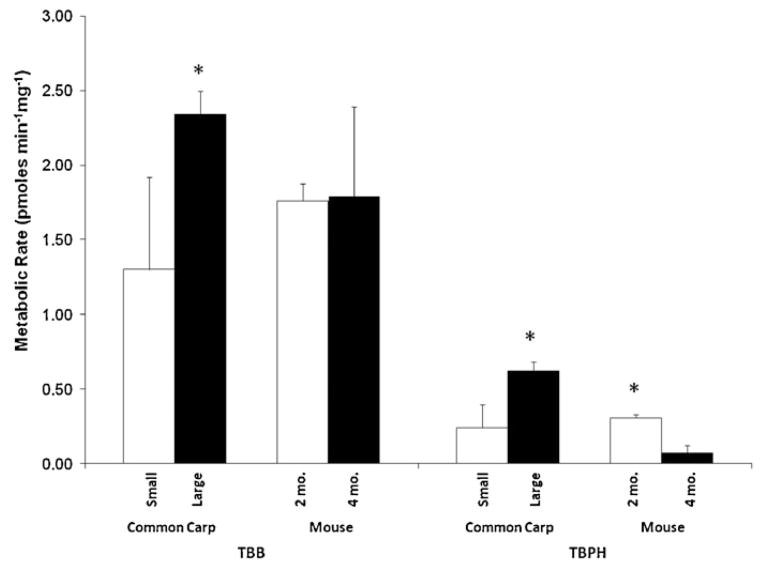
Metabolism differed between age/size groups tested, but it was not consistent with respect to age between the carp and mice. In a previous preliminary study it appears that there are metabolic differences in the S9 incubations in the two age classes of carp and mice investigated. The smaller (and likely younger carp population) metabolized TBB (1.30 ± 0.62 pmoles TBB mg−1 min−1) and TBPH (0.243 ± 0.154 pmoles TBPH mg−1 min−1) at a significantly slower rate than the larger carp (Fig. 3; 2.34 ± 0.12 pmoles TBB mg−1 min−1; 0.620 ± 0.103 pmoles TBPH mg−1 min−1; p < 0.05). In contrast, a comparison between 4-month-old and 2-month-old male mice showed that the older mouse population metabolized TBPH (0.076 ± 0.050 pmoles TBPH mg−1 min−1) at a significantly slower rate than the younger mice (0.309 ± 0.21 pmoles TBPH mg−1 min−1). No difference was observed in TBB metabolism for the mice. Age may be a factor affecting the rate of metabolism, but is likely species-specific and warrants further investigation. Enzyme activity (e.g., CYP, GST) was not assessed for the additional age classes, which would help in determining if enzyme activity in general was a contributing factor for the differences observed. Traditional toxicity testing has often considered early life stages of organisms to be most sensitive. It is unclear what variables may be affecting these different results. This difference in metabolism between age/size groups tested highlights an area of future research.
Fig. 3.
Comparison of TBB and TBPH metabolism in the S9 of different sized populations of carp and mice of different ages (2 months and 4 months). Results presented as the mean ± SD (N = 3). Asterisks denote significant differences between age classes for each species and compound (t-test, p < 0.05).
The use of subcellular fractions is useful for screening chemicals for toxicity, assessing potential metabolism and understanding environmental fate. By comparing metabolism in different subcellular fractions, catalyzing enzymes groups may be deduced. These observations may also help determine if the toxicological model species being used are actually responding to the chemical exposure in a way that is representative to the interests of the study. Finally, in vitro incubations may be an effective way to predict whether the parent compounds are likely to accumulate in the environment or if they can be biotransformed to metabolites that have different physical and chemical characteristics, which can affect their fate and transport.
Acknowledgments
Funding for this research was provided by the Drach-Mellody Navigator Research Fellowship, Chesapeake Biological Laboratory, University of Maryland – Baltimore, and the National Institute of Environmental Health Sciences (R01-ES016099). This is Contribution No. 4666 of the University of Maryland Center for Environmental Science.
References
- Barron MS, Albro PW, Hayton WL. Biotransformation of di(2-ethylhexyl)phthalate by rainbow trout. Environmental Toxicology and Chemistry. 1995;14:873–876. [Google Scholar]
- Bearr JS, Stapleton HM, Mitchelmore CL. Accumulation and DNA damage in fathead minnows (Pimephales promelas) exposed to 2 brominated flame retardant mixtures, Firemaster® 550 and Firemaster® BZ-54. Environmental Toxicology and Chemistry. 2010;29:722–729. doi: 10.1002/etc.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RT, Stapleton HM, Letcher RJ, Mitchelmore CL. Debromination of polybrominated diphenyl ether-99 (BDE-99) in carp (Cyprinus carpio) microflora and microsomes. Chemosphere. 2007;69:987–993. doi: 10.1016/j.chemosphere.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Browne EP, Stapleton HM, Kelly SM, Tilton SC, Gallagher EP. In vitro hepatic metabolism of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE 99) in Chinook Salmon (Onchorhynchus tshawytscha) Aquatic Toxicology. 2009;92:281–287. doi: 10.1016/j.aquatox.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemtura. Firemaster® 550; MSDS00896. Middlebury, CT, USA: 2006a. [Google Scholar]
- Chemtura. Firemaster® BZ-54; MSDS 00074. Middlebury, CT, USA: 2006b. [Google Scholar]
- Chen F, D’Auria JC, Tholl D, Ross JR, Gershenzon J, Noel JP, Pichersky E. An Arabidopsis thaliana gene for methylsalicylate biosynthesis, identified by a biochemical genomics approach, has a role in defense. Plant Journal. 2003;36:577–588. doi: 10.1046/j.1365-313x.2003.01902.x. [DOI] [PubMed] [Google Scholar]
- Gibbons GF, Wiggins D. Intracellular triacylglycerol lipase: its role in the assembly of hepatic very-low-density lipoprotein (VLDL) Advances in Enzyme Regulation. 1995;35:179–198. doi: 10.1016/0065-2571(94)00006-o. [DOI] [PubMed] [Google Scholar]
- Gilham D, Ho S, Rasouli M, Martres P, Vance DE, Lehner R. Inhibitors of hepatic microsomal triacylglycerol hydrolase decrease VLDL secretion. FASEB Journal. 2003;17:1685–1687. doi: 10.1096/fj.02-0728fje. [DOI] [PubMed] [Google Scholar]
- Graves TL, Zhang Y, Scott JE. A universal competitive fluorescence polarization activity assay for S-adenosylmethionine utilizing methyltransferases. Analytical Biochemistry. 2008;373:296–306. doi: 10.1016/j.ab.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig WH, Jakoby WB. Assay for differentiation of glutathione S-transferases. Methods in Enzymology. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Harada N, Omura T. Participation of cytochrome-P-450 in the reduction of nitro-compounds by rat liver microsomes. Journal of biochemistry. 1980;87 (5):1539–1554. doi: 10.1093/oxfordjournals.jbchem.a132895. [DOI] [PubMed] [Google Scholar]
- Health and Environmental Horizons. High Production Volume (HPV) Challenge Program Test Plan for Phthalic Acid Tetrabromo Bis 2-Ethylhelxyl Ester (CAS#26040-51-7) Prepared for the Brominated Phthalate Ester Panel, American Chemical Council. 2004 Jul;1:2004. [Google Scholar]
- Kavlock R, Boeckelheide K, Chapin R, Cunningham M, Faustman E, Foster P. NTP center for the evaluation of risks to human reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl)phthalate. Reproductive Toxicology. 2002;16:529–653. doi: 10.1016/s0890-6238(02)00032-1. [DOI] [PubMed] [Google Scholar]
- Kennedy SW, Jones SP. Simultaneous measurement of cytochrome P4501A catalytic activity and total protein concentration with a fluorescence plate reader. Analytical Biochemistry. 1994;222:217–223. doi: 10.1006/abio.1994.1476. [DOI] [PubMed] [Google Scholar]
- LaGuardia MJ, Hale RC, Harvey E, Chen D. Flame-retardants and other organohalogens detected in sewage sludge by electron capture negative ion mass spectrometry. Environmental Science and Technology. 2010;44:4658–4664. doi: 10.1021/es9039264. [DOI] [PubMed] [Google Scholar]
- Lam JCW, Lau RKF, Murphy MB, Lam PKS. Temporal trends of hexabro-mocyclododecanes (HBCDs) and polybrominated diphenyl ethers (PBDEs) and detection of two novel flame retardants in marine mammals from Hong Kong, South China. Environmental Science and Technology. 2009;43:6944–6949. doi: 10.1021/es901408t. [DOI] [PubMed] [Google Scholar]
- Nilsen BM, Berg K, Goksøyr A. Induction of cytochrome P450 1A (CYP1A) in fish. In: Phillips IR, Shephard EA, editors. Methods in Molecular Biology. Vol. 107. Humana Press; Totowa: 1998. pp. 423–438. Cytochrome P450 protocols. [DOI] [PubMed] [Google Scholar]
- Noyes PD, Kelly SM, Mitchelmore CL, Stapleton HM. Characterizing the in vitro hepatic biotransformation of the flame retardant BDE 99 by common carp. Aquatic Toxicology. 2010;97:142–150. doi: 10.1016/j.aquatox.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrzebowksi J, Chance W. Great Lakes Chemical Corporation: Report on Firemaster 550. 2003 http://www.pu2pu.com/htdocs/Customers/greatlakes/Firemaster.htm.
- Qiu X, Mercado-Feliciano M, Bigsby RM, Hites RA. Measurement of polybrominated diphenyl ethers and metabolites in mouse plasma after exposure to a commercial pentabromodiphenyl ether mixture. Environmental Health Perspectives. 2007;115:1052–1058. doi: 10.1289/ehp.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SC, Noyes PD, Gallagher EP, Stapleton HS. Species-specific differences and structure–activity relationships in the debromination of PBDE congeners in three fish species. Environmental Science and Technology. 2011a;45:1999–2005. doi: 10.1021/es103934x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SC, Dishaw LV, Patisaul HB, Stapleton HS. Dioxin 2011. Brussels, Belgium: 2011b. Aug 21, In vitro biotrans-formation of the pentaBDE replacement Firemaster® 550 and in vivo effects on thyroid hormone levels in rats. [Google Scholar]
- Stapleton HM, Letcher RJ, Baker JE. Debromination of polybrominated diphenyl ether congeners BDE 99 and BDE 183 in the intestinal tract of the common carp (Cyprinus carpio) Environmental Science and Technology. 2004a;38:1054–1061. doi: 10.1021/es0348804. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Letcher RJ, Li J, Baker JE. Dietary accumulation and metabolism of polybrominated diphenyl ethers by juvenile carp (Cyprinus carpio) Environmental Toxicology and Chemistry. 2004b;23:1939–1946. doi: 10.1897/03-462. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Alaee M, Letcher RJ, Baker JE. Debromination of the flame retardant decabromodiphenyl ether by juvinle carp (Cyprinus carpio) following dietary exposure. Environmental Science and Technology. 2004c;38:112–119. doi: 10.1021/es034746j. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF. Alternate and new brominated flame retardants detected in US house dust. Environmental Science and Technology. 2008;42:6910–6916. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- Stewart KR, Keller JM, Templeton R, Kucklick JR, Johnson C. Monitoring persistent organic pollutants in leatherback turtles (Dermochelys coriacea) confirms maternal transfer. Marine Pollution Bulletin. 2011;62 (7):1396–1409. doi: 10.1016/j.marpolbul.2011.04.042. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Short-term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms. 4. 2002. EPA-821-R-02-013. [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annual Review of Pharmacology and Toxicology. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]