Abstract
Objective
Laminin is a major component of the vascular basal lamina, implying that laminin receptors such as α6β1 and α6β4 integrins may regulate vascular remodeling and homeostasis. Previous studies in the central nervous system (CNS) have shown that β4 integrin is expressed by only a fraction of cerebral vessels, but defining the vessel type and cellular source of β4 integrin has proved controversial. The goal of this study was to define the class of vessel and cell type expressing β4 integrin in cerebral vessels, and to examine its potential role in vascular remodeling.
Approach and Results
Dual-immunofluorescence showed that β4 integrin is expressed predominantly in arterioles, both in the CNS and in peripheral organs. Cell-specific knockouts of β4 integrin revealed that β4 integrin expression in cerebral vessels is derived from endothelial cells, not astrocytes or smooth muscle cells. Lack of endothelial β4 integrin had no effect on vascular development, integrity, or endothelial proliferation, but in the hypoxic CNS its absence led to defective arteriolar remodeling and associated TGF-β signaling.
Conclusions
These results define high levels of β4 integrin in arteriolar endothelial cells, and demonstrate a novel link between β4 integrin, TGF-β signaling and arteriolar remodeling in cerebral vessels.
Keywords: cell adhesion molecule, endothelial cell, extracellular matrix, hypoxia, vascular remodeling
INTRODUCTION
Cell adhesion mechanisms play critical roles in the growth, establishment, and maintenance of blood vessels. In particular, extracellular matrix (ECM) proteins such as fibronectin, collagen and laminin provide important instructional cues in directing vasculogenesis and angiogenesis, both during development and in remodeling events in the adult 1. Blood vessels in the central nervous system (CNS) are unique compared to those in other organs, having extremely low permeability and high electrical resistance, defined as the blood-brain barrier (BBB). The cellular and molecular basis of the BBB is thought to lie in the extremely tight apposition of neighbouring endothelial cells, as a result of extensive tight junction protein expression, and the influence of astrocyte end-feet and pericytes 2. Within blood vessels, laminin is a major component of the vascular basal lamina, implying that cell surface laminin receptors such as α6β1 and α6β4 integrins and dystroglycan may play important functions in regulating blood vessel modeling and stability of mature vessels. In particular, the α6β4 integrin warrants special attention for three reasons. First, in contrast to α6β1 integrin and dystroglycan, α6β4 integrin is detected on only a small fraction of cerebral vessels 3. Second, the number of cerebral vessels expressing α6β4 integrin is strongly increased, both during neuroinflammatory conditions 4, 5, and hypoxic-induced angiogenic remodeling 6. Third, the cytoplasmic domain of the β4 integrin subunit (~1000 amino acids) is much longer than those of other integrin subunits (~50 amino acids) 7, implying potential for unique interactions with cytoskeletal adaptor proteins and intracellular signaling pathways.
While α6β4 expression on cerebral vessels has been well demonstrated, it is still unclear which cell type/s in cerebral vessels expresses the β4 integrin subunit. Though studies of vessels outside the CNS have described α6β4 integrin expression in endothelial cells 8 and smooth muscle cells 9, this has not been demonstrated on cerebral vessels; in fact the majority of CNS studies have suggested that α6β4 integrin is expressed by astrocyte end-feet that run along the vascular basal lamina 3, 5, 10. One of the reasons this analysis has proven so elusive is due to the very tight apposition of all the different cellular components of cerebral vessels, which include endothelial cells, pericytes, smooth muscle cells and astrocyte end-feet. Interestingly, a previous study of non-CNS tissue showed that α6β4 integrin is expressed by endothelial cells in mature vessels, but not within angiogenic capillaries, prompting the suggestion that α6β4 may be a negative regulator of the angiogenic switch 8. Based on its distribution, the authors also suggested that α6β4 integrin may provide higher levels of endothelial adhesion, necessary for the maintenance of vascular integrity in mature vessels.
The importance of α6β4 integrin in providing extra adhesion at sites requiring high adhesive strength is best illustrated by the finding that global murine knockouts of either β4 or α6 integrin subunits result in perinatal mortality, due to defective epidermal integrity 11, 12. This manifests as a skin blistering condition that is analogous to the human disease junctional epidermolysis bullosa (JEB), of which some are due to mutations in the human β4 integrin gene 13. In light of the essential adhesive role for α6β4 integrin in maintaining epidermal integrity, the limited expression pattern of this integrin on cerebral vessels and its strong upregulation during hypoxic vascular remodeling, and the controversy over which cell type expresses α6β4 integrin in cerebral vessels, we embarked on a study to address three main questions. First, which part of the cerebral vascular tree expresses β4 integrin? Second, which specific vascular cells express β4 integrin; is it astrocytes, endothelial cells, or smooth muscle cells? Third, does absence of the β4 integrin in cerebral vessels lead to alterations in vascular development, integrity, or remodeling?
RESULTS
β4 integrin is detected on a sub-population of cerebral vessels
Laminin is expressed at high levels in the basal lamina of cerebral vessels, and we and others have described the expression of several laminin receptors on cerebral vessels, including the integrins α6β1, α6β4, and dystroglycan 3, 10, 14. To investigate how laminin and its receptors are regulated during development of cerebral vessels, we performed dual-immunofluorescent (IF) studies on frozen brain sections derived from mice that were 1 day, 1 week, 4 weeks or 8 weeks old. As shown in Figure 1, while laminin, α6 integrin and dystroglycan were expressed on all vessels throughout this developmental period, β4 integrin was expressed by only a small fraction of vessels, and this situation was maintained into adulthood. Thus, consistent with previous results, while all vessels in the adult CNS expressed dystroglycan (Figure 1C) 10, 14, β4 integrin was detected on only ~10% of cerebral vessels (Figure 1B), which generally were larger diameter vessels.
Figure 1.
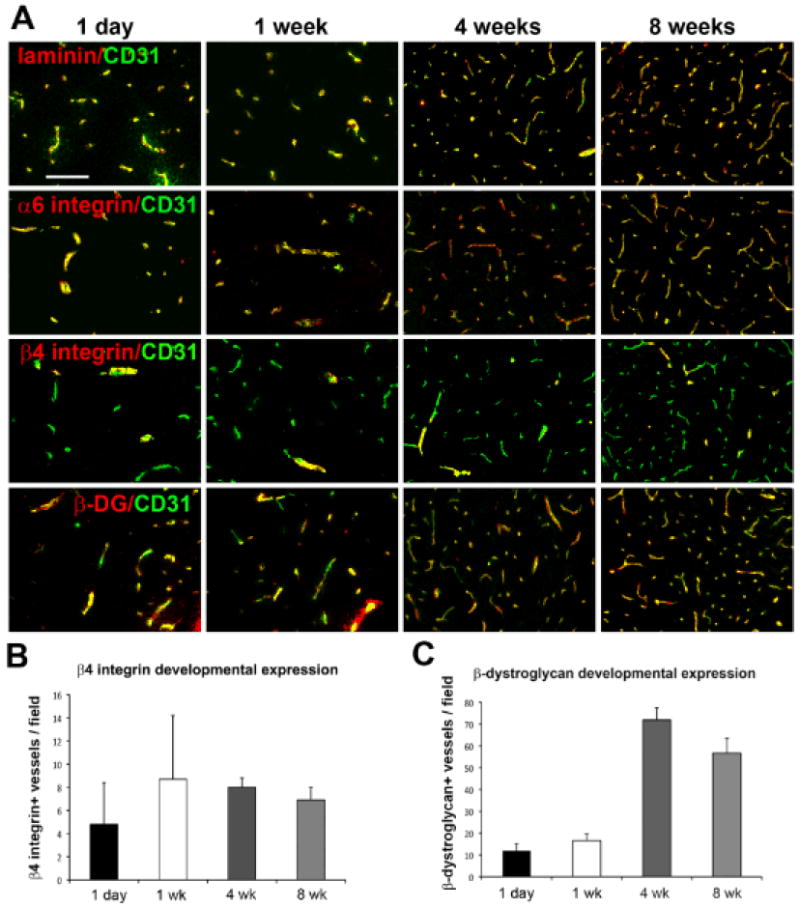
Expression profile of laminin and its receptors during cerebrovascular development. A. Dual-IF was performed on frozen sections of the frontal lobe from 1 day, 1 week, 4 week or 8 week old mice using antibodies specific for CD31 (AlexaFluor-488, green) and the adhesion molecules laminin, α6 integrin, β4 integrin or β-dystroglycan (Cy3, red). Scale bar = 100 μm. B and C. Quantification of the number of β4 integrin (B) or β-dystroglycan-positive (C) vessels per field of view. Analysis was performed with four different animals per condition, and the results expressed as the mean ± SEM of β4 integrin or β-dystroglycan-positive vessels per field. Note that while laminin, α6 integrin and β-dystroglycan were expressed on all vessels throughout this developmental period, β4 integrin subunit was expressed by only a small fraction of vessels (~10%), and this situation was maintained into adulthood.
β4 integrin co-localizes with α-smooth muscle actin (α-SMA)
The majority of studies within the CNS have suggested that β4 integrin expression within cerebral vessels is contributed by astrocyte end-feet 3, 5, 10. In contrast, studies of blood vessels outside the CNS have described β4 integrin expression on endothelial cells 8 and on smooth muscle cells (SMC) 9. To determine which cell type expresses β4 integrin in cerebral vessels, we performed dual-IF for β4 integrin and markers for each of the three different cell types present within cerebral vessels: endothelial cells (CD31), astrocytes (glial fibrillary acidic protein (GFAP)), and SMC (alpha-smooth muscle actin (α-SMA)). Dual-IF with β4 integrin/CD31 showed that β4 integrin expression was always intimately associated with the endothelial marker CD31, but confirmed that β4 integrin was expressed by only a fraction of CD31-positive vessels (Figure 2). In contrast to the close relationship with CD31, β4 integrin was only occasionally associated with GFAP. The tightest co-localization of all was found between β4 integrin and α-SMA, which revealed that virtually every α-SMA-positive vessel expressed β4 integrin. The close association between β4 integrin and α-SMA suggests that β4 integrin is expressed predominantly by vessels in the arterial side of the circulation, which include arteries and arterioles. This finding is consistent with the work of Hiran et al, who described β4 integrin expression on a subset of endothelial cells within arterial vessels that invest veins, known as the vasa vasorum, but not by endothelial cells lining the walls of veins 8. However, yet to be clarified is whether β4 integrin is expressed by endothelial cells, by the SMC that surround them, or by a sub-population of astrocyte end-feet.
Figure 2.
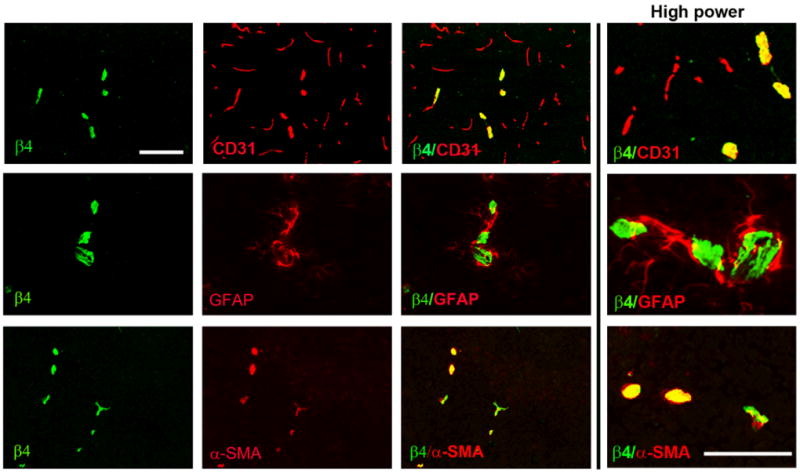
Co-localization of β4 integrin with cell-specific markers in cerebral vessels. Dual-IF was performed on frozen sections of the frontal lobe from adult mice using antibodies specific for β4 integrin (AlexaFluor-488, green), endothelial marker CD31 (Cy3, red), astrocyte marker GFAP (Cy3, red) or smooth muscle cell marker α-SMA (Cy3, red). Scale bar = 100 μm. Note that β4 integrin was expressed by only a fraction of CD31-positive vessels, but was expressed by virtually every α-SMA-positive vessel.
β4 integrin is expressed by endothelial cells, not astrocytes
Considering the difficulty in identifying the cellular source of β4 integrin in cerebral vessels by conventional IF studies, we set out to unambiguously answer this question by deleting the β4 integrin gene selectively, from either astrocytes or endothelial cells using Cre-Lox. To knockout β4 integrin from astrocytes, mice expressing nestin-Cre were crossed with mice homozygous for the floxed β4 integrin gene 15. Nestin is expressed in all cells of neural lineage at an early stage of development, including neurons, astrocytes and oligodendrocytes 16 and the nestin-Cre mouse line has been used successfully to delete β1 integrins from astrocytes 17. Mice expressing nestin-Cre and one copy of the β4-flox gene (nestin-cre; β4+/f) were crossed with mice homozygous for flox β4 integrin (β4f/f). From this breeding strategy, approximately 25% of the offspring carried the combination of nestin-Cre and 2 alleles of flox β4 integrin (referred to as β4-astro-KO mice), as shown in Supplemental Figure IA. Littermate mice that had two copies of β4-flox and no nestin-Cre (β4f/f) or one copy of the β4-flox gene and nestin-Cre (nestin-Cre; β4+/f) were used as controls. A similar approach was taken to delete β4 integrin from endothelial cells using Tie2-Cre mice, to produce mice lacking β4 integrin expression specifically in endothelial cells (referred to as β4-EC-KO mice), as shown in Supplemental Figure IB. We successfully used this approach recently to examine the function of α5 integrin in regulating brain endothelial cell proliferation and cerebral angiogenesis 18. When we examined the proportions of different genotypes of mice generated by nestin-Cre x flox β4 and Tie2-Cre x flox β4, we found that the numbers of mice expressing the different genotypes closely matched the expected Mendelian ratios (data not shown). Thus, deletion of β4 integrin in either astrocytes or endothelial cells did not produce a lethal phenotype, and further inspection of these two strains of mice failed to reveal any obvious phenotype. Next, we performed IF of brain sections to determine whether vascular β4 integrin was absent in either of these strains of mice. As shown in Figure 3, this revealed a very clear result. While β4 integrin expression was not noticeably different in β4-astro-KO mice compared to littermate controls, β4 integrin expression on cerebral vessels was totally eliminated in β4-EC-KO mice. This provides unequivocal evidence that β4 integrin expression on cerebral blood vessels derives from endothelial cells, not astrocytes or SMC.
Figure 3.
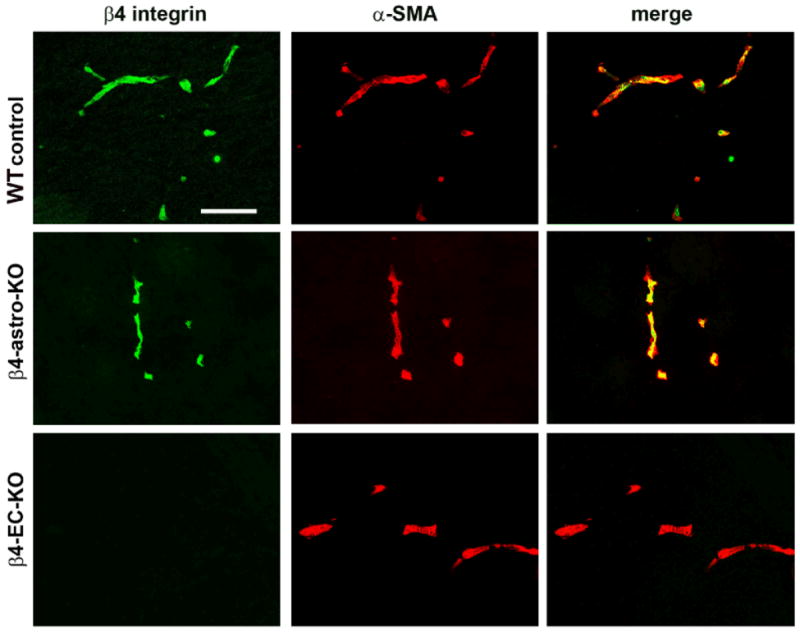
Identification of the cerebrovascular cell type expressing β4 integrin. Dual-IF was performed on frozen sections of the frontal lobe from adult mice using antibodies specific for β4 integrin (AlexaFluor-488, green) and SMC marker α-SMA (Cy3, red). Scale bar = 100 μm. Note that while β4 integrin-positive vessels were still present in β4-astro-KO mice, cerebral vessels in β4-EC-KO mice totally lacked this integrin, definitive evidence that β4 integrin expression on cerebral blood vessels derives from endothelial cells, not astrocytes or SMC.
Arterial endothelial cells in peripheral organs also express high levels of β4 integrin
To investigate whether β4 integrin expression, located predominantly within arterial vessels is specific to the CNS, or more of a global phenomenon, we examined β4 integrin/ α-SMA co-localization in other organs, including heart, kidney, and skeletal muscle. As shown in Supplemental Figure II, in all three organs examined, there existed a very close association between α-SMA and β4 integrin expression in blood vessels. This demonstrates that endothelial β4 integrin expression in arterial vessels is not specific to the CNS, but a general phenomenon of vessels in different organs.
Endothelial β4 integrin expression in cerebral vessels is localized to arterioles
As β4 integrin is expressed predominantly by endothelial cells in the arterial side of the circulation, we next performed a detailed analysis to determine if β4 integrin is expressed at high levels at all stages of the arterial circulation, or limited to specific stages of the arterial tree. Frozen sections of heart, aorta, and brain were examined by dual-IF for β4 integrin/CD31. As shown in Figure 4, β4 integrin was not detected on endothelial cells lining the high pressure left ventricle, though interestingly, smaller diameter vessels within the ventricular myocardium expressed β4 at high levels. Likewise, β4 integrin was not detected on endothelial cells lining the thoracic aorta, but smaller caliber vessels in adjacent skeletal muscle stained strongly for β4 integrin. In the brain, endothelial cells lining large diameter arterial vessels (> 30μm) were strikingly negative for β4 integrin, while smaller caliber, adjacent vessels showed strong β4 integrin expression. Taken together, these results demonstrate that β4 integrin is expressed predominantly by small caliber vessels of the arterial circulation, i.e.: arterioles. Within the brain, the diameter of vessels expressing β4 integrin was in the range 7-25 μm. Figure 4B provides an example of the largest diameter cerebral vessel expressing β4 integrin, and also illustrates that β4 integrin expression within the vessel shows a striated appearance, with lines of β4 integrin protein orientated along the direction of blood flow.
Figure 4.
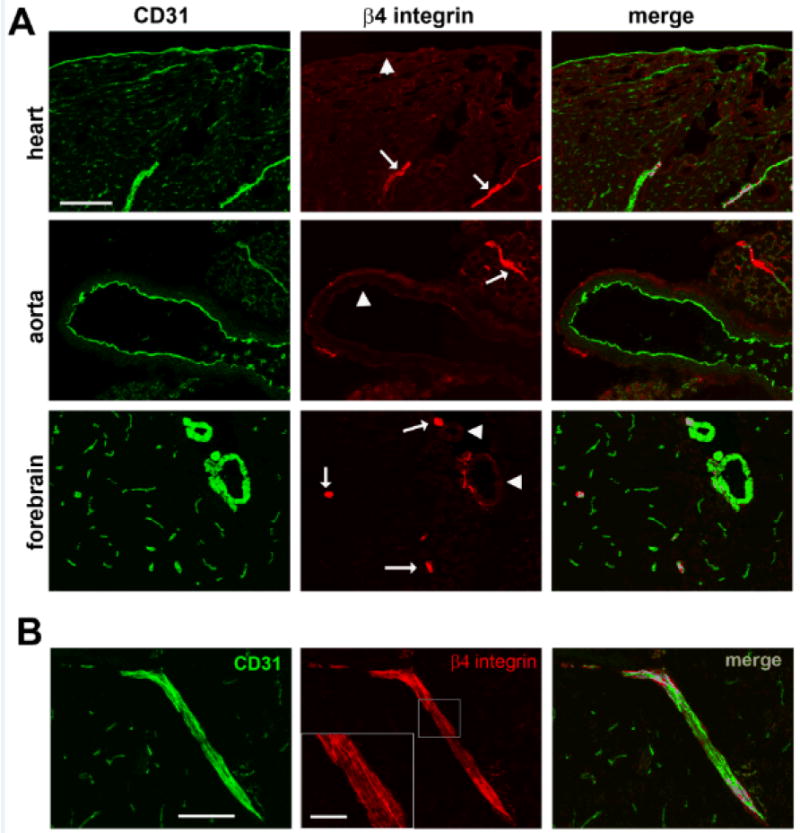
A. Characterization of β4 integrin expression at different stages of the arterial circulation. Dual-IF was performed on frozen sections of the left ventricle of the heart, aorta, and forebrain from adult mice using antibodies specific for the endothelial marker CD31 (AlexaFluor-488, green) and β4 integrin (Cy3, red). Scale bar = 100 μm. Note that β4 integrin was not detected on endothelial cells lining the left ventricle of the heart, aorta, or cerebral arteries (arrowheads), but was strongly expressed by endothelial cells lining cerebral arterioles, and also present within small diameter arterioles within cardiac myocardium and skeletal muscle adjacent to the aorta (arrows). B. Example of β4 integrin expression on cerebral arterioles. CD31/β4 integrin dual-IF was performed on frozen sections of the frontal lobe. Scale bar = 100 μm. Cerebral vessels expressing β4 integrin were in the range 7-25 μm diameter. In larger vessels, such as the one shown (inset scale bar = 25 μm), β4 integrin expression showed a striated appearance, with visible lines of β4 protein orientated along the direction of blood flow.
β4-EC-KO mice show no major abnormalities in cerebrovascular development
The high level of β4 integrin expression by endothelial cells in cerebral arterioles suggests a unique function for this integrin in this location. Previous studies suggest a potential role in regulating endothelial cell proliferation and angiogenesis 8, 19. To investigate this we examined the brains of 8-week old β4-EC-KO mice for evidence of alterations in vessel density, vessel integrity, density of α-SMA-positive vessels, as well as expression of laminin, the physiological ECM ligand for the α6β4 integrin 12. Surprisingly, we found no differences between the brains of β4-EC-KO mice and littermate controls in any of the parameters investigated. Using CD31 as a marker of endothelial cells, quantification of total vessel area (Figure 5A) and size distribution of cerebral vessels (Figure 5C: normoxic bars) revealed no differences between β4-EC-KO mice and littermate controls. Albumin IF failed to reveal any extravascular leak in β4-EC-KO mice, implying that BBB permeability in these mice is not perturbed. Furthermore, the density of α-SMA-positive vessels was equivalent between the two strains, and there was no difference in the distribution pattern of laminin within the basal lamina of blood vessels, or in the expression of alternative laminin receptors such as α6β1 integrin, or dystroglycan (data not shown). To exclude the possibility that lack of endothelial β4 integrin may cause a delay in cerebrovascular maturation, we also performed similar analyses in mice that were 2 or 4 weeks old, but this revealed no obvious differences in any of the parameters described above (data not shown). Taken together, this demonstrates that β4 integrin is not essential for cerebrovascular development, or for the maintenance of BBB integrity under the conditions tested.
Figure 5.
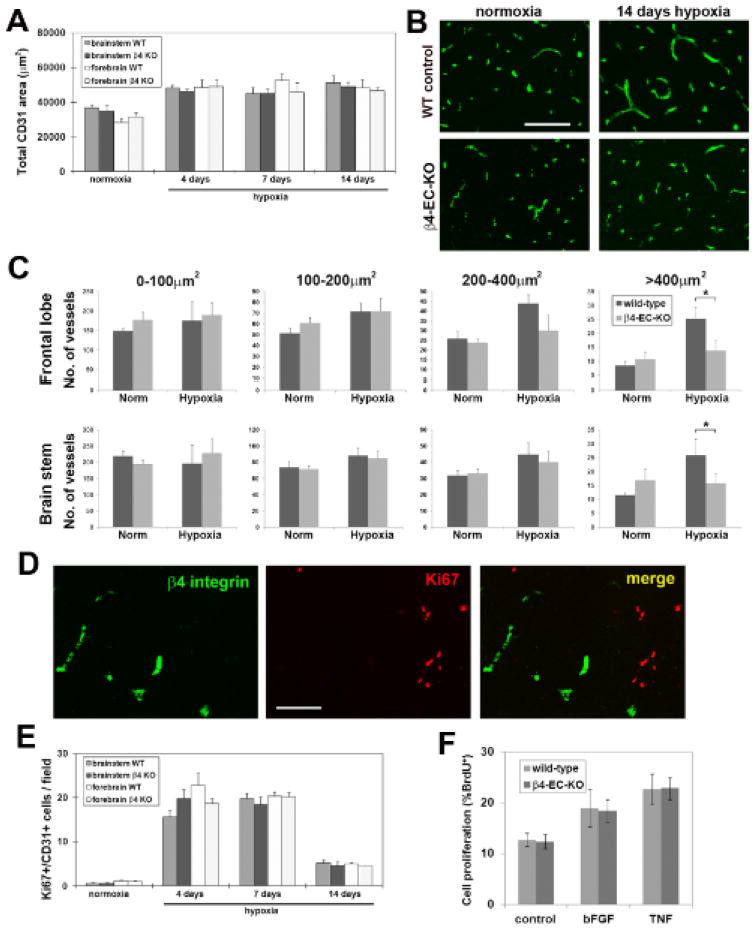
Comparison of hypoxic-induced vascular remodeling in the brains of wild-type and β4-EC-KO mice. β4-EC-KO and wild-type mice were maintained at normoxia or exposed to mild hypoxia (8% O2) for 4, 7 or 14 days before frozen brain sections were immunostained to determine the influence of hypoxia on: A. total vascular area, B and C. cerebral vessel size distribution, and E. endothelial cell proliferation. Analysis was performed with four different animals per condition, and the results expressed as the mean ± SEM. Note that cerebral hypoxia promoted similar increases in total CD31 area (A), and in the number of proliferating BEC in the brains of wild-type and β4-EC-KO mice (E), with no significant differences observed between the two groups at any time-point. However, vessel size distribution revealed an important difference between wild-type and β4-EC-KO mice (C). In wild-type mice, 14 days hypoxia induced a preferential increase in the number of vessels in the size range of arterioles (area > 400μm2) in both brain areas examined. In contrast, β4-EC-KO mice failed to show this response. * P< 0.05. D. β4 integrin/Ki67 dual-IF of 7 day hypoxic wild-type brain. Scale bars in panels B and D = 100μm. Note that despite the presence of many Ki67-positive cells in the hypoxic remodeling brain, dual-labeled β4 integrin/Ki67 cells were never detected. F. Comparison of cell proliferation by BrdU incorporation in BEC derived from β4-EC-KO or littermate control mice. Note that while bFGF and TNF increased BEC proliferation relative to control conditions, no differences were observed in the mitotic rates of BEC derived from β4-EC-KO or littermate control mice.
β4-EC-KO mice show a specific defect in arteriolar remodeling in the hypoxic adult CNS
To investigate whether β4 integrin is required for vascular remodeling in the adult brain, we employed a mouse model of mild hypoxia, in which chronic exposure to mild hypoxia (8% O2) induces a strong angiogenic response in the CNS 20, 21. β4-EC-KO and wild-type littermate control mice were exposed to hypoxia for 0, 4, 7 or 14 days. As shown in Figure 5A, hypoxia promoted similar increases in the total vascular area in the brains of β4-EC-KO and wild-type mice, as determined by total CD31 area, with no significant differences observed between the two groups at any time point. Recently, we demonstrated that chronic cerebral hypoxia stimulates generation of new arterial vessels, corresponding to a preferential increase in the number of large area vessels 22. Vessel size distribution analysis revealed that β4 integrin is expressed specifically by large area α-SMA-positive vessels in the range 200-400 μm2 and >400 μm2 (not shown). As vascular expression of β4 integrin is strongly increased during hypoxic remodeling 6, we next examined the potential role of β4 integrin in the remodeling response by comparing the size distribution of cerebral vessels in β4-EC-KO and wild-type mice. As illustrated in Figure 5B and quantified in Figure 5C, in both areas of the brain examined (frontal lobe and brain stem), wild-type mice exposed to 14 days hypoxia showed a preferential increase in the number of vessels in the arteriolar size range (areas of 200-400 μm2 and >400 μm2), with only relatively minor increases in smaller vessels. Strikingly, while β4-EC-KO mice showed a similar lack of change in small diameter vessels, the increase in number of arteriole-size vessels (area >400 μm2) was almost totally absent, and the increase in vessels of area range 200-400 μm2 showed a marked flattened response. Thus, 14 days hypoxia resulted in a significantly greater number of arteriole-size vessels (area >400 μm2) in wild-type compared to β4-EC-KO mice, both in the frontal lobe (25.3 ± 4.0 vs. 13.8 ± 3.5, p < 0.05) and in the brainstem (25.9 ± 5.8 vs. 15.7 ± 3.7, p < 0.05). This demonstrates that β4-EC-KO mice show a specific defect in arteriolar remodeling in response to chronic hypoxia. Further analysis revealed that in the hypoxic CNS, β4-EC-KO mice showed no alteration in vessel integrity (assessed by albumin IF) or laminin expression (data not shown).
To determine whether lack of β4 integrin impacted endothelial cell proliferation, we performed CD31/Ki67 dual-IF. Consistent with previous findings 6, this revealed that hypoxia triggered strong endothelial cell proliferation, which peaked between 4 and 7 days hypoxia, but showed no difference between β4-EC-KO and wild-type mice (Figure 5E). To investigate whether β4 integrin is expressed by proliferating or post-mitotic endothelial cells, we performed β4 integrin/Ki67 dual-IF on brains of hypoxic mice mounting an angiogenic response. As shown in Figure 5D, despite the presence of numerous Ki67-positive cells in the hypoxic remodeling brain, we never detected dual-labeled β4 integrin/Ki67 cells. This supports the notion that β4 integrin is expressed by terminally differentiated endothelial cells, consistent with the findings of Hiran et al 8. Furthermore, in vitro studies revealed that the proliferation rate of β4 integrin-deficient brain endothelial cells (BECs) cultured with a number of different mitogenic stimuli including bFGF and TNF was no different to wild-type cells (Figure 5F). Taken together, these combined in vivo and in vitro studies demonstrate that β4 integrin has no direct influence on endothelial cell proliferation.
Absence of β4 integrin results in attenuation of endothelial TGF-β signaling
Other studies have demonstrated that integrin β subunits influence activation of TGF-β signaling pathways 23, 24, and directly relevant to the current studies, manipulation of β4 integrin levels have been shown to result in alterations in TGF-β signaling in epithelial cells 25, 26. Taken with previous reports of strong upregulation of specific TGF-β receptors in growing arterial vessels 27, and the proarteriogenic influence of TGF-β1 in animal models of peripheral vascular disease 28, we next examined the possibility that lack of β4 integrin may disrupt TGF-β signaling in endothelial cells. Dual-IF with CD31 and the type I TGF-β receptor activin receptor-like kinase 1 (ALK1) revealed strong upregulation of ALK1 on large-diameter arterial vessels during the hypoxic remodeling response in wild-type mice (Figure 6A), consistent with previous reports of elevated ALK1 on growing arterial vessels 27. Whereas in the normoxic CNS, ALK1 was expressed at equivalent levels by neurons and blood vessels, hypoxia promoted strong upregulation of vascular ALK1 expression. Quantification of fluorescent intensity showed that in wild-type mice, mean vascular ALK1 expression was maximal at 7 days hypoxia, before declining towards pre-hypoxic levels (Figure 6C). In contrast, while β4-EC-KO mice showed equivalent ALK1 levels on neurons and vessels in the normoxic CNS, cerebral vessels in these mice failed to show ALK1 upregulation as seen in wild-type mice (Figure 6B and quantified in 6C). Next, we investigated the activation of the ALK1 signaling pathway by performing dual-IF with CD31 and phospho-Smad1/5/8. Interestingly, in the normoxic CNS, phospho-Smad1/5/8 was present only in neurons, and this was also true in the β4-EC-KO CNS (Figure 7). However, after 7 days hypoxia, vascular cells in the wild-type CNS labeled positive for phospho-Smad1/5/8 (arrows), but vessels in β4-EC-KO mice failed to show this response. To seek confirmation of these findings, we employed a flow cytometry-based approach, in which brains from wild-type or β4-EC-KO mice exposed to normoxia or 7 days hypoxia were dissociated and CD31-positive endothelial cells analyzed for expression of ALK1 or phospho-Smad1/5/8 (Figure 8). This revealed that under normoxic conditions, there were no differences between wild-type and β4-EC-KO mice, either in the proportion of brain endothelial cells (BEC) expressing ALK1 or phospho-Smad1/5/8 (Figure 8C and E), or in the mean expression levels of these two proteins (Figure 8D and F). However, 7 days hypoxia induced large increases in endothelial expression of these markers in wild-type but not β4-EC-KO mice. In wild-type mice, 7 days hypoxia significantly increased both the percentage of ALK1-positive BECs (from 24.1 ± 2.8 to 52.9 ± 8.8, p < 0.01) and mean fluorescent intensity (MFI) of BEC ALK1 (from 3.8 ± 0.7 to 7.3 ± 0.9, p < 0.01), but had no effect on β4-EC-KO mice (Figure 8A, C-D). In a similar manner, 7 days hypoxia in wild-type mice significantly increased both the percentage of phospho-Smad1/5/8-positive BECs (from 10.2 ± 2.1 to 65.8 ± 8.4, p < 0.001) and mean fluorescent intensity (MFI) of BEC phospho-Smad1/5/8 (from 3.2 ± 0.6 to 12.7 ± 2.4, p < 0.01), but had no effect on β4-EC-KO mice (Figure 8B, E-F). Based on these findings, we conclude that absence of endothelial β4 integrin disrupts TGF-β mediated signaling during hypoxic-induced vascular remodeling.
Figure 6.
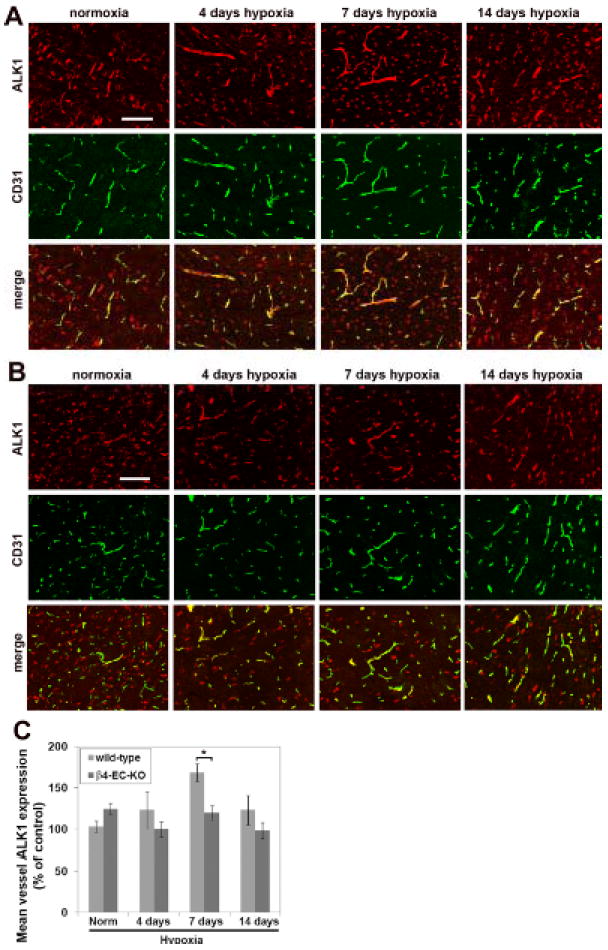
Comparison of hypoxic-induced endothelial expression of ALK1 in the brains of wild-type and β4-EC-KO mice. Wild-type litter-mate (A) and β4-EC-KO (B) mice were maintained at normoxia or exposed to mild hypoxia (8% O2) for 4, 7 or 14 days before frozen sections of brainstem were subject to CD31/ALK1 dual-IF. Scale bar = 100μm. C. Quantification of endothelial ALK1 expression. The fluorescent intensity of vessels was measured using the Volocity software program and the results presented as mean vessel ALK1 expression (% of control level in normoxic wild-type mice). Results represent mean ± SEM of four different mice per condition. Note that while hypoxia induced a significant increase in endothelial ALK1 expression in wild-type mice, β4-EC-KO mice failed to show this response. * P< 0.05.
Figure 7.
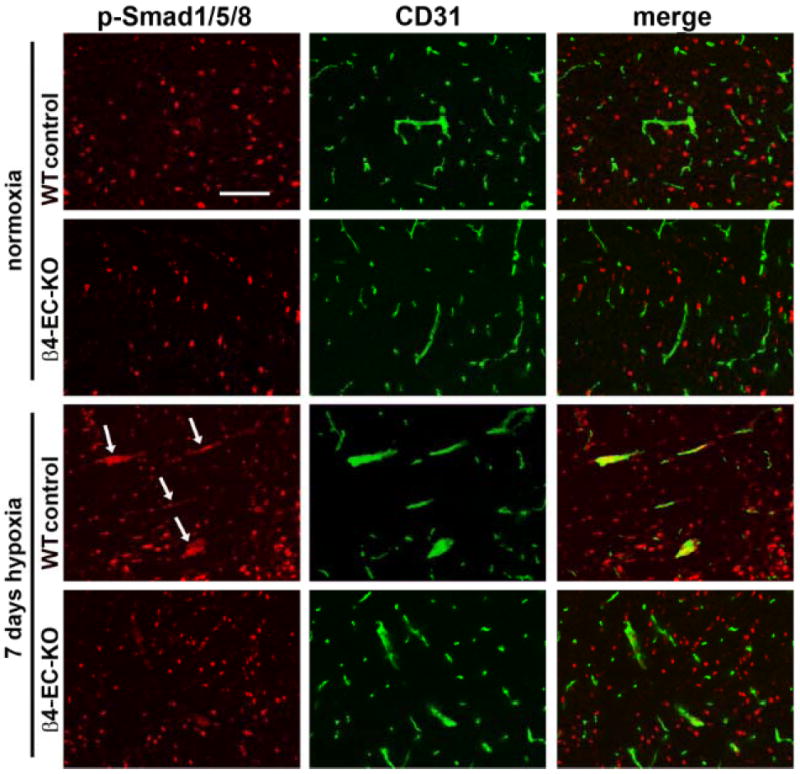
Comparison of hypoxic-induced endothelial activation of Smad1/5/8 in the brains of wild-type and β4-EC-KO mice. Wild-type litter-mate and β4-EC-KO mice were maintained at normoxia or exposed to mild hypoxia (8% O2) for 7 days before frozen sections of brainstem were subject to CD31/phospho-Smad1/5/8 dual-IF. Scale bar = 100μm. Note that while 7 days hypoxia promoted strong induction of phospho-Smad1/5/8 in endothelial cells in wild-type brain (arrows), β4-EC-KO mice failed to show this response.
Figure 8.
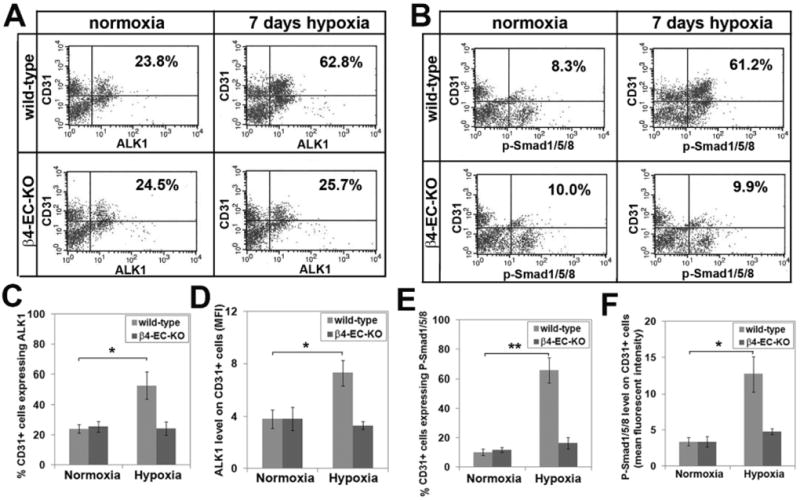
Flow cytometry analysis of ALK1 and phospho-Smad1/5/8 expression on brain endothelial cells (BEC) freshly isolated from mouse brain. Single cell suspensions from the brains of 8-10 week old wild-type or β4-EC-KO mice exposed to normoxia or 7 days mild hypoxia (8% O2) were prepared as described in Materials and Methods, and CD31-positive BEC expression of ALK1 (A) or phospho-Smad1/5/8 (B) analyzed by flow cytometry. Data are presented as the percentage of BEC expressing ALK1 (C) or phospho-Smad1/5/8 (E), or as the mean BEC expression levels of ALK1 (D) or phospho-Smad1/5/8 (F), and represent the mean ± SEM of three different experiments. Note that under normoxic conditions, no difference existed between wild-type and β4-EC-KO mice, either in the percentage or expression levels of brain BEC expressing ALK1 or phospho-Smad1/5/8. However, 7 days hypoxia induced large increases in BEC expression of these markers in wild-type but not β4-EC-KO mice. * P< 0.01, ** P< 0.001.
DISCUSSION
The aim of this study was to identify the class of vessel and cell type that expresses β4 integrin in cerebral vessels, and to examine the potential role of this integrin in regulating vascular development, integrity and remodeling in the CNS. Interestingly, we found that β4 integrin is expressed predominantly by endothelial cells in arterioles, both in the CNS and in peripheral organs, and that astrocytes and SMC do not express β4 integrin. Lack of endothelial β4 integrin had no effect on vascular development, integrity, or endothelial cell proliferation, but in the hypoxic CNS its absence led to specific defects in arteriolar remodeling and associated TGF-β-mediated signaling.
Unique expression pattern of β4 integrin on cerebral vessels
When we began this study, the prevailing school of thought was that β4 integrin expression in cerebral vessels was localized to the astrocyte end-feet that run along cerebral vessels 3, 5, 10. In this study, we definitively show for the first time that endothelial cells are the sole source of β4 integrin in cerebral vessels, and that astrocytes or SMC in this location do not express β4 integrin. While prior studies have described β4 integrin expression on only a fraction of cerebral vessels 3, 10, the question of why this expression is limited has not been previously addressed. Our data show a very tight correlation between α-SMA and β4 integrin expression, demonstrating that endothelial cells within arterial vessels express high levels of this integrin. Furthermore, all peripheral organs examined also revealed a close association between α-SMA and β4 integrin expression in small vessels, indicating that this correlation is not brain-specific, but a widespread phenomenon. That endothelial β4 integrin expression was detected predominantly in arterial vessels suggests that this integrin may be induced by high blood pressure or shear stress. To differentiate between these two possibilities, we examined β4 integrin expression at all stages of the arterial circulation, from the lining of the left ventricle of the heart, which is exposed to the highest blood pressure, through the aorta, carotid arteries, cerebral arteries, to the cerebral arterioles. Surprisingly, β4 integrin was not detected on endothelial cells lining the left ventricle of the heart, aorta, carotid or cerebral arteries, but was strongly expressed by cerebral arterioles, with a vessel diameter ranging between 7-25μm. These findings suggest that β4 integrin is not induced by high blood pressure, but rather, as shear stress levels in arterioles are much greater than in larger vessels (shear stress is inversely proportional to the cube of vessel radius), it seems more likely that β4 integrin expression is triggered by high shear stress in arterioles. This expression pattern is consistent with the findings of Hiran et al, who described β4 integrin expression on small arterial vessels supplying large veins, but not by endothelial cells lining large veins 8, and also with those of Cremona et al who described β4 integrin expression on small vessels in human tissue 9.
A role for α6β4 integrin in vascular modeling
While our data demonstrates a role for β4 integrin in mediating arteriogenic remodeling, previous studies examining β4 integrin function in vascular remodeling have generated conflicting results. Hiran et al showed that β4 integrin is expressed by well-differentiated, angiostatic endothelial cells, suggesting that α6β4 integrin may be a negative regulator of angiogenesis 8. However, Nikolopoulos et al demonstrated that mice with a truncated version of β4 integrin lacking the cytoplasmic signaling domain showed reduced angiogenesis, suggesting that β4 integrin signaling may drive angiogenesis 19. Both studies were consistent in showing that β4 integrin does not directly promote endothelial proliferation, and our results confirm these findings. While it is clear that β4 integrin is not required for endothelial proliferation, our results demonstrate a novel role for β4 integrin in mediating arteriolar remodeling. Endothelial proliferation is not part of this response, as we never detected proliferating endothelial cells within remodeling arterial vessels or proliferating endothelial cells that were β4 integrin-positive. These findings are remarkably similar to those of Nikolopoulos et al, who found that mice with mutated β4 integrin showed reduced vascular remodeling, but no defect in endothelial proliferation 19.
β4 integrin influence on TGF-β signaling
Our results demonstrate that absence of β4 integrin led to disruption of TGF-β mediated signaling in endothelial cells. This finding is in keeping with other studies describing regulation of TGF-β signaling by β integrin subunits 23-26. TGF-β plays a critical role in regulating vascular remodeling during development and in the adult 29, and emerging evidence suggests that the influence of TGF-β on endothelial remodeling is determined by the relative balance of specific type I TGF-β receptors expressed by endothelial cells, with ALK1 promoting vascular remodeling and ALK5 suppressing it 30. Consistent with this notion, we found that remodeling arterial vessels in the hypoxic CNS strongly upregulated ALK1 expression, and this correlated with activation of the downstream Smad 1/5/8 signaling pathway. In keeping with an absent arterial remodeling response, β4-EC-KO mice failed to show these changes in ALK1 expression and Smad 1/5/8 activation. Taken together, these results define a novel link between endothelial β4 integrin, TGF-β signaling and arteriolar remodeling in cerebral vessels. They also suggest that manipulation of this pathway may provide a means of promoting arteriogenic remodeling in patients pre-disposed to cerebral ischemia.
Supplementary Material
SIGNIFICANCE.
Laminin is a major component of the vascular basal lamina, suggesting that laminin receptors such as α6β4 integrin may regulate vascular remodeling and homeostasis. In this study, we defined the class of vessel and cell type expressing β4 integrin in cerebral vessels, and examined its potential role in vascular remodeling. Cell-specific knockouts of β4 integrin revealed that β4 integrin expression in cerebral vessels is derived from endothelial cells, not astrocytes or smooth muscle cells. Lack of endothelial β4 integrin had no effect on vascular development, integrity, or endothelial proliferation, but in the hypoxic CNS its absence led to defective arteriolar remodeling and associated TGF-β signaling. These results reveal high levels of β4 integrin in arteriolar endothelial cells, and demonstrate a novel link between β4 integrin, TGF-β signaling and arteriolar remodeling in cerebral vessels.
Acknowledgments
None
Sources of Funding
This work was supported by the National Multiple Sclerosis Society: by a Harry Weaver Neuroscience Scholar Award to RM (JF 2125A1/1), and by a Post-Doctoral Fellowship to JVW (FG 1879-A-1), and by the NIH RO1 grant NS060770. This is manuscript number 21737 from The Scripps Research Institute.
Footnotes
Disclosures
None
References
- 1.Hynes RO, Lively JC, McCarty JH, Taverna D, Francis SE, Hodivala-Dilke K, Xiao Q. The diverse roles of integrins and their ligands in angiogenesis. Cold Spring Harb Symp Quant Biol. 2002;67:143–153. doi: 10.1101/sqb.2002.67.143. [DOI] [PubMed] [Google Scholar]
- 2.del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol. 2006;26:1966–1975. doi: 10.1161/01.ATV.0000232525.65682.a2. [DOI] [PubMed] [Google Scholar]
- 3.Wagner S, Tagaya M, Koziol J, Quaranta V, del Zoppo GJ. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin α6β4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28:858–865. doi: 10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- 4.Milner R, Campbell IL. Increased expression of the beta 4 and alpha 5 integrin subunits in cerebral blood vessels of transgenic mice chronically producing the pro-inflammatory cytokines IL-6 or IFN-alpha in the central nervous system. Mol Cell Neurosci. 2006;33:429–440. doi: 10.1016/j.mcn.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Previtali S, Archelos J, Hartung H-P. Modulation of the expression of integrins on glial cells during experimental autoimmune encephalomyelitis. Am J Pathol. 1997;151:1425–1435. [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Welser JV, Dore-Duffy P, Del Zoppo GJ, LaManna JC, Milner R. In the hypoxic central nervous system, endothelial cell proliferation is followed by astrocyte activation, proliferation, and increased expression of the α6β4 integrin and dystroglycan. Glia. 2010;58:1157–1167. doi: 10.1002/glia.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feltri ML, Arona M, Scherer SS, Wrabetz L. Cloning and sequence of the cDNA encoding the beta 4 integrin subunit in rat peripheral nerve. Gene. 1997;186:299–304. doi: 10.1016/s0378-1119(96)00725-1. [DOI] [PubMed] [Google Scholar]
- 8.Hiran TS, Mazurkiewicz JE, Kreienberg P, Rice FL, LaFlamme SE. Endothelial expression of the α6β4 integrin is negatively regulated during angiogenesis. J Cell Sci. 2003;116:3771–3781. doi: 10.1242/jcs.00681. [DOI] [PubMed] [Google Scholar]
- 9.Cremona O, Savoia P, Marchisio PC, Gabbiani G, Chapponier C. The α6β4 integrin subunits are expressed by smooth muscle cells of human small vessels; a new localization in mesenchymal cells. J Histochem Cytochem. 1994;42:1221–1228. doi: 10.1177/42.9.8064129. [DOI] [PubMed] [Google Scholar]
- 10.Milner R, Hung S, Wang X, Spatz M, del Zoppo G. The rapid decrease in astrocyte-associated dystroglycan expression by focal cerebral ischemia is protease-dependent. J Cereb Blood Flow Metab. 2008;28:812–823. doi: 10.1038/sj.jcbfm.9600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:3370–3373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- 12.van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nature Genetics. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- 13.Nakano A, Pulkkinen L, Murrell D, Rico J, Lucky AW, Garzon M, Stevens CA, Robertson S, Pfendner E, Uitto J. Epidermolysis bullosa with congenital pyloric atresia: novel mutations in the beta 4 integrin gene (ITGB4) and genotype/phenotype correlations. Pedatr Res. 2001;49:618–626. doi: 10.1203/00006450-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, Sorokin LM. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203:1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nodari A, Previtali SC, Dati G, Occhi S, Court FA, Colombelli C, Zambroni D, Dina G, Del Carro U, Campbell KP, Quattrini A, Wrabetz L, Feltri ML. α6β4 integrin and dystroglycan cooperate to stablize the myelin sheath. J Neurosci. 2008;28:6714–6719. doi: 10.1523/JNEUROSCI.0326-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nature Genetics. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 17.Graus-Porta G, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Muller U. β1-class integrins regulate the development of laminae and folia in the cerebral cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Welser-Alves JV, van der Flier A, Boroujerdi A, Hynes RO, Milner R. An angiogenic role for the α5β1 integrin in promoting endothelial cell proliferation during cerebral hypoxia. Exp Neurol. 2012;237:46–54. doi: 10.1016/j.expneurol.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG. Integrin β4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6:471–483. doi: 10.1016/j.ccr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 20.LaManna JC, Vendel LM, Farrell RM. Brain adaptation to chronic hypobaric hypoxia in rats. J Appl Physiol. 1992;72:2238–2243. doi: 10.1152/jappl.1992.72.6.2238. [DOI] [PubMed] [Google Scholar]
- 21.Milner R, Hung S, Erokwu B, Dore-Duffy P, LaManna JC, del Zoppo GJ. Increased expression of fibronectin and the α5β1 integrin in angiogenic cerebral blood vessels of mice subject to hypobaric hypoxia. Mol Cell Neurosci. 2008;38:43–52. doi: 10.1016/j.mcn.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boroujerdi A, Welser-Alves J, Tigges U, Milner R. Chronic cerebral hypoxia promotes arteriogenic remodeling events that can be identified by reduced endoglin (CD105) expression and a switch in β1 integrins. J Cereb Blood Flow Metab. 2012;32:1820–1830. doi: 10.1038/jcbfm.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alphav beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 25.Rodius S, Indra G, Thibault C, Plister V, Georges-Labouesse E. Loss of alpha6 integrins in keratinocytes leads to an increase in TGFbeta and AP1 signaling and in expression of differentiation genes. J Cell Physiol. 2007;212:439–449. doi: 10.1002/jcp.21040. [DOI] [PubMed] [Google Scholar]
- 26.Owens DM, Romero MR, Gardner C, Watt FM. Suprabasal alpha6 beta4 integrin expression in epidermis results in enhanced tumourigenesis and disruption of TGFbeta signalling. J Cell Sci. 2003;116:3783–3791. doi: 10.1242/jcs.00725. [DOI] [PubMed] [Google Scholar]
- 27.Seki T, Yun J, Oh P. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res. 2003;93:682–689. doi: 10.1161/01.RES.0000095246.40391.3B. [DOI] [PubMed] [Google Scholar]
- 28.van Royen N, Hoefer I, Buschmann IR, Heil M, Kostin S, Deindl E, Vogel S, Korff T, Augustin H, Bode C, Piek JJ, Schaper W. Exogenous application of transforming growth factor beta 1 stimulates arteriogenesis in the peripheral circulation. FASEB J. 2002;16:432–434. doi: 10.1096/fj.01-0563fje. [DOI] [PubMed] [Google Scholar]
- 29.ten Dijke P, Arthur HM. Extracellular control of TGFβ signaling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 30.Goumans M-J, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch. Trends Cardiovasc Med. 2003;13:301–307. doi: 10.1016/s1050-1738(03)00142-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


