Abstract
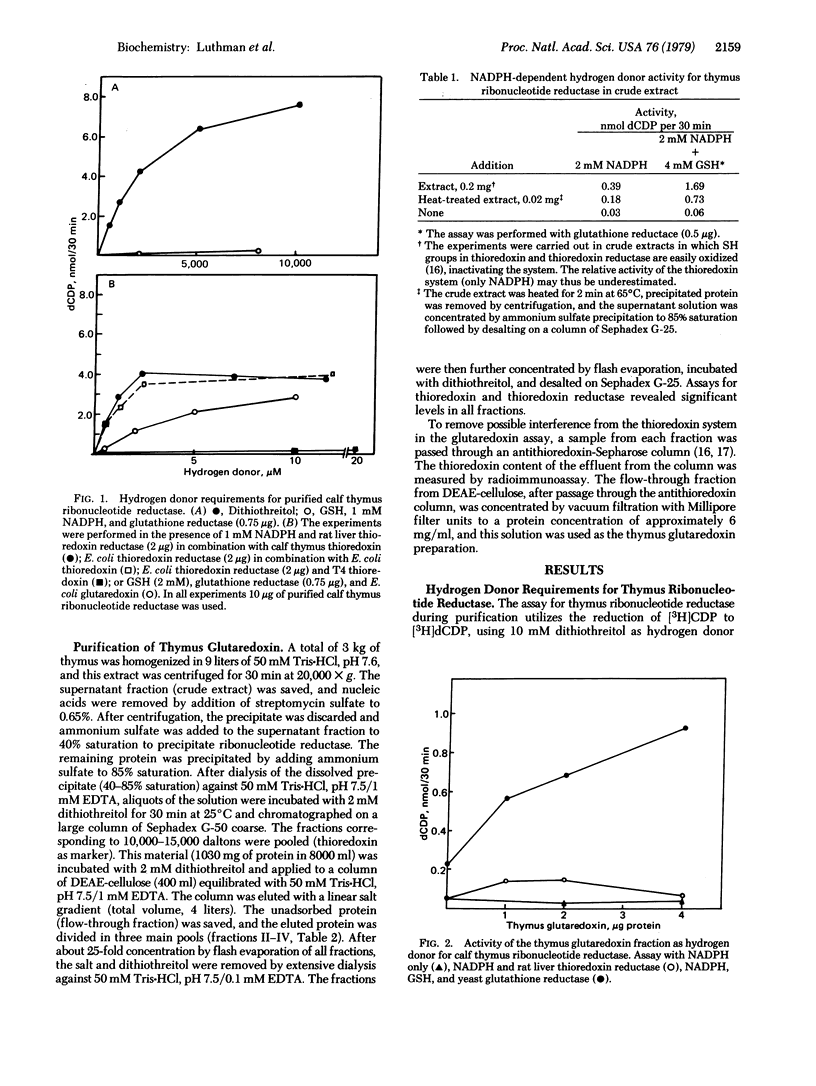
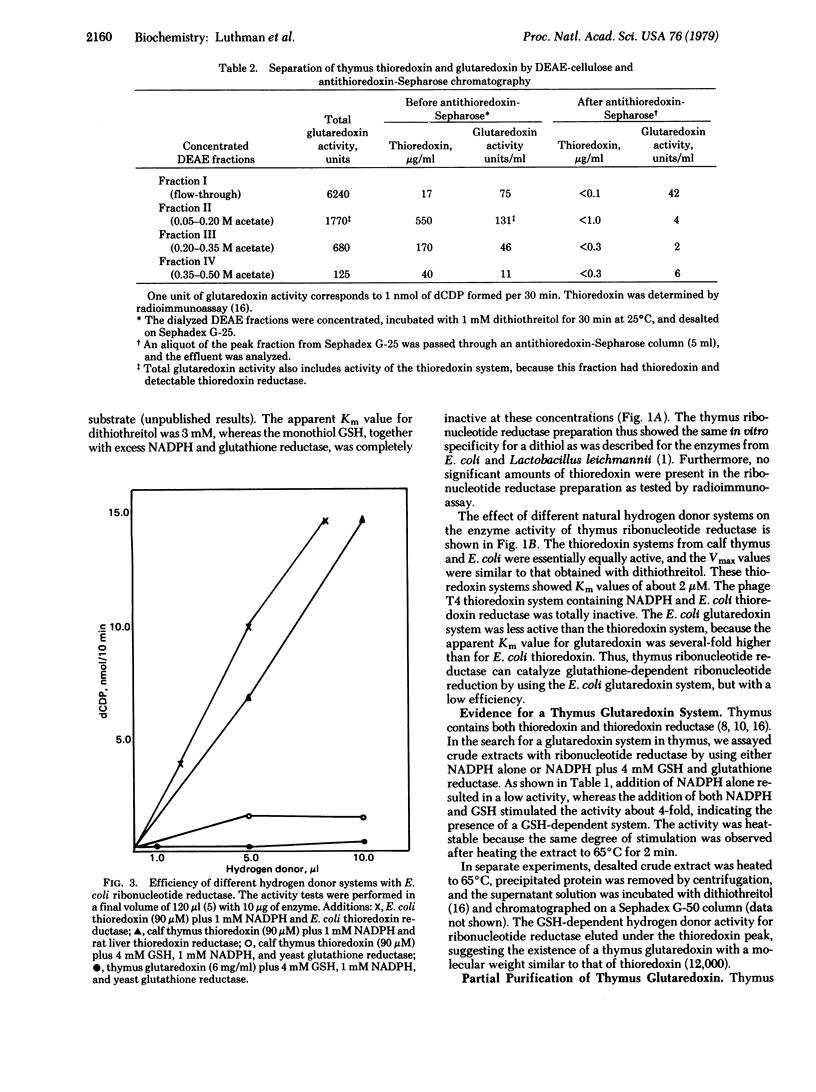
Purified calf thymus ribonucleoside-diphosphate reductase (2'-deoxyribonucleoside-diphosphate:oxidized-thioredoxin 2'-oxidoreductase, EC 1.17.4.1), showed an absolute requirement for a dithiol as hydrogen donor, whereas the natural monothiol glutathione (GSH) was inactive per se. However, a protein partially purified from thymus coupled the oxidation of GSH to the formation of deoxyribonucleotides by ribonucleotide reductase. In analogy with the ribonucleotide reductase system of Escherichia coli this protein was called glutaredoxin [Holmgren, A. (1976) Proc. Natl. Acad. Sci. USA 73, 2275-2279]. Thymus glutaredoxin had the following properties: (i) its molecular weight determined by gel chromatography was about 12,000; (ii) it was active iwth ribonucleotide reductase in the presence of GSH, NADPH, and glutathione reductase but had no activity with NADPH and thioredoxin reductase; and (iii) it was immunologically different from thioredoxin because it did not bind to antithioredoxin immunoadsorbents. Experiments on the crossreactivity of thymus and E. coli ribonucleotide reductases and the corresponding thioredoxin and glutaredoxin systems showed essentially no specificity for the homologous thioredoxin but a high species specificity for the homologous glutaredoxin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berglund O., Sjöberg B. M. A thioredoxin induced by bacteriophage T4. II. Purification and characterization. J Biol Chem. 1970 Nov 25;245(22):6030–6035. [PubMed] [Google Scholar]
- Chamberlin M. Isolation and characterization of prototrophic mutants of Escherichia coli unable to support the intracellular growth of T7. J Virol. 1974 Sep;14(3):509–516. doi: 10.1128/jvi.14.3.509-516.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., McCall B. L., Moore E. C. Purification of thioredoxin reductase from the Novikoff rat tumor. Prep Biochem. 1977;7(2):165–177. doi: 10.1080/00327487708061633. [DOI] [PubMed] [Google Scholar]
- Conley R. R., Pigiet V. In vivo distribution of phosphothioredoxin and thioredoxin in Escherichia coli. J Biol Chem. 1978 Aug 25;253(16):5568–5572. [PubMed] [Google Scholar]
- Elford H. L., Freese M., Passamani E., Morris H. P. Ribonucleotide reductase and cell proliferation. I. Variations of ribonucleotide reductase activity with tumor growth rate in a series of rat hepatomas. J Biol Chem. 1970 Oct 25;245(20):5228–5233. [PubMed] [Google Scholar]
- Engström N. E., Holmgren A., Larsson A., Söderhäll S. Isolation and characterization of calf liver thioredoxin. J Biol Chem. 1974 Jan 10;249(1):205–210. [PubMed] [Google Scholar]
- Eriksson S., Sjöberg B. M., Hahne S. Ribonucleoside diphosphate reductase from Escherichia coli. An immunological assay and a novel purification from an overproducing strain lysogenic for phage lambdadnrd. J Biol Chem. 1977 Sep 10;252(17):6132–6138. [PubMed] [Google Scholar]
- Fuchs J. Isolation of an Escherichia coli mutant deficient in thioredoxin reductase. J Bacteriol. 1977 Feb;129(2):967–972. doi: 10.1128/jb.129.2.967-972.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann E. C., Moore E. C. Purification of thioredoxin from rat Novikoff ascites hepatoma. J Biol Chem. 1973 Feb 25;248(4):1219–1223. [PubMed] [Google Scholar]
- Holmgren A. Bovine thioredoxin system. Purification of thioredoxin reductase from calf liver and thymus and studies of its function in disulfide reduction. J Biol Chem. 1977 Jul 10;252(13):4600–4606. [PubMed] [Google Scholar]
- Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2275–2279. doi: 10.1073/pnas.73.7.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A., Luthman M. Tissue distrubution and subcellular localization of bovine thioredoxin determined by radioimmunoassay. Biochemistry. 1978 Sep 19;17(19):4071–4077. doi: 10.1021/bi00612a031. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Ohlsson I., Grankvist M. L. Thiroedoxin from Escherichia coli. Radioimmunological and enzymatic determinations in wild type cells and mutants defective in phage T7 DNA replication. J Biol Chem. 1978 Jan 25;253(2):430–436. [PubMed] [Google Scholar]
- Holmgren A., Reichard P. Thioredoxin 2: cleavage with cyanogen bromide. Eur J Biochem. 1967 Sep;2(2):187–196. doi: 10.1111/j.1432-1033.1967.tb00125.x. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Söderberg B. O., Eklund H., Brändén C. I. Three-dimensional structure of Escherichia coli thioredoxin-S2 to 2.8 A resolution. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2305–2309. doi: 10.1073/pnas.72.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. 6. The amino acid sequence of the protein from escherichia coli B. Eur J Biochem. 1968 Dec 5;6(4):475–484. doi: 10.1111/j.1432-1033.1968.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Mark D. F., Richardson C. C. Escherichia coli thioredoxin: a subunit of bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1976 Mar;73(3):780–784. doi: 10.1073/pnas.73.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigiet V., Conley R. R. Isolation and characterization of phosphothioredoxin from Excherichia coli. J Biol Chem. 1978 Mar 25;253(6):1910–1920. [PubMed] [Google Scholar]
- Sjöberg B. M., Holmgren A. Purification of thioredoxin from Escherichia coli and bacteriophage T4 by immunoadsorbent affinity chromatography. Biochim Biophys Acta. 1973 Jul 5;315(1):176–180. doi: 10.1016/0005-2744(73)90140-x. [DOI] [PubMed] [Google Scholar]
- Thelander L. Reaction mechanism of ribonucleoside diphosphate reductase from Escherichia coli. Oxidation-reduction-active disulfides in the B1 subunit. J Biol Chem. 1974 Aug 10;249(15):4858–4862. [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Thelander L. Thioredoxin reductase. Characterization of a homogenous preparation from Escherichia coli B. J Biol Chem. 1967 Mar 10;242(5):852–859. [PubMed] [Google Scholar]



