Abstract
Objectives
The purpose of this study was to test the hypothesis that local renal delivery of B-type natriuretic peptide (BNP) will overcome renal resistance to BNP without systemic hypotension.
Background
BNP has vasodilating, natriuretic, and renin-inhibiting properties. In overt heart failure (HF), there is development of renal resistance to BNP.
Methods
We defined the cardiorenal and humoral effects of systemic (n = 6) or local renal (n = 7) administration of canine BNP (0.01 f.Lg/kg/min) in 2 separate groups of dogs with pacing-induced subacute overt HF complicated by renal dysfunction. We used a commercially available small (3.1-F) bifurcated renal catheter (FlowMedica Inc., Fremont, California) for direct bilateral infusion of BNP into both renal arteries.
Results
With systemic BNP at this clinically used dose (without the bolus), urine flow increased, but there was only a trend for an increase in urinary sodium excretion and glomerular filtration rate (GFR). In contrast, local renal delivery of BNP resulted in significant diuresis and natriuresis and an increase in GFR. These diuretic and natriuretic responses were greater with local renal BNP compared with systemic BNP, and were associated with increased delivery of BNP to the renal tubules as evident by a greater urinary BNP excretion resulting in a decrease in distal reabsorption of sodium. Importantly, local renal BNP did not result in a significant decrease in mean arterial pressure that was observed with systemic BNP.
Conclusions
We conclude that local renal BNP delivery is a novel strategy that may overcome renal assistance to BNP in overt HF by increasing local delivery of BNP to the renal tubules.
Keywords: natriuretic peptides, renal function, heart failure
B-type natriuretic peptide (BNP) is a 32-amino acid peptide with vasodilating natriuretic, diuretic, lusitropic, growth-suppressing, and anti-inflammatory properties (1,2). Unlike conventional diuretic agents, BNP may preserve or enhance the glomerular filtration rate (GFR) and suppress plasma renin release (3–6). In 2 recent placebo-controlled clinical trials involving patients undergoing cardiovascular surgery with either left ventricular dysfunction or chronic renal insufficiency, intravenously administered BNP enhanced renal function (7,8). Although BNP has demonstrated renal-enhancing actions in humans and animal models of heart failure (HF), the renal response may also be markedly attenuated or even absent, underscoring a renal resistance to BNP in some patients or under certain experimental conditions (9,10). Furthermore, analysis of clinical trials with BNP in human HF has even suggested a worsening of renal function (11).
Mechanisms that limit the renal actions of the natriuretic peptides in overt HF may be linked to humoral and hemodynamic factors. Our group and others have recently reported data in overt experimental HF that phosphodiesterase V (PDEV) inhibition may enhance the renal response to BNP (12). This indicates that PDEV activity, which degrades BNP-mediated generation of its second messenger 3′,5′ cyclic guanosine monophosphate (cGMP), may limit the full renal actions of BNP in overt HF. Another important braking action on the renal actions of the natriuretic peptides is renal perfusion pressure. Specifically, studies have clearly demonstrated that a reduction in renal perfusion pressure by supra-aortic constriction in experimental studies in canines blunts the renal response to natriuretic peptide administration (13). This concept of the importance of renal perfusion pressure is underscored in human HF in a retrospective study of patients hospitalized with acute HF and hypotension whose renal function improved in response to infusion of nonhypotensive doses of BNP (14).
The goal of the current study was to test the hypothesis that increasing the local renal delivery of BNP directly into the kidney, avoiding systemic hypotension and reductions in renal perfusion pressure, would result in renal-enhancing actions exceeding systemic BNP administration. We tested this hypothesis in a well-characterized canine model of subacute overt HF characterized by avid sodium and water retention, marked activation of the renin-angiotensin-aldosterone system (RAAS), severe renal vasoconstriction, and reductions in GFR, thus mimicking renal and neuro-humoral characteristics of the cardiorenal syndrome. If such an approach proved successful, it would provide insight into optimizing the use of a natriuretic peptide for renal dysfunction associated with HF through local and not systemic peptide delivery. The clinical importance of testing this hypothesis is supported by epidemiological data from the ADHERE (Acute Decompensated Heart Failure National Registry) study that reported that a major unmet need in the management of acute decompensated HF is a pharmacological approach to treat marked renal dysfunction, which occurs in at least 65% of patients hospitalized for acute HF and which is associated with excess mortality and morbidity (3,5).
Methods
Studies were conducted in 3 groups of anesthetized male mongrel dogs (18 to 23 kg). The first group (n = 6) were normal dogs that served as a reference group to characterize cardiorenal and neurohumoral function in the absence of HF. The 2 other groups were groups with subacute overt HF produced by rapid ventricular pacing at 240 beats/min for 10 days on a fixed sodium diet. The 2 HF groups consisted of a systemic delivery (systemic) group (n = 6) and a local renal delivery (local) group (n = 7). Studies were performed in accordance with the Animal Welfare Act and with approval of the Mayo Clinic Institutional Animal Care and Use Committee.
As stated above, we utilized a well-characterized model of overt HF in canines, produced by rapid ventricular pacing. All HF dogs underwent implantation of a programmable cardiac pacemaker (Medtronic, Minneapolis, Minnesota). Under pentobarbital sodium anesthesia (30 mg/kg, intravenous) and artificial ventilation (Harvard respirator, Harvard Apparatus, Millis, Massachusetts) with 5 l/min supplemental oxygen, a left lateral thoracotomy, and a pericardiotomy were performed. With the heart exposed, a screw-in epicardial pacemaker lead was implanted into the right ventricle. The pacemaker generator was implanted subcutaneously into the left chest wall and connected to the pacemaker lead. Dogs received pre- and postoperative prophylactic antibiotic treatment with 225 mg of clindamycin subcutaneous and 400,000 U of procaine penicillin G plus 500 mg of dihydrostreptomycin intramuscularly (Combiotic, Pfizer, Inc., New York, New York). Post-operative prophylactic antibiotic was continued through the first 2 postoperative days. Dogs were fed a fixed sodium diet (58 mEq/day, Hill’s ID, Topeka, Kansas) and allowed water ad lib. All dogs were walked daily. Appetite, activity, body temperature, and condition of surgical skin sites were documented. Following a 14-day post-operative recovery period, the pacemaker was turned on at 240 beats/min.
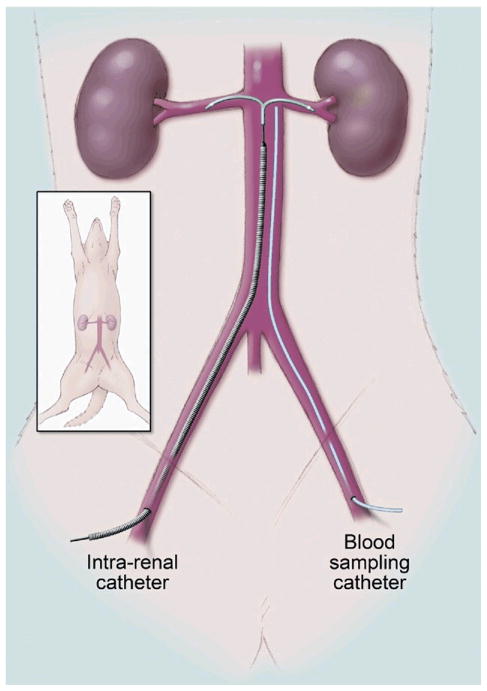
On Day 11 of rapid ventricular pacing at 240 beats/min, an acute experiment was carried out to determine the cardiorenal and humoral effects of systemic (n = 6) or local (n = 7) administration of canine BNP (0.01 f.Lg/kg/min). On the night before experimentation, animals were fasted and given 300 mg of lithium carbonate for assessment of renal tubular function. On the day of the experiment, dogs were anesthetized with sodium pentobarbital (15 mg/kg, intravenous), intubated, and mechanically ventilated with supplemental oxygen (Harvard respirator) at 12 cycles/min. A flow-directed balloon-tipped thermodilution catheter (Ohmeda, Criticath, Madison, Wisconsin) was advanced into the pulmonary artery via the external jugular vein for cardiac hemodynamic measurement. The left femoral artery was cannulated for blood pressure monitoring and blood sampling; the catheter was placed just below the level of the renal arteries to allow us to estimate the concentration of BNP that is delivered to the kidneys (Fig. 1). The femoral vein was also cannulated for inulin and normal saline infusion. The left kidney was exposed via a flank incision, and the ureter was cannulated for urine collection. A calibrated electromagnetic flow probe was placed around the renal artery to measure renal blood flow (RBF). In the local group, the FlowMedica (Fremont, California) bifurcated infusion catheter was inserted as under fluoroscopy, over a 0.035-inch guidewire up the aorta to the renal arteries via a right femoral arterial access after injection of 3 ml of contrast (Visipaque, GE Healthcare, Waukesha, Wisconsin) via a pigtail catheter (Fig. 1). Fluoroscopy and direct palpation of the exposed renal artery confirmed the position of the catheter.
Figure 1.
Illustration of the Benephit CV Intrarenal Catheter and the Infrarenal Catheter for Blood Sampling
Figure illustration by Mayo Clinic.
The experiment began after a 60-min equilibration period, with a 30-min baseline urinary clearance. After the 30-min baseline urinary clearance, systemic or local infusion of 0.01 f.Lg/kg/min of canine BNP was started. Following a 15-min lead-in period, a 30-min clearance period was performed. Cardiovascular parameters measured during the acute experiment included mean arterial blood pressure (MAP), right atrial pressure (RAP), pulmonary artery pressure (PAP), cardiac output (CO), and pulmonary capillary wedge pressure (PCWP). CO was determined by thermodilution in triplicate and averaged (Cardiac Output model 9510-A computer, American Edwards Laboratories, Irvine, California). MAP was assessed via direct measurement from the femoral arterial catheter. Renal perfusion pressure was calculated as MAP – RAP in mm Hg. Inulin was administered intravenously at the start of the equilibration period as a calculated bolus, followed by a 1 ml/min continuous infusion to achieve plasma levels of 40 to 60 mg/dl. GFR was calculated by inulin clearance.
Cardiovascular hemodynamics was measured at the start of each clearance. Arterial blood was collected in heparin and EDTA tubes and immediately placed on ice midway through each clearance. After centrifugation at 2,500 rpm at 4°C, plasma was decanted and stored at −20°C until analysis. Urine was collected on ice during the entire period of each clearance for assessment of urine volume, electrolytes, and inulin. Urine collected for cGMP analysis was heated to more than 90°C before storage.
Plasma and urinary samples for BNP, renin, aldosterone, angiotensin II, and cGMP were measured by radioimmunoassay using the methods previously described (12). Urinary and plasma inulin concentrations were measured by the anthrone method. Urinary and plasma lithium levels were determined by flame emission spectrophotometry (model 357, Instrumentation Laboratory, Wilmington, Massachusetts). Employing the lithium clearance (CLLi) technique, distal factional reabsorption of sodium (DFRNa) was calculated utilizing the following equation: DFRNa − (CLLi − CLNa)/CLLi × 100, where CLLi = (urine Li × urine flow)/plasma Li and CLNa = (urine Na × urine flow)/plasma Na.
Sample size
Our sample size calculation was based on our previous studies in the model of overt experimental HF. On the basis of our previous experimental data, we calculated that a sample size of 6 to 7 animals in each group will give us 89% power to detect a difference of 15 mm Hg in MAP, 80% power to detect a difference of 25 ml/min in GFR, 73% power to detect a difference of 40 f.LEq/min in urinary sodium excretion, and 77% power to detect a difference of 0.6 ml/min in urine flow (UV). This calculation was based on a paired t test with a significance level of p < 0.05.
Statistical analysis
Results are expressed as mean ± SD. Comparisons between groups were completed with a 2-sample t test if the data were normally distributed or a Wilcoxon rank sum test for non-normal factors. Within-group measurements were compared using a paired t test for normally distributed differences or Wilcoxon signed rank was used to evaluate the difference between the groups, adjusting for the baseline measurements of those same factors. Statistical significance was accepted as p < 0.05.
Results
Cardiorenal and humoral function in experimental overt HF at baseline
Table 1 reports cardiorenal and hormonal function for normal dogs (n = 6) and for the overt HF groups combined (n = 13). Compared with the normal group, the overt HF group had a significant decrease in CO and MAP associated with significant increases in cardiac filling pressures. Renal blood flow, GFR, urinary sodium excretion, and UV were all significantly reduced in the overt HF group. There was intense activation of RAAS and a strong trend for increased plasma BNP levels in the overt HF group.
Table 1.
Cardiorenal and Humoral Characteristics of Normal and Overt HF Dogs
| Normal (n = 6) | Overt HF (n = 13) | |
|---|---|---|
| MAP, mm Hg | 112 ± 3 | 98 ± 7* |
| CO, l/min | 3.3 ± 0.3 | 1.7 ± 0.3* |
| PCWP, mm Hg | 5 ± 0.5 | 21 ± 3† |
| RAP, mm Hg | 3 ± 0.5 | 11 ± 2* |
| UNaV, f.LEq/min | 69 ± 59 | 3 ± 4† |
| Urine flow, ml/min | 0.6 ± 0.4 | 0.16 ± 0.1† |
| GFR, ml/min | 47 ± 20 | 30 ± 11* |
| RBF, ml/min | 240 ± 67 | 152 ± 60* |
| Plasma BNP, pg/ml | 23 ± 4 | 53 ± 25‡ |
| Plasma renin, ng/ml/h | 3 ± 2 | 20 ± 7* |
| Aldosterone, ng/dl | 16 ± 12 | 53 ± 31† |
| Angiotensin II, pg/ml | 10 ± 4 | 74 ± 40* |
Data are presented as mean ± SD.
p < 0.05 versus normal (2-sample t test);
p < 0.05 versus normal (Wilcoxon rank sum test).
p = 0.08 versus normal (Wilcoxon rank sum test).
BNP = B-type natriuretic peptide; CO = cardiac output; GFR = glomerular filtration rate; HF = heart failure; MAP = mean arterial blood pressure; PCWP = pulmonary capillary wedge pressure; RAP = right atrial pressure; RBF = renal blood flow; UNaV = urinary sodium excretion.
Cardiovascular hemodynamic responses to systemic versus local renal BNP delivery in experimental HF
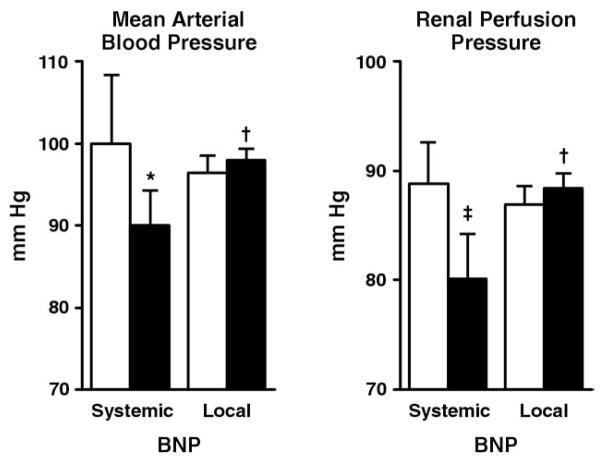
Systemic infusion of BNP resulted in a significant decrease in MAP, whereas local renal BNP infusion did not change MAP (Fig. 2). This was associated with a strong trend (p = 0.07) for a decrease in renal perfusion pressure with systemic BNP, whereas renal perfusion pressure was maintained with local renal BNP. At the end of the 30-min infusion period, MAP and renal perfusion pressure were significantly higher in the local renal BNP group compared with the systemic BNP group. Systemic BNP delivery significantly decreased RAP, PCWP, and PAP, which was greater compared with local renal BNP delivery. Local renal BNP delivery only resulted in a very modest decrease in PCWP and PAP (Table 2). Renal blood flow increased similarly in both groups.
Figure 2.
Mean Arterial Blood Pressure and Renal Perfusion Pressure in the Systemic and Local BNP Delivery Groups
Open bars represent at baseline, and solid bars represent after 30 min of infusion. *p < 0.05 versus baseline (paired t test); †p < 0.05 local BNP versus systemic B-type natriuretic peptide (BNP) (analysis of covariance [ANCOVA]); ‡p= 0.07 versus baseline (paired t test).
Table 2.
Cardiovascular Hemodynamics
| Baseline | 0.01 f..g/kg/min | |
|---|---|---|
| Systemic BNP (n = 6) | ||
| MAP, mm Hg | 100 ± 8 | 90 ± 10*† |
| CO, l/min | 1.8 ± 0.4 | 1.8 ± 0.3 |
| PAP, mm Hg | 27 ± 3 | 23 ± 2†‡ |
| PCWP, mm Hg | 22 ± 2 | 19 ± 1†‡ |
| RAP, mm Hg | 11 ± 3 | 9 ± 2†‡ |
| RBF, ml/min | 138 ± 73 | 153 ± 80* |
| Local BNP (n = 7) | ||
| MAP, mm Hg | 96 ± 6 | 98 ± 4 |
| CO, l/min | 1.8 ± 0.3 | 1.8 ± 0.3 |
| PAP, mm Hg | 24 ± 4 | 23 ± 4‡ |
| PCWP, mm Hg | 20 ± 4 | 19 ± 4‡ |
| RAP, mm Hg | 10 ± 1 | 9.6 ± 0.8 |
| RBF, ml/min | 163 ± 48 | 186 ± 45* |
Data are presented as mean ± SD.
p < 0.05 versus baseline (paired t test);
p < 0.05 systemic versus local (analysis of covariance);
p < 0.05 versus baseline (Wilcoxon signed rank test).
PAP = mean pulmonary arterial pressure; other abbreviations as in Table 1.
Renal excretory and humoral function to systemic versus local renal BNP delivery in experimental HF
Figure 3 illustrates the response in urinary sodium excretion (UNaV), UV, and GFR to systemic versuslocal renal BNP delivery. Systemic BNP delivery increased UV but did not produce a significant change in UNaV or GFR. In contrast, local renal BNP delivery increased UV, UNaV, and GFR significantly. The diuretic and natriuretic actions of local renal BNP delivery were significantly greater compared with systemic BNP delivery. These renal effects of local renal BNP delivery were associated with increased delivery of BNP to the kidney, as evidenced by a greater infrarenal arterial BNP concentration (see the Methods section) and urinary BNP excretion with a trend for urinary cGMP excretion to be greater compared with systemic BNP administration (Fig. 4). Local renal delivery of BNP suppressed plasma renin activity (23 ± 3 ng/ml/h to 16 ± 3 ng/ml/h, p < 0.05) and angiotensin II (69 ± 36 pg/ml to 37 ± 15 pg/ml, p < 0.05), whereas systemic BNP did not (plasma renin activity15 ± 8 ng/ml/h to 12 ± 5 ng/ml/h, p > 0.05; angiotensin II 79 ± 45 pg/ml to 119 ± 144 pg/ml, p > 0.05). Local BNP also decreased sodium reabsorption (from 99 ± 1% to 96 ± 1.7%, p < 0.05) in the distal tubules, the site in the renal tubules that the natriuretic peptides act on, whereas there was only a trend for distal tubular sodium reabsorption to decrease (from 99 ± 1% to 98 ± 1.8%, p = 0.4) with systemic BNP.
Figure 3.
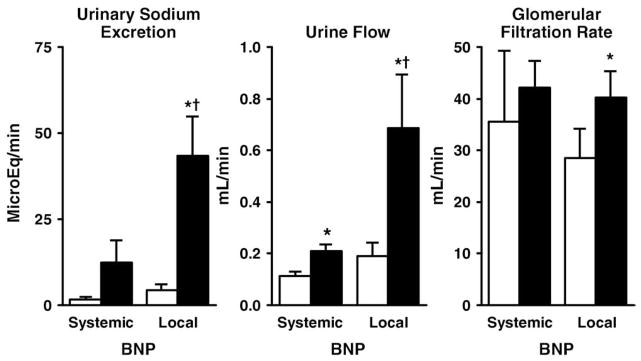
Urinary Sodium Excretion, Urine Flow, and Glomerular Filtration Rate in the Systemic and Local BNP Delivery Groups
Open bars represent at baseline, and solid bars represent after 30 min of infusion.
*p < 0.05 versus baseline (paired t test); †p < 0.05 local B-type natriuretic peptide (BNP) versus systemic BNP (rank sum test).
Figure 4.

Infrarenal Arterial BNP Concentration, Urinary BNP Excretion, and Urinary cGMP Excretion in the Systemic and Local BNP Delivery Groups
Open bars represent at baseline, and solid bars represent after 30 min of infusion. *p < 0.05 versus baseline (paired t test); †p < 0.05 local B-type natriuretic peptide (BNP) versus systemic BNP (rank sum test); ‡p < 0.05 versus baseline (signed rank test); #p = 0.1 local BNP versus systemic BNP (t test). cGMP = 3′,5′ cyclic guanosine monophosphate.
Discussion
The goal of the current study was to test the hypothesis that local renal delivery of BNP directly into the kidneys via increasing renal concentrations of BNP in the absence of systemic hypotension would augment the renal-enhancing actions of BNP. These studies demonstrated that systemic infusion of the clinical dose of BNP (without the bolus) was associated with a decrease in blood pressure and renal perfusion pressure, and only a trend for sodium excretion or GFR to increase. In contrast, local renal BNP delivery into both kidneys did not result in a decrease in blood pressure, maintained renal perfusion pressure, and was associated with significant increases in urinary sodium excretion and GFR, and suppression of the RAAS. Previous experimental studies have demonstrated that renal excretory function is dependent on renal perfusion pressure; hence, the greater increase in UV and urinary sodium excretion with local renal BNP delivery may be due in part to the maintenance of renal perfusion pressure. Indeed, there was a strong trend for correlation between changes in renal perfusion pressure and UV; however, it did not reach statistical significance (r = 0.52, p = 0.07). Despite the decrease in renal perfusion pressure with systemic infusion of BNP, there was still an increase in UV and a trend for sodium excretion and GFR to increase. This suggests that BNP has direct action on the nephron in addition to hemodynamic actions. Indeed, previous studies have demonstrated that BNP acts on the mesangial cells to increase the glomerular ultrafiltration coefficient, which in turn increases the GFR (15–17).
Our group and others have demonstrated in pre-clinical studies that the renal actions of BNP are attenuated in overt HF compared with mild HF and normal controls (18). The mechanisms that mediate the attenuated response to natriuretic peptides in overt HF remain poorly defined and are most likely multifactorial. These include increased degradation of the peptide by neutral endopeptidase, decreased number or reduced affinity of biological receptors, or post-receptor events leading to reduced production of cGMP or increased cGMP degradation by PDEV (10).
It is known that clearance of BNP occurs by several routes, all of which exist within the kidneys, and these routes include degradation by neutral endopeptidase 24.11 (NEP) and clearance by NRP-C. NEP is a metalloprotease that is localized in greatest abundance in the kidney and degrades the natriuretic peptides. It is found at highest abundance in the proximal tubules and has been shown to be up-regulated in overt HF (19). In the current study, the improvement in sodium excretion and GFR with local renal delivery of BNP was associated with greater urinary BNP excretion compared with systemic infusion. This suggests that local renal BNP infusion delivered more BNP to the kidneys, which may have overwhelmed the different degradative and clearance pathways for BNP in the kidneys, and thus more BNP reached the glomerulus and renal tubules to act on the natriuretic peptide receptors found there (20). This is further supported by the observation that only in the local renal delivery group did we observe a decrease in distal tubular sodium reabsorption, a site that is rich in natriuretic peptide receptors.
As expected, systemic BNP delivery reduced cardiac filling pressures in association with hypotension. Of note, despite the lack of change in arterial pressure with local renal delivery of BNP, there was a very modest decrease in cardiac filling pressures, which was less than with systemic delivery. This most likely reflects a combination of systemic spillover and the increased natriuresis with the local renal BNP delivery.
The current study has clinical implications. The Benephit CV Infusion System (FlowMedica, Inc.) is a Food and Drug Administration 510(k)-cleared device indicated for the infusion of physician-specified agents into the renal arteries (21). This system is currently used in the coronary catheterization laboratory for the direct renal infusion of agents to the kidney to prevent contrast nephropathy. On the basis of the findings of the current pre-clinical study, we are conducting a pilot clinical study to assess the safety and efficacy of intrarenal BNP infusion in HF patients with cardiorenal syndrome.
Conclusions
In overt experimental HF with renal dysfunction, local renal delivery of BNP has greater renal-enhancing effects compared with systemic BNP delivery. These favorable renal effects include greater natriuretic and diuretic responses with increases in GFR and suppression of the RAAS. These renal-enhancing responses were associated with no change in arterial pressure and the maintenance of renal perfusion pressure, which we speculate also contributed to renal responsiveness to local delivery. We conclude that local renal delivery of BNP is a novel strategy that may enhance renal function in HF by increasing local delivery of BNP to the renal glomerulus and tubules.
Acknowledgments
This research was supported by FlowMedica Inc. However, this is an investigator-initiated study, and the sponsor had no role in the design of the study and analysis of the data. Drs. Chen and Burnett have received honoraria and research grants from FlowMedica Inc. and Scios Inc. Drs. Chen and Burnett have patented and received royalties for designer natriuretic peptides. Dr. Chen has received royalties from Uptodate Inc. Mayo Clinic has patented and licensed designer natriuretic peptides.
The authors gratefully acknowledge the assistance of Denise M. Heublein, Sharon S. Sandberg, and Gail Harty.
Abbreviations and Acronyms
- BNP
B-type natriuretic peptide
- cGMP
3′,5′ cyclic guanosine monophosphate
- CO
cardiac output
- GFR
glomerular filtration rate
- HF
heart failure
- MAP
mean arterial blood pressure
- PAP
pulmonary artery pressure
- PCWP
pulmonary capillary wedge pressure
- PDEV
phosphodiesterase V
- RAAS
renin-angiotensin-aldosterone system
- RAP
right atrial pressure
References
- 1.Silver MA, Horton DP, Ghali JK, Elkayam U. Effect of nesiritide versus dobutamine on short-term outcomes in the treatment of patients with acutely decompensated heart failure. J Am Coll Cardiol. 2002;39:798–803. doi: 10.1016/s0735-1097(01)01818-6. [DOI] [PubMed] [Google Scholar]
- 2.Chen HH, Burnett JC., Jr Therapeutic potential for existing and novel forms of natriuretic peptides. Heart Fail Clin. 2006;2:365–73. doi: 10.1016/j.hfc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–30. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Chen HH, Redfield MM, Nordstrom LJ, Horton DP, Burnett JC., Jr Subcutaneous administration of the cardiac hormone BNP in symptomatic human heart failure. J Card Fail. 2004;10:115–9. doi: 10.1016/j.cardfail.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Ho A, Kiger E, Mathur VS, Heywood JT. Renal and hemodynamic effects of intra-renal nesiritide (B-type natriuretic peptide) in heart transplant patients. J Am Soc Nephrol. 2005;16:618A. [Google Scholar]
- 7.Mentzer RM, Jr, Oz MC, Sladen RN, et al. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: the NAPA trial. J Am Coll Cardiol. 2007;49:716–26. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 8.Chen HH, Sundt TM, Cook DJ, Heublein DM, Burnett JC., Jr Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary-bypass surgery: a double-blind placebo-controlled pilot study. Circulation. 2007;116:I134–8. doi: 10.1161/CIRCULATIONAHA.106.697250. [DOI] [PubMed] [Google Scholar]
- 9.Forfia PR, Lee M, Tunin RS, Mahmud M, Champion HC, Kass DA. Acute phosphodiesterase 5 inhibition mimics hemodynamic effects of B-type natriuretic peptide and potentiates B-type natriuretic peptide effects in failing but not normal canine heart. J Am Coll Cardiol. 2007;49:1079–88. doi: 10.1016/j.jacc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 10.Chen HH. Heart failure: a state of brain natriuretic peptide deficiency or resistance or both! J Am Coll Cardiol. 2007;49:1089–91. doi: 10.1016/j.jacc.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–91. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 12.Chen HH, Huntley BK, Schirger JA, Cataliotti A, Burnett JC., Jr Maximizing the renal cyclic 3′-5′-guanosine monophosphate system with type V phosphodiesterase inhibition and exogenous natriuretic peptide: a novel strategy to improve renal function in experimental overt heart failure. J Am Soc Nephrol. 2006;17:2742–7. doi: 10.1681/ASN.2006020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redfield MM, Edwards BS, Heublein DM, Burnett JC., Jr Restoration of renal response to atrial natriuretic factor in experimental low-output heart failure. Am J Physiol. 1989;257:R917–23. doi: 10.1152/ajpregu.1989.257.4.R917. [DOI] [PubMed] [Google Scholar]
- 14.Riter H, Redfield MM, Burnett JC, Chen HH. Non-hypotensive low dose nesiritide has differential renal effects compared to standard dose nesiritide in patients with acute decompensated heart failure and renal dysfunction. J Am Coll Cardiol. 2006;47:2334–5. doi: 10.1016/j.jacc.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Barrio V, De Arriba G, Lopez-Novoa JM, Rodriguez-Puyol D. Atrial natriuretic peptide inhibits glomerular contraction induced by angiotensin II and platelet activating factor. Eur J Pharmacol. 1987;135:93– 6. doi: 10.1016/0014-2999(87)90762-x. [DOI] [PubMed] [Google Scholar]
- 16.Butlen D, Mistaoui M, Morel F. Atrial natriuretic peptide receptors along the rat and rabbit nephrons: [125I] alpha-rat atrial natriuretic peptide binding in microdissected glomeruli and tubules. Pflugers Arch. 1987;408:356–65. doi: 10.1007/BF00581129. [DOI] [PubMed] [Google Scholar]
- 17.Umemura S, Toya Y, Hirawa N, et al. Inhibitory effect of human atrial natriuretic peptide on cyclic AMP levels in microdissected human glomeruli. J Cardiovasc Pharmacol. 1989;13 (Suppl 6):S36–8. [PubMed] [Google Scholar]
- 18.Chen HH, Schirger JA, Chau WL, et al. Renal response to acute neutral endopeptidase inhibition in mild and severe experimental heart failure. Circulation. 1999;100:2443–8. doi: 10.1161/01.cir.100.24.2443. [DOI] [PubMed] [Google Scholar]
- 19.Margulies KB, Barclay PL, Burnett JC., Jr The role of neutral endopeptidase in dogs with evolving congestive heart failure. Circulation. 1995;91:2036–42. doi: 10.1161/01.cir.91.7.2036. [DOI] [PubMed] [Google Scholar]
- 20.Millul V, Ardaillou N, Placier S, Baudouin B, Ronco PM. Receptors for natriuretic peptides in a human cortical collecting duct cell line. Kidney Int. 1997;51:281–7. doi: 10.1038/ki.1997.34. [DOI] [PubMed] [Google Scholar]
- 21.Allie DE, Lirtzman MD, Wyatt CH, et al. Targeted renal therapy and contrast-induced nephropathy during endovascular abdominal aortic aneurysm repair: results of a feasibility pilot trial. J Endovasc Ther. 2007;14:520–7. doi: 10.1177/152660280701400413. [DOI] [PubMed] [Google Scholar]