Abstract
The surface properties of materials contribute to host cellular response and play a significant role in determining the overall success or failure of an implanted biomaterial. Rough titanium (Ti) surface microtopography and high surface free energy have been shown to enhance osteoblast maturation in vitro and increase bone formation in vivo. While the surface properties of Ti are known to affect osteoblast response, host bone quality also plays a significant role in determining successful osseointegration. One factor affecting host bone quality is patient age. We examined both in vitro and in vivo whether response to Ti surface features was affected by animal age. Calvarial osteoblasts isolated from 1-, 3-, and 11-month-old rats all displayed a reduction in cell number and increases in alkaline phosphatase specific activity and osteocalcin in response to increasing Ti surface microtopography and surface energy. Further, osteoblasts from the three ages examined displayed increased production of osteocalcin and local factors osteoprotegerin, VEGF-A, and active TGF-β1 in response to increasing Ti surface roughness and surface energy. Latent TGF-β1 only increased in cultures of osteoblasts from 1- and 3-month-old rats. Treatment with the systemic osteotropic hormone 1α,25(OH)2D3 further enhanced the response of osteoblasts to Ti surface features for all three age groups. However, osteoblasts derived from 11-month-old animals had a reduced response to 1α,25(OH)2D3 as compared to osteoblasts derived from 1-or 3-month-old animals. These results were confirmed in vivo. Ti implants placed in the femoral intramedullary canal of old (9-month) mice yielded lower bone-to-implant contract and neovascularization in response to Ti surface roughness and energy compared to younger (2-month) mice. These results show that rodent osteoblast maturation in vitro as well as new bone formation in vivo is reduced with age. Whether comparable age differences exist in humans needs to be determined.
INTRODUCTION
The surface properties of materials and the quality of the bone contribute to host cellular response and ultimately determine the overall success or failure of an implanted biomaterial. Cells interact with the surface of a material through an adsorbed layer of proteins, ions, sugars, and lipids present in the blood and tissue fluid. Surface properties including topography, surface energy, chemistry, and surface charge are largely responsible for this interaction (1–3).
In vitro studies show that modifications to titanium (Ti) substrate micro-architecture have effects on the attachment and differentiation of osteoblast-like cells, including MG63 and MC3T3-E1 cell lines, as well as fetal rat calvarial cells and normal human osteoblasts (4). On micron- and submicron-scale rough Ti substrates, cell proliferation is decreased and differentiation is increased, as these cells display changes in alkaline phosphatase specific activity and increased osteocalcin production (5). Further, MG63 osteoblast-like cells cultured on these rough Ti surfaces release increased levels of local bone regulatory factors, including osteoprotegerin (OPG), prostaglandin E2 (PGE2), and transforming growth factor β1 (TGF-β1). These surface dependent effects are mediated by alpha-2, beta-1 (α2β1) integrin signaling (6). Osteoblastic differentiation of human mesenchymal stem cells (HMSCs) is also increased on Ti surfaces with micron- and submicron scale rough topographies, and this is mediated by α2β1 integrin signaling (7). Taken together, these results indicate that rough Ti substrates promote osteoblast differentiation and maturation more than smooth Ti surfaces.
In addition to surface roughness, surface free energy of Ti substrates also plays a role in regulating bone cell responsiveness. Ti implant surfaces have a low surface free energy due to the adsorption of hydrocarbons and carbonates from the ambient atmosphere onto the TiO2 surface layer. This adsorption also makes the Ti surfaces hydrophobic. Clean TiO2 surfaces are hydrophilic with a high surface free energy (8), and this can be maintained by limiting exposure to ambient air conditions. MG63 cells cultured on high surface free energy substrates show an enhanced response to Ti surface microtopography. On microrough, high surface free energy Ti substrates, these cells show increases in alkaline phosphatase specific activity, osteocalcin production, and levels of TGF-β1 and OPG compared to rough Ti surfaces alone (9).
Human osteoblasts and HMSCs also exhibit enhanced differentiation and osteogenic factor production on the high-energy surfaces (10,11). Importantly, factors secreted into the conditioned media of osteoblasts grown on the high-energy microtextured Ti surfaces can act on HMSCs cultured on tissue culture polystyrene to induce their osteoblastic differentiation (7). These results suggest that peri-implant bone formation in vivo may be modulated by events associated with surface design and that the cells populating the surface will contribute to the peri-implant milieu.
In vivo, Ti substrates with a rough microtopography support greater bone-to-implant contact than smooth surfaces do, resulting in greater removal torque strength (12–14). Preliminary studies have indicated that high surface free energy Ti surfaces have the potential to improve the early stages of soft and hard tissue integration of implants (15–17). One contributing factor to this may be an increase in vascularization. Both osteoblasts and HMSCs produce increased levels of angiogenic growth factors including vascular endothelial growth factor A (VEGF-A), basic fibroblast growth factor (FGF-2), and endothelial growth factor (EGF) (7,10,18). Whether angiogenesis is increased in vivo, however, is not known.
Host bone quality also plays a significant role in determining successful osseointegration. Bone quality and subsequent bone formation can be affected by several factors including smoking, diabetes, cancer and other diseases (19,20). Another factor that is known to play a role in bone formation is age. Several studies have shown that both natural bone formation and bone fracture healing in response to osteoinductive factors is impaired in an aged population (21,22). The results of these studies suggest that new bone formation surrounding Ti implants may also be affected by patient age.
In the present study, osteoblast maturation in response to Ti surface microtopography and surface free energy was assessed with respect to donor age. To do this, osteoblasts were isolated from 1-month, 3-month, and 11-month-old Sprague-Dawley rats, representing young, adult, and old age groups, respectively, and were cultured on Ti substrates with two different surface morphologies and identical morphology but different surface hydrophilicity. In addition, we investigated whether donor age had an effect on the surface-dependent responses to the systemic regulatory hormone 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3]. To investigate potential mechanisms underlying age-specific differences in cell response, the expression of mRNAs for integrin subunits and receptors for 1α,25(OH)2D3 in osteoblasts derived from young and old populations in response to Ti substrate surface properties was also examined. Finally, the effects of surface microstructure and surface free energy on bone formation and vascularity in vivo were examined using a novel peri-implant bone formation model in which implants were placed in the femoral intramedullary canal of young and old C57BL/6 mice.
MATERIALS & METHODS
In Vitro Studies
Titanium Disk Preparation
Ti disks (15mm diameter) were prepared from 1mm thick sheets of grade 2 unalloyed Ti as described previously (23) and were supplied by Institut Straumann AG (Basel, Switzerland). Fabrication methods and resulting morphology of the surfaces have been reported previously (8,11,24). Pretreatment (PT) surfaces are smooth, with a mean peak to valley roughness (Ra) of 600 nm. Sandblasted with large grit and acid etched (SLA) surfaces were created from PT disks that were coarse grit-blasted with 0.25–0.50 mm corundum until the surface reached a uniform gray tone, followed by acid etching, resulting in a combination of micron and sub-micron scale features (Ra= 3.2 µm). Modified SLA (modSLA) surfaces were created in the same manner as SLA but they were processed with limited exposure to the ambient atmosphere, resulting in a hydrophilic substrate with the same topographical characteristics. modSLA surfaces were rinsed under nitrogen during fabrication and stored in isotonic sodium chloride until use. PT, SLA and modSLA Ti disks all have a TiO2 surface layer, with the PT and SLA surfaces being hydrophobic due to the adsorption of atmospheric hydrocarbons while the modSLA surface is hydrophilic. Advancing contact angles were used to calculate the hydrophilicity of the surfaces as PT (95.8°), SLA (139.80°), and modSLA (~0°). Surface free energy for PT, SLA, and modSLA surfaces was calculated according to Zisman (critical surface tension), equation of state (EOS), and geometric mean approaches and is described in detail elsewhere (8). Terminal sterilization of all Ti disks was by overnight gamma irradiation at 25 kGy. Control cultures were grown on standard tissue culture polystyrene (TCPS).
Cell Culture
Osteoblasts were isolated from the calvaria (frontal and parietal) bones of 1-month, 3-month, and 11-month-old male Sprague-Dawley rats using an explant technique (10,25). Briefly, bones were cleaned of soft tissue and periosteum. Bones pieces were digested in 0.25% trypsin-EDTA (Invitrogen, Carlsbad, CA) for 15 minutes at 37°C to avoid fibroblast contamination. They were then cultured in 100 cm × 20 cm Petri dishes (BD Biosciences, Franklin Lakes, NJ) in DMEM (Cellgro®, Mediatech, Inc., Manassas, VA) supplemented with 10% fetal bovine serum (Hyclone, South Logan, UT) and 1% penicillin-streptomycin (Invitrogen) at 37°C, 5% CO2, and 100% humidity. The resulting cultures were subpassaged and seeded on tissue culture polystyrene (TCPS), PT, SLA, or modSLA substrates at an initial cell density of 20,000 cells/cm2.
Biochemical Assays
When cultures reached confluence on TCPS, cells were incubated with media containing either vehicle (0.01% ethanol) or 10−7M 1α,25(OH)2D3 (Enzo Life Sciences, Plymouth Meeting, PA) for 24 hours. At harvest, cells were released by two sequential 10-minute incubations in 0.25% trypsin-EDTA at 37°C. Cell number was determined using a Z2 Cell Counter (Beckman Coulter, Fullerton, CA). Cells were lysed in Triton X-100 (Sigma Aldrich, St. Louis, MO) and cellular alkaline phosphatase specific activity measured by assaying the release of p-nitrophenol from p-nitrophenylphosphate at pH 10.2. Alkaline phosphatase specific activity was normalized to total protein content of the cell lysates (BCA Protein Assay, Thermo Fisher Scientific).
Levels of secreted factors in the conditioned media were normalized to cell number. Osteocalcin was measured using a commercially available radioimmunoassay following manufacturer’s instructions (Biomedical Technologies, Stoughton, MA). Osteoprotegerin (OPG) and vascular endothelial growth factor A (VEGF-A) were measured using commercially available enzyme-linked immunosorbent assays (ELISA, DuoSet, R&D Systems, Minneapolis, MN). Active TGF-β1 was measured in the conditioned media using an ELISA kit (DuoSet, R&D Systems). Media was acidified and total TGF-β1 measured. Latent TGF-β1 was determined by subtracting active TGF-β1 from total TGF-β1.
Gene Expression
Osteoblasts isolated from 3-month and 11-month-old animals were cultured on TCPS or Ti substrates. When cells reached confluence on TCPS, cells were incubated with fresh media for 12 hours. RNA was harvested using a TRIzol® (Invitrogen) extraction method. The resulting RNA was quantified (Nanodrop Spectrophotometer, Thermo Scientific) and 1µg RNA reversed transcribed to cDNA (OmniScript Reverse Transcription Kit, Qiagen, Valencia, CA) using random oligomers (Promega, Madison, WI). Starting mRNA quantities were determined using SybrGreen (BioRad, Hercules, CA) in an iQ5 Real-time PCR machine (BioRad) and a standard curve method. Expression of mRNA was measured for the following integrin subunits: alpha 2 (ITGA2, F: 5’ACTGTTCAAGGAGGAGAC-3’; R: 5’-GGTCAAAGGCTTGTTTAGG-3’); alpha 5 (ITGA5, F: 5’-ATCTGTGTGCCTGACCTG-3-‘; R: 5’-AAGTTCCCTGGGTGTCTG-3’); and beta 1 (ITGB1, F: 5’-ATTACTCAGATCCAACCAC-3’; R: 5’-TCCTCCTCATTTCATTCATC-3’); as well as for the membrane associated receptor for 1α,25(OH)2D3, protein disulfide isomerase family A, member 3 (PDIA3) (26,27) (F: 5’-CCAATGATGTGCCTTCTC-3’; R: 5’-TGTGCCTTCTTCTTCTTC-3’); and the classical vitamin D receptor (VDR) (F: 5’-CATCTTGGCAGTGAGTGAGC-3’; R: 5’-ACACAACCTACCCATCATCCC-3’). All genes are presented as normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH, F: 5’-CATACTCAGCACCAGCATCACC-3’; R: 5’-AAGTTCAACGGCACAGTCAAGG-3’).
In Vivo Study
Titanium Implant Fabrication and Characterization
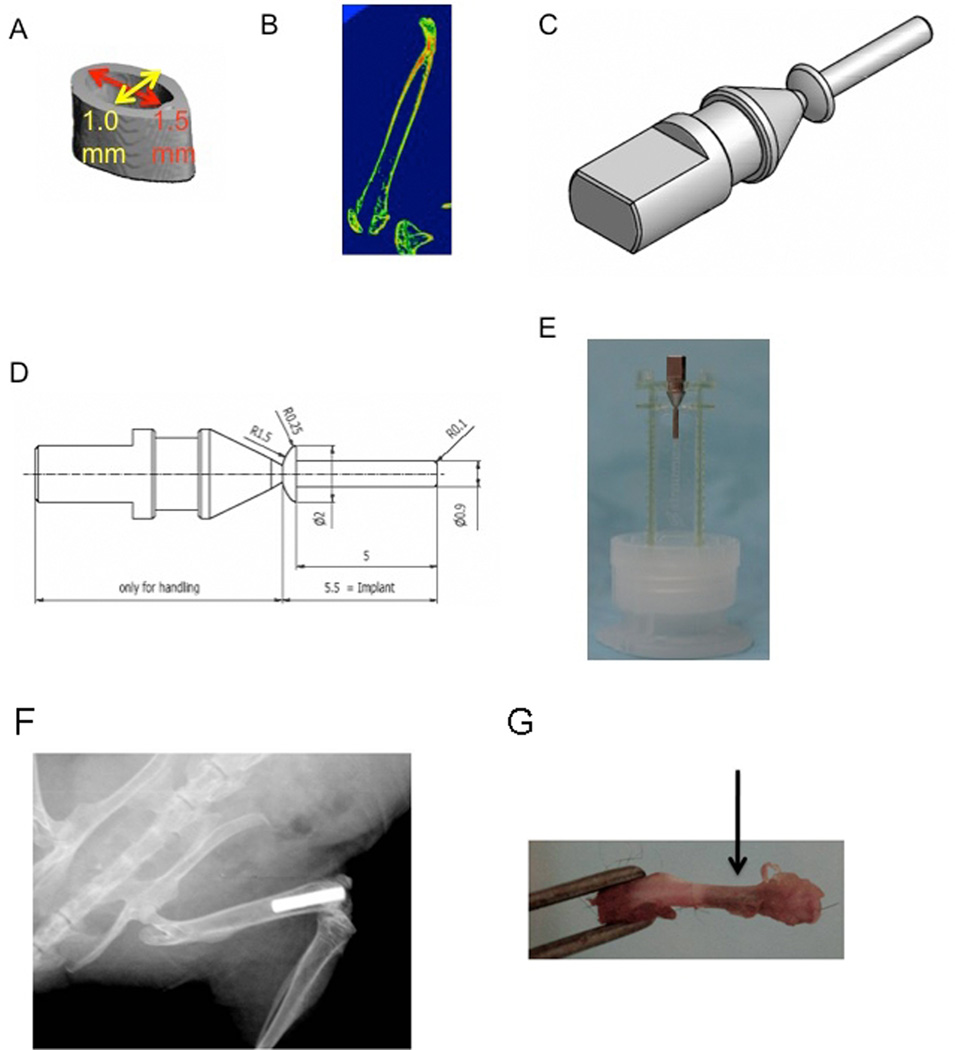
Bone formation and osseointegration of PT, SLA, and modSLA in vivo were examined using a femoral intramedullary bone formation model. Micro-computed tomography (microCT) imaging of 6-week-old mice was done in order to determine the size of the femoral medullary canal (Fig. 1A, 1B) and custom designed Ti implants were manufactured to fit into the femoral medullary space of C57BL/6 mice (Fig. 1C, 1D). The implants were fabricated from ASTM grade 4 Ti using the same processing methods used for commercially available dental implants. Surface treatments for Ti implants for in vivo studies were prepared in a manner similar to those for Ti surfaces described above. Ti implants consisted of two parts: a handling device and the Ti implant rod (Fig. 1C, 1D, 1E). The cylindrical Ti implants were manufactured to be 5 mm in length and 0.9 mm in diameter. All implants were sterilized by gamma irradiation at 25 kGy prior to use.
Figure 1.
Titanium implant design. Three-dimensional microCT reconstructions of the mouse femoral medullary canal (A) and microCT images of mouse femurs (B) were used to inform implant design. (C) Three-dimensional CAD representation and (D) mechanical drawing showing dimensions of Ti implant with handling device. (E) Image of custom manufactured Ti implant. (F) X-ray image showing an implant that has been inserted into the femoral medullary space without breaking of the bone. (G) Gross morphology of a mouse femur with an implant (arrow).
To characterize the surface morphology of Ti implants, surfaces that were not coated with gold were examined using an Ultra 60 field emission (FE) scanning electron microscope (SEM, Carl Zeiss SMT Ltd., Cambridge, UK). Images were recorded using a 5 kV accelerating voltage.
Surface wettability of Ti implants was determined with a Ramé-Hart goniometer (model 250-F1, Ramé-Hart Instrument Co., Mountain Lakes, NJ). Because the Ti implants had a cylindrical shape, it was not possible to measure the surface contact angle directly. Therefore, Ti implant surface wettability was qualitatively determined by immersing Ti implants into ultra pure water while recording the process. Images were recorded with DROPimage CA software package (Ramé-Hart Instrument Co.).
Surface chemistry was analyzed by using X-ray photoelectron spectroscopy (XPS) on a Thermo K-Alpha (Thermo Fisher Scientific, Waltham, MA). XPS was performed using monochromatic Al Kα X-ray source (hv = 1486.6 eV photons) at a 90° take-off angle in an analysis chamber evacuated to a pressure of 10−9 Torr or lower. Data analysis was performed using the Thermo Advantage 4.43 software package (Thermo Fisher Scientific, Inc).
Surface roughness of Ti implants was characterized by using a LEXT 3D Material Confocal Laser Microscope (CLM, Olympus America Inc., Center Valley, PA). Roughness was evaluated using the LEXT OLS4000 software provided by Olympus. Three measurements were made on two implants per surface topography. All measurements were performed in an organic clean room.
Animals
All animal handling and procedures were approved by the Georgia Institute of Technology Institutional Animal Care and Use Committee (IACUC), and were conducted in accordance with NIH guidelines. Custom-made Ti implants were inserted into the femoral medullary canal of mice via a medial parapatellar arthrotomy. Briefly, male C57BL/6 mice (aged 2-months or 9-months) were anesthetized with 5% isoflurane gas inhalation. The right hind limb was prepared by shaving and cleaning using ethanol and chlorhexidine. Anesthesia was maintained with 2% isoflurane gas inhalation for the duration of the surgical procedure. Cleaned, anesthetized animals were placed in a supine position and were covered with a sterile surgical drape. Using a scalpel, an 8 mm incision was made over the distal side of the knee. Blunt dissection was used to move aside the ligament and patella to expose the intercondylar notch of the distal femur. Once the femoral cartilage was exposed, a 1 mm round dental bur was used to penetrate the distal intercondylar notch of the femur to access the medullary cavity. A 22-gauge needle was gently pushed into the femur to confirm penetration of the medullary cavity. Cylindrical Ti implants were inserted into the femoral medullary canal and then were broken from the handling device. To confirm successful insertion of the implant into the femoral medullary canal, x-ray imaging was done prior to closure of the surgical incision (Fig. 1F, 1G). If the Ti implant was not inserted into the medullary canal or if insertion of the Ti implant resulted in a broken femur, the mouse was withdrawn from the study and euthanized using CO2 inhalation. This was done to ensure an appropriate number of successful surgeries for each Ti implant type. Six successful implants were performed for each surface type to ensure adequate sample sizes for statistical analysis as determined by power analysis. Following successful implant insertion, periosteal tissue was closed using resorbable sutures and the surgical incision was closed with wound clips. After recovery from anesthesia, mice were injected with 0.03 mg/kg buprenorphine to relieve post-operative pain. Animals were monitored every 12 hours for 48 hours post-surgery and once per day thereafter for the duration of the study. Wound clips were removed 10–14 days post-surgery. All animals had access to food and water ad libitum for the duration of the study.
Implant Harvest and Vascular Perfusion
Limbs from the 2-month-old mice were harvested 35 days post-implantation. The limbs containing Ti implants were stored in 10% neutral buffered formalin for histological processing. Limbs from the 9-month-old animals were harvested at 28 days post-implantation for blood vessel quantification and histological analysis. Mice were euthanized via CO2 inhalation. Immediately after death, the thoracic cavity was opened and a 22G butterfly needle was inserted into the left ventricle. The inferior vena cava was severed to allow the vasculature to be flushed. Heparinized saline (100 U/mL) was used to flush the vasculature followed by perfusion with 10% neutral buffered formalin to fix the tissue specimens. The vasculature was perfused using a radio-opaque silicone rubber compound (Microfil, Flow Tech, Inc., Carver, MA) mixed in a 9:1 v/v ratio with Microfil curing agent. Following vascular perfusion, the injected Microfil compound was allowed to polymerize overnight at 4°C before histological processing.
Histology
Fixed limbs were embedded in plastic. Sagittal tissue sections were cut and polished to a final thickness of 10–20 µm and were stained with haematoxylin and eosin for histological analysis. Using commercially available image analysis software (Image-Pro Plus, Media Cybernetics, Bethesda, MD), stained tissue sections were analyzed for bone-to-implant contact (BIC) using two different methods. First, the BIC where the implant surface was in direct contact with the femoral cortical bone surface was calculated for each animal for each implant type and the average total BIC was determined. We also measured the BIC where new bone had formed within the femoral intramedullary space for each animal for each implant surface type.
Statistical Analysis
For each in vitro experiment, data presented are mean ± SEM for six independent cultures per variable. Experiments were repeated to ensure validity. Data are presented from one representative experiment of two experiments. For each in vivo study, six implants were harvested for each implant type. In vitro and in vivo data were analyzed by ANOVA and when statistical differences were detected, Student’s t-test for multiple comparisons using Bonferroni’s modification was used. P-values < 0.05 were considered significant.
RESULTS
In Vitro Results
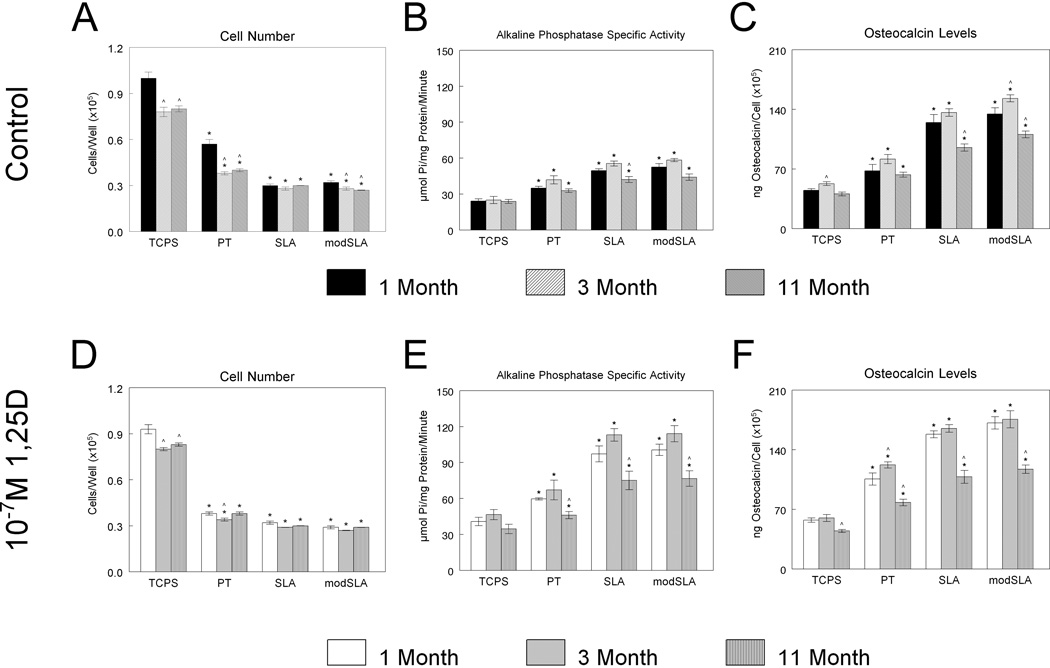
Total osteoblast cell number was reduced on all three Ti surfaces compared to TCPS control surfaces for all three donor ages examined (Fig. 2A). On TCPS and PT surfaces, osteoblasts derived from 1-month-old rats had a significantly higher cell number compared to osteoblasts from both 3- and 11-month-old rats while total cell number on SLA surfaces were similar amongst cells derived from all three age groups. 1α,25(OH)2D3 treatment did not affect cell number on any of the surfaces for any of the ages examined (Fig. 2B).
Figure 2.
Response of osteoblasts derived from 1-month, 3-month, and 11-month-old rats to Ti surface roughness and hydrophilicity and treatment with 1α,25(OH)2D3. Cells were cultured on TCPS, PT, SLA, and modSLA Ti surfaces with or without 10−7M 1α,25(OH)2D3. (A,D) Cell number, (B,E) alkaline phosphatase specific activity in the cell lysate, and (C,F) osteocalcin levels were determined. Values presented are mean ± SEM of six independent cultures. *p<0.05 vs. TCPS, ^p<0.05 vs. 1-month-old rats.
Alkaline phosphatase specific activity was higher in osteoblasts of all three age groups on all three Ti surfaces compared to TCPS (Fig. 2C). . The addition of 1α,25(OH)2D3 increased enzyme activity on all surfaces but the magnitude of the stimulatory effect was donor age dependent (Fig. 2D). Osteoblasts from 11-month-old animals did not respond to treatment to the same extent as cells from 1-month or 3-month-old animals. Secreted levels of osteocalcin, which is a late marker of osteoblast maturation, were higher with increased surface roughness and energy for all three age groups examined (Fig. 2E). The increase on SLA and modSLA was significantly less robust in cells from aged animals. 1α,25(OH)2D3 caused a further increase in osteocalcin levels in cultures grown on PT but this increase was less robust in cells from 11-month-old donors (Fig. 2F).
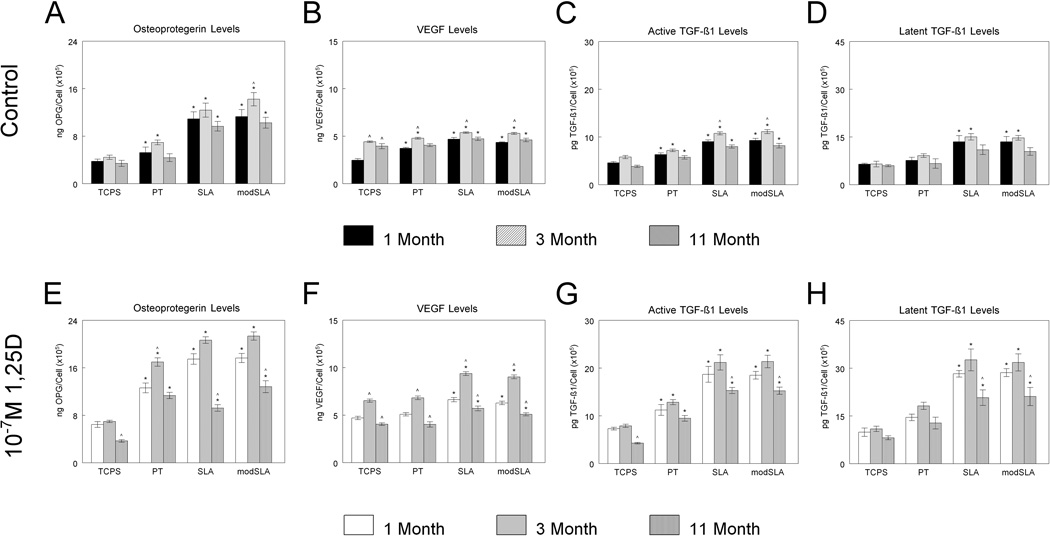
Local factor production was also sensitive to donor age. In cells from younger animals, osteoprotegerin (OPG) levels were higher on all three Ti surfaces compared to TCPS control surfaces. In cells from 11-month-old animals, OPG levels were increased only in cultures grown on microstructured Ti surfaces (Fig. 3A). The addition of 1α,25(OH)2D3 significantly increased OPG secretion on all surfaces examined, however the effect was donor age dependent (Fig. 3B). Cells derived from younger donors responded to 1α,25(OH)2D3 to a greater extent than cells derived from the older donors. Similar to OPG production, VEGF-A levels were higher in cultures grown on Ti surfaces compared to TCPS control surfaces in cells from young animals, whereas, secreted levels of VEGF-A in cells from 11-month-old animals were only increased on microstructured Ti surfaces (Fig. 3C). 1α,25(OH)2D3 enhanced the stimulated VEGF-A secretion on all surfaces in cultures from 1- and 3-month-old animals but did not have a stimulatory effect in cultures from 11-month-old animals (Fig. 3D). VEGF-A levels were highest in cells from 3-month-old animals. Active TGF-β1 levels were increased on all three Ti surfaces compared to TCPS in all three age groups. 1α,25(OH)2D3 increased levels of active TGF-β1 in osteoblasts derived from all donor age groups with levels being higher on all three Ti surfaces compared to TCPS control surfaces (Fig. 3F). Latent TGF-β1 levels were higher only in response to growth on microtextured Ti surfaces in cells from 1- and 3-month-old animals while levels in cells from 11-month-old animals were unaffected by either Ti surface roughness or energy (Fig. 3G). 1α,25(OH)2D3 increased latent TGF-β1 levels on all surfaces examined and this effect was greatest in cells from younger animals (Fig. 3H).
Figure 3.
Response of osteoblasts derived from 1-month, 3-month, and 11-month-old rats to Ti surface roughness and hydrophilicity and treatment with 1α,25(OH)2D3. Cells were cultured on TCPS, PT, SLA, and modSLA Ti surfaces with or without 10−7M 1α,25(OH)2D3. (A,E) Osteoprotegerin, (B,F) VEGF-A, (C,G) active TGF-β1, and (D,H) latent TGF-β1 levels were determined. Values presented are mean ± SEM of six independent cultures. *p<0.05 vs. TCPS, ^p<0.05 vs. 1-month-old rats.
Expression of integrin receptor subunits α2 and α5 were higher on all three Ti surfaces examined compared to TCPS control surfaces for both 3- and 11-month-old animals, however expression of both integrins α2 and α5 were lower in cells derived from 11-month-old animals on all substrates examined when compared to 3-month-old donor cells (Fig. 4A, 4B). Levels of β1 integrin mRNA were higher for cells on microrough SLA and microrough, hydrophilic modSLA Ti surfaces compared to TCPS substrates for cells from both 3- and 11-month-old animals (Fig. 4C). Expression of the β1 integrin was similar between cells from 3- and 11-month-old animals except on modSLA, where cells from 11-month-old animals displayed a reduction in β1 expression.
Figure 4.
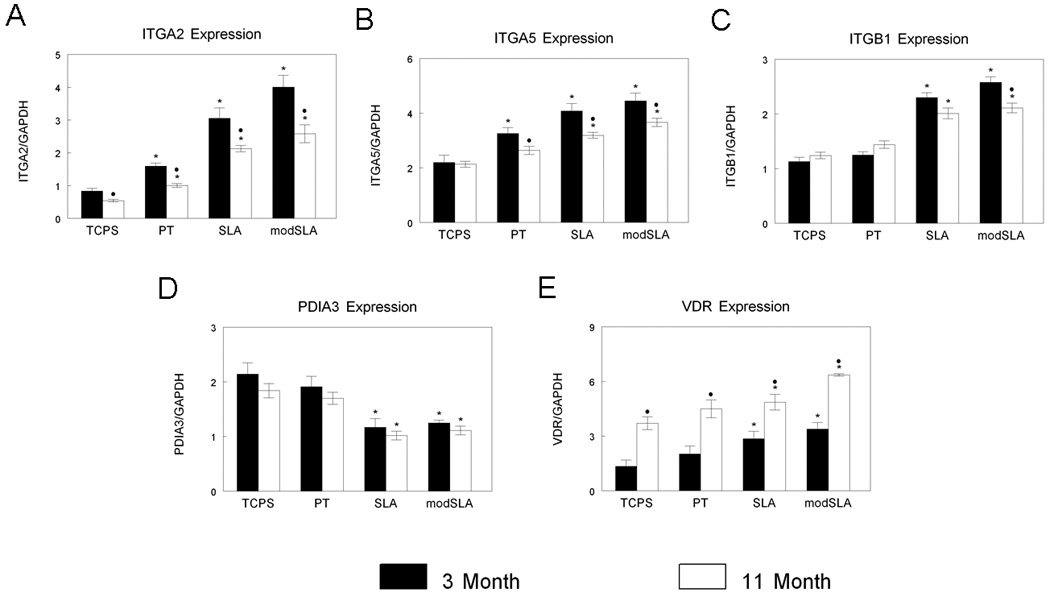
Gene expression in adult (3-month) and old (11-month) rats. Osteoblasts isolated from 3- and 11-month-old rats were cultured on TCPS and PT, SLA, and modSLA Ti surfaces and expression of Integrin α2 (ITGA2, A), Integrin α5 (ITGA5, B), and Integrin β1 (ITGB1, C) and vitamin D receptors PDIA3 (D) and VDR (E) measured. Values presented are mean ± SEM of six independent cultures. *p<0.05 vs. TCPS, •p<0.05 vs. 3-month-old rats.
Receptors for 1α,25(OH)2D3 were expressed in osteoblasts isolated from both young and old donors. Levels of expression were specific to receptor type and varied with the surface and donor age. mRNAs for PDIA3, the membrane receptor for 1,25(OH)2D3, were reduced in response to increasing Ti surface roughness and surface hydrophilicity, but no differences in expression between osteoblasts from the different aged animals were observed (Fig. 4D). In contrast, mRNAs for VDR, the nuclear receptor for 1,25(OH)2D3, increased on SLA and modSLA surfaces compared to TCPS for osteoblasts from both age groups (Fig. 4E). In cell cultures from 11-month-old animals, expression of VDR was significantly higher than that observed in cell cultures from 3-month-old animals on all surfaces examined.
Implant Characterization
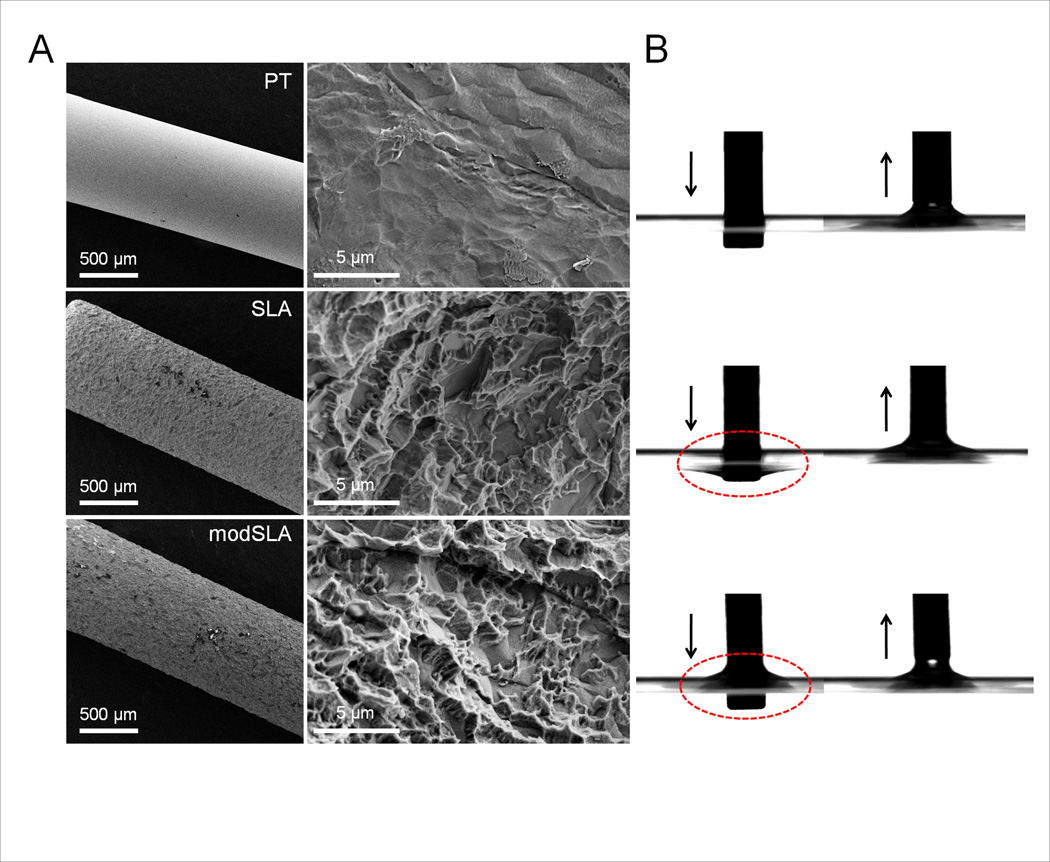
The surface morphology of PT, SLA, and modSLA implants was obtained at low and high magnification with SEM (Fig. 5A). The surfaces of PT implants were relatively smooth with more irregular surface features than SLA and modSLA implants. Both SLA and modSLA surfaces exhibit complex features such as shallow micron-scale craters (10–60 µm) and submicron-scale pits (1–3 µm). There was no clear boundary between craters and pits. Unmodified areas characterized by flat and smooth sections were also found on SLA and modSLA surfaces. However, there were no observed topographical differences between SLA and modSLA surfaces.
Figure 5.
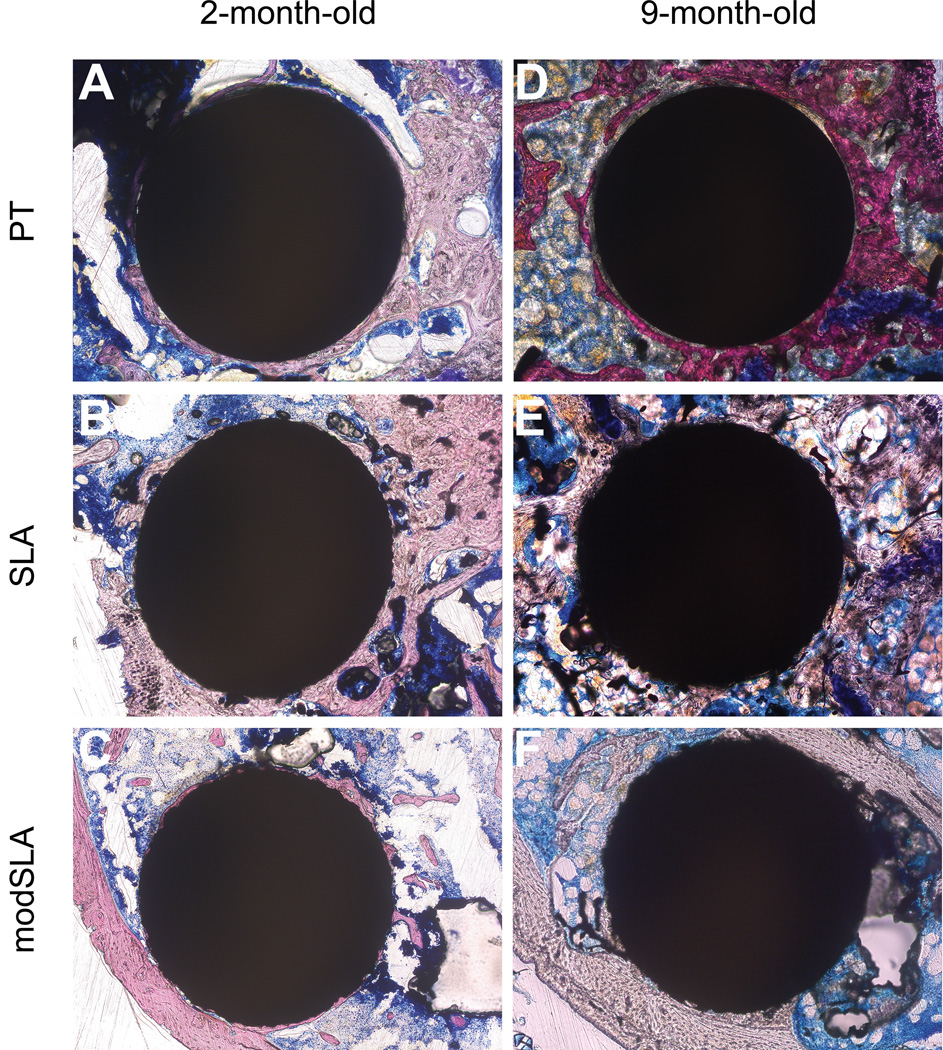
Representative histology sections. Representative histology sections PT, SLA, and modSLA titanium implants placed in of 2-month-old (A, B, C) or 9-month-old mice (D, E, F).
The modSLA surface showed excellent wettability compared to the SLA surface with poor wettability (Fig. 5B). Titanium (Ti2p), oxygen (O1s), and carbon (C1s) were detected on PT, SLA, and modSLA surfaces (Table 1). Trace nitrogen (N1s) was detected on PT and SLA surfaces. Atomic percentages for the PT, SLA, and modSLA surfaces are summarized in Table 1. More oxygen and titanium with less carbon was found on modSLA surfaces compared to PT and SLA surfaces.
Table 1.
Surface chemical composition and roughness parameters of titanium cylinder surfaces (mean value ± SD).
| Concentration (atomic % ± std.) | Roughness parameter (mean µm ± std.) |
|||||
|---|---|---|---|---|---|---|
| C1s | N1s | O1s | Ti2p | Ra | Rt | |
| PT | 31.7 ± 2.3 | 2.4 ± 0.1 | 49.5 ± 1.2 | 16.4 ± 1.0 | 0.21 ± 0.03 | 1.39 ± 0.32 |
| SLA | 40.3 ± 1.1 | 3.0 ± 0.4 | 43.5 ± 0.7 | 13.3 ± 0.7 | 3.39 ± 0.63 | 19.71 ± 5.09 |
| modSLA | 24.8 ± 2.1 | 54.5 ± 1.5 | 20.6 ± 0.6 | 3.47 ± 0.71 | 21.15 ± 4.96 | |
Surface roughness determined by CLM was significantly different between PT (Ra = 0.21 µm) and SLA (Ra = 3.39 µm). In contrast, there was no difference between SLA (Ra = 3.39 µm) and modSLA (Ra = 3.47 µm) (Table 1). SEM and CLM suggest that no morphological difference was observed between SLA and modSLA surfaces. However, higher surface wettability of the modSLA surface than the SLA surface was induced by different surface chemistry.
In Vivo Results
Implant osseointegration was quantified by evaluating transverse histology sections. An example of such a cut is presented in Figure 6. The amount of bone and the BIC was different when the implant was in contact with cortical bone compared to bone in the marrow compartment. Accordingly, the morphometric analysis was performed separately on the areas contacting cortical bone versus the bone contact in the marrow area. In both age groups, neither Ti implant surface microstructure nor surface energy had an effect on BIC where the implant surface came in direct contact with the femoral cortical bone surface (Table 2). Neither Ti surface microstructure nor surface energy had an effect on total marrow to implant contact in young (2-month-old) male C57BL/6 mice (Fig. 7A). In 9-month-old male C57BL/6 mice, surface microtopography alone had no significance effect on total BIC within the femoral marrow cavity, however the BIC was significantly increased in response to the surface microtopography and high surface energy seen on modSLA Ti implants compared to smooth PT Ti implants (Fig. 7A). The total number of blood vessels within the marrow space surrounding Ti implants within the femoral medullary space was also increased in the modSLA implant group relative to the smoother PT controls (Fig.7B).
Figure 6.
In vivo bone formation. Bone formation and neovascularization surrounding PT, SLA, and modSLA Ti implants was examined using a novel murine intramedullary bone formation model. The percent bone to implant contact (% BIC) was measured for both 2-month-old and 9-month-old animals (A). *p<0.05 vs. PT. (B) The total number of blood vessels within the marrow space of 9-month-old animals. *p<0.05 vs. PT. Values presented are the mean ± SEM of six implants per group.
Table 2.
Bone-to-implant contact for 8-week and 9-month-old mice for PT, SLA, and modSLA Ti implants.
| Cortical Bone to Implant Contact | ||
|---|---|---|
| Surface Type | 8 Week | 9 Month |
| PT | 0.88 ± 0.06 | 0.89 ± 0.06 |
| SLA | 0.87 ± 0.05 | 0.89 ± 0.07 |
| modSLA | 0.92 ± 0.07 | 0.93 ± 0.07 |
Fig. 7.
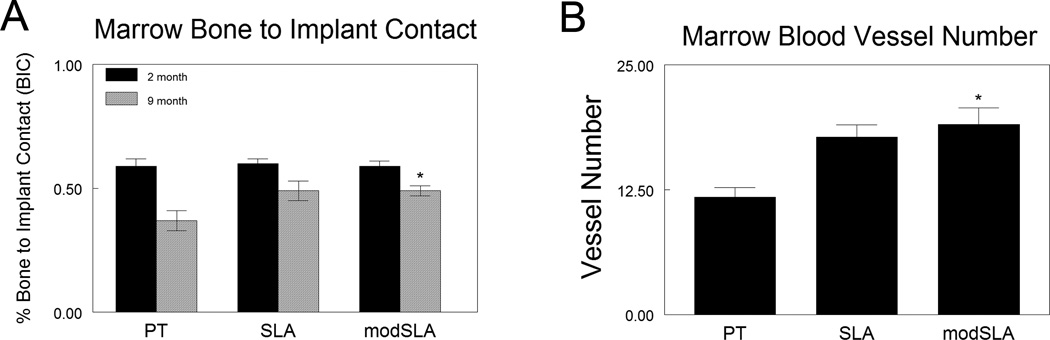
In vivo bone formation. Bone formation and neovascularization surrounding PT, SLA, and modSLA Ti implants was examined using a novel murine intramedullary bone formation model. (A) The percent bone to implant contact (% BIC) was measured for both 2-month-old and 9-month-old animals. p<0.05 versus PT. (B) The total number of blood vessels within the marrow space of 9-month-old animals. p<0.05 versus PT. Values presented are the mean SEM of six implants per group.
DISCUSSION
The success of implants in compromised patients in general, and in older patients specifically, has attracted research attention. Several studies have reported conflicting results on whether the healing capacity diminishes with increasing age (21,22). Endosteal bone formation and marrow regeneration following tibial marrow ablation are delayed with increasing age in immunocompromised rats (22). Healing of segmental fracture defects was delayed in older animals with demineralized bone matrix (DBM) treatment compared to younger animals (21). Diminished healing capabilities with increasing age have been reported in other organ systems as well. Bone marrow derived mesenchymal stem cell homing to sites of burn injury was significantly reduced in older animals (28). In contrast, compensatory bone formation during tooth movement is maintained at similar levels in both young and old rats (29). The results of the present study indicate that responses of osteoblasts from aged animals to surface roughness and the addition of 1α,25(OH)2D3 as well as in vivo osseointegration in older animals were reduced when compared to younger animals.
Ti implant surface properties are known to affect osteoblast maturation in vitro and new bone formation in vivo. The response of osteoblasts to microstructured Ti surfaces is similar to their response to bone wafer surfaces that have been pre-conditioned by osteoclasts to create microtextured resorption pits (30), suggesting that the results of this study may have broader application to our understanding of the role aging in bone physiology. Consistent with previous results, the present study found that osteoblasts display reduced proliferation and increased maturation in response to increasing surface roughness and surface free energy (3,5,15,31–33). It was also observed that treatment with the systemic osteotropic hormone 1α,25(OH)2D3 further enhanced osteoblast response to Ti substrate features as has been reported previously (34). While osteoblasts isolated from 1-, 3-, and 11-month-old Sprague-Dawley rats showed surface roughness and energy dependent increases in maturation and local factor production, osteoblasts isolated from 11-month-old animals had a less robust response to both Ti surface roughness properties. Cells from older animals also had a diminished response to treatment with 10−7M 1α,25(OH)2D3 compared to osteoblasts isolated from 1- and 3-month-old animals.
Our results suggest that integrin signaling may play a role in the reduced response observed in cells from aged rats. We have previously shown that α5β1 is required for osteoblast attachment and proliferation, but α2β1 is required for osteoblast differentiation of HMSCs and osteoblasts (6,7,35). Expression levels of α2 and α5 as well as β1 were lower in osteoblasts derived from older animals.
Age-dependent differences in expression of PDIA3 were not observed, suggesting that signaling via PDIA3-dependent pathways may not mediate the age-dependent differences in response to 1α,25(OH)2D3. In contrast, osteoblasts from older animals had higher expression of mRNAs for VDR compared to osteoblasts from younger animals. This suggests that the diminished responsiveness of osteoblasts from older animals to treatment with 1α,25(OH)2D3 is not caused by a lack of receptors in these cells but rather by some other mechanism. Alternatively, increased VDR may be a compensatory mechanism in the older animals.
mRNAs for PDIA3 were reduced in osteoblasts cultured on the microtextured surfaces whereas mRNAs for VDR were increased, regardless of the age of the donor rats. We have observed similar changes in expression of these two receptors during osteogenic differentiation of mouse embryoid bodies or cultures of HMSCs (36). Embryonic stem cells (ESCs) expressed mRNA and protein for both receptors for 1α,25(OH)2D3 and they continued to be present as the ESCs differentiated in the form of embryoid bodies. Interestingly, the expression of Vdr increased in embryoid bodies after incubation in osteogenic medium, while Pdia3 expression decreased. In adult human MSCs, osteogenic induction increased VDR mRNA whereas levels of PDIA3 mRNA remained stable after osteogenic induction. Taken together with the results of the present study, these findings suggest that expression levels of these receptors are modulated during the course of osteogenic differentiation but it is not known if changes in mRNA are required to initiate a change in osteoblast differentiation state or if they are a consequence of that state.
The novel murine intramedullary bone formation model used in this study enabled us to compare BIC and vasculogenesis as a function of age in two different conditions. Injury to the medullary canal caused by insertion of the implant induced endosteal bone formation and with it new blood vessel formation (37). In the absence of a biomaterial within the canal, the endosteal bone will be remodeled, restoring the contours of the canal within 28 days. The rate and extent of remodeling and the quality of the regenerated marrow are sensitive to biomaterial properties (38) and vary with animal age (22).
The results of the present study show that both age as well as biomaterial surface features affect osseointegration and the neovasculature, not only in the peri-implant cortical bone but also in the endosteal bone in the medullary canal. In young, skeletally mature C57BL/6 mice, neither Ti surface roughness nor wettability had a significant effect on osseointegration as determined by histological examination of total bone to implant contact at 35 days post-implantation. However, in adult animals smooth and SLA surfaces had less BIC and new blood vessels compared to mod SLA. Moreover, BIC and neovasculature in older animals with modSLA implants were comparable to levels found in young animals. modSLA had higher BIC after 4 weeks of implantation compared to SLA.
Age-dependent differences in peri-implant bone formation may be resolved over time. Similar to our results, modSLA implants supported significantly greater BIC compared to SLA implants in minipigs at 14 days post-implantation (16). It may also be possible that in the young mice, new bone formation around modSLA implants occurs more rapidly than around PT or SLA implants, however at our harvest time of 35 days post-implantation, these differences may not have been observed.
Our results are consistent with results found previously demonstrating that new bone formation capacity is diminished with age (22) and support the hypothesis that implant surface properties can enhance osseointegration in sites of compromised bone. In 9-month-old C57BL/6 mice, which are an aged model representing compromised bone, the combination of a rough surface microtopography and high surface energy on modSLA substrates resulted in a significant increase in total bone to implant contact surrounding these implants. In addition, when the vasculature of these animals was perfused and the total number of blood vessels surrounding the implants within the marrow space determined, it was found that modSLA implants had significantly increased total blood vessel number. These results are in agreement with our previous findings in vitro, which indicated that surface roughness enhanced production of angiogenic and vasculogenic factors (18).
In a clinical setting, establishment of a vasculature preceding or concomitant with bone formation allows for the delivery of oxygen, systemic hormones, and nutrients to the injury site, as well as the migration of mesenchymal stem cells. In the absence of neovascularization, the implant may be surrounded by a fibrous capsule, resulting in implant loosening and ultimately failure, demonstrating the importance of the initial reaction of the first cells to encounter an implanted material. The results from this study have shown that microstructured, hydrophilic Ti surfaces can improve neovascularization and subsequently implant osseointegration in sites of compromised bone formation, such as in an older patient population.
In addition to the data presented here, this novel mouse model of intramedullary bone formation provides several advantages to evaluate the numerous factors involved in peri-implant bone formation. The numerous genetically modified mouse strains readily available, such as vitamin D receptor knockout mice will make it easier to isolate and examine the specific roles of these factors in influencing implant osseointegration, particularly in an aged population.
CONCLUSIONS
In the present study, it was determined that osteoblast maturation in response to Ti implant surface roughness and surface free energy diminishes with age. Furthermore, osteoblast responsiveness to the systemic osteotropic hormone 1α,25(OH)2D3 was shown to be reduced in osteoblasts derived from aged animals. However, VDR expression was significantly higher in osteoblasts derived from older animals compared to osteoblasts derived from a younger population, indicating that the diminished responsiveness of cells to treatment with 1α,25(OH)2D3 was not caused by a lack of these vitamin D receptors in these cells. Further, we describe here the development of a novel mouse intramedullary bone formation model and show that both new bone formation and neovascularization are reduced in response to Ti implants in older animals. This effect can be avoided by titanium implants with roughened and high-energy surfaces. Taken together, these results indicate that osteoblast maturation in vitro and new bone formation in vivo are diminished with age, suggesting that these factors should be taken into consideration clinically when selecting appropriate biomaterials.
ACKNOWLEGDMENTS
This study was funded by NIH USPHS AR052102, the ITI Foundation, and Children’s Healthcare of Atlanta. ALR was supported by funding from Cell and Tissue Engineering (CTEng) NIH Biotechnology Training Grant (TG GM08433). Implants were provided as a gift from Institut Straumann AG (Basel, Switzerland). We thank Ms. Sonja Bustamante for her excellent technical help.
RON and ZS designed the in vitro studies. RON, SLH, and DLH performed the in vitro studies. ALR and ZS designed the in vivo studies. JHP performed implant surface characterization. ALR, SLH, and ZS performed in vivo studies. RON, DLC, BDB, and ZS performed data analysis and interpretation. RON and ALR drafted the manuscript, and all authors have critically revised it. BDB accepts responsibility for the integrity of the data analysis for the work described.
Contributor Information
Rene Olivares-Navarrete, Email: rene.olivares-navarrete@bme.gatech.edu.
Andrew L. Raines, Email: araines@sjha.org.
Sharon L. Hyzy, Email: sharon.hyzy@bme.gatech.edu.
Jung Hwa Park, Email: jpark87@mail.gatech.edu.
Daphne L. Hutton, Email: dhutton4@gmail.com.
David L. Cochran, Email: cochran@uthscsa.edu.
Barbara D. Boyan, Email: barbara.boyan@bme.gatech.edu.
Zvi Schwartz, Email: zvi.schwartz@bme.gatech.edu.
REFERENCES
- 1.Ratner BDHA, Schoen FJ, Lemons JE. Biomaterials Science: An Introduction to Materials in Medicine. 2 ed. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- 2.Ellingsen JE. A study on the mechanism of protein adsorption to TiO2. Biomaterials. 1991;12(6):593–596. doi: 10.1016/0142-9612(91)90057-h. [DOI] [PubMed] [Google Scholar]
- 3.Kieswetter K, Schwartz Z, Dean DD, Boyan BD. The role of implant surface characteristics in the healing of bone. Crit Rev Oral Biol Med. 1996;7(4):329–345. doi: 10.1177/10454411960070040301. [DOI] [PubMed] [Google Scholar]
- 4.Goto T, Yoshinari M, Kobayashi S, Tanaka T. The initial attachment and subsequent behavior of osteoblastic cells and oral epithelial cells on titanium. Biomed Mater Eng. 2004;14(4):537–544. [PubMed] [Google Scholar]
- 5.Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, Lankford J, Jr, Dean DD, Cochran DL, Boyan BD. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63) J Biomed Mater Res. 1995;29(3):389–401. doi: 10.1002/jbm.820290314. [DOI] [PubMed] [Google Scholar]
- 6.Olivares-Navarrete R, Raz P, Zhao G, Chen J, Wieland M, Cochran DL, Chaudhri RA, Ornoy A, Boyan BD, Schwartz Z. Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proc Natl Acad Sci U S A. 2008;105(41):15767–15772. doi: 10.1073/pnas.0805420105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olivares-Navarrete R, Hyzy SL, Hutton DL, Erdman CP, Wieland M, Boyan BD, Schwartz Z. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials. 2010;31(10):2728–2735. doi: 10.1016/j.biomaterials.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006;76(2):323–334. doi: 10.1002/jbm.a.30518. [DOI] [PubMed] [Google Scholar]
- 9.Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, Boyan BD. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005;74(1):49–58. doi: 10.1002/jbm.a.30320. [DOI] [PubMed] [Google Scholar]
- 10.Olivares-Navarrete R, Hyzy SL, Chaudhri RA, Zhao G, Boyan BD, Schwartz Z. Sex dependent regulation of osteoblast response to implant surface properties by systemic hormones. Biol Sex Differ. 2010;1(1):4. doi: 10.1186/2042-6410-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivares-Navarrete R, Hyzy SL, Park JH, Dunn GR, Haithcock DA, Wasilewski CE, Boyan BD, Schwartz Z. Mediation of osteogenic differentiation of human mesenchymal stem cells on titanium surfaces by a Wnt-integrin feedback loop. Biomaterials. 2011;32(27):6399–6411. doi: 10.1016/j.biomaterials.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991;25(7):889–902. doi: 10.1002/jbm.820250708. [DOI] [PubMed] [Google Scholar]
- 13.Gotfredsen K, Wennerberg A, Johansson C, Skovgaard LT, Hjorting-Hansen E. Anchorage of TiO2-blasted, HA-coated, and machined implants: an experimental study with rabbits. J Biomed Mater Res. 1995;29(10):1223–1231. doi: 10.1002/jbm.820291009. [DOI] [PubMed] [Google Scholar]
- 14.Cochran DL, Schenk RK, Lussi A, Higginbottom FL, Buser D. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: a histometric study in the canine mandible. J Biomed Mater Res. 1998;40(1):1–11. doi: 10.1002/(sici)1097-4636(199804)40:1<1::aid-jbm1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz F, Wieland M, Schwartz Z, Zhao G, Rupp F, Geis-Gerstorfer J, Schedle A, Broggini N, Bornstein MM, Buser D, Ferguson SJ, Becker J, Boyan BD, Cochran DL. Potential of chemically modified hydrophilic surface characteristics to support tissue integration of titanium dental implants. J Biomed Mater Res B Appl Biomater. 2009;88(2):544–557. doi: 10.1002/jbm.b.31233. [DOI] [PubMed] [Google Scholar]
- 16.Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, Hoffmann B, Lussi A, Steinemann SG. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004;83(7):529–533. doi: 10.1177/154405910408300704. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson SJ, Broggini N, Wieland M, de Wild M, Rupp F, Geis-Gerstorfer J, Cochran DL, Buser D. Biomechanical evaluation of the interfacial strength of a chemically modified sandblasted and acid-etched titanium surface. J Biomed Mater Res A. 2006;78(2):291–297. doi: 10.1002/jbm.a.30678. [DOI] [PubMed] [Google Scholar]
- 18.Raines AL, Olivares-Navarrete R, Wieland M, Cochran DL, Schwartz Z, Boyan BD. Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy. Biomaterials. 2010;31(18):4909–4917. doi: 10.1016/j.biomaterials.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Jaffin RA, Berman C. The effect of smoking on achieving osseointegration of surface-modified implants: a clinical report. Int J Oral Maxillofac Implants. 2002;17(6):816–819. [PubMed] [Google Scholar]
- 20.Cochran DL. A comparison of endosseous dental implant surfaces. J Periodontol. 1999;70(12):1523–1539. doi: 10.1902/jop.1999.70.12.1523. [DOI] [PubMed] [Google Scholar]
- 21.Tucci M, Lancaster R, Wingerter S, Woodall J, Jr, Russell G, Benghuzzi H. Comparison of segmental fracture healing in young and old rats that were treated with bone stimulators - biomed 2009. Biomed Sci Instrum. 2009;45:407–412. [PubMed] [Google Scholar]
- 22.Fisher M, Hyzy S, Guldberg RE, Schwartz Z, Boyan BD. Regeneration of bone marrow after tibial ablation in immunocompromised rats is age dependent. Bone. 2010;46(2):396–401. doi: 10.1016/j.bone.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 23.Zinger O, Zhao G, Schwartz Z, Simpson J, Wieland M, Landolt D, Boyan B. Differential regulation of osteoblasts by substrate microstructural features. Biomaterials. 2005;26(14):1837–1847. doi: 10.1016/j.biomaterials.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 24.Zhao G, Raines AL, Wieland M, Schwartz Z, Boyan BD. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials. 2007;28(18):2821–2829. doi: 10.1016/j.biomaterials.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellows CG, Aubin JE, Heersche JN, Antosz ME. Mineralized bone nodules formed in vitro from enzymatically released rat calvaria cell populations. Calcif Tissue Int. 1986;38(3):143–154. doi: 10.1007/BF02556874. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Olivares-Navarrete R, Wang Y, Herman TR, Boyan BD, Schwartz Z. Protein-disulfide isomerase-associated 3 (Pdia3) mediates the membrane response to 1,25-dihydroxyvitamin D3 in osteoblasts. J Biol Chem. 2010;285(47):37041–37050. doi: 10.1074/jbc.M110.157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Chen J, Lee CS, Nizkorodov A, Riemenschneider K, Martin D, Hyzy S, Schwartz Z, Boyan BD. Disruption of Pdia3 gene results in bone abnormality and affects 1alpha,25-dihydroxy-vitamin D3-induced rapid activation of PKC. J Steroid Biochem Mol Biol. 2010;121(1–2):257–260. doi: 10.1016/j.jsbmb.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Sarkar K, Rey S, Sebastian R, Andrikopoulou E, Marti GP, Fox-Talbot K, Semenza GL, Harmon JW. Aging impairs the mobilization and homing of bone marrow-derived angiogenic cells to burn wounds. J Mol Med. 2011 doi: 10.1007/s00109-011-0754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimpo S, Horiguchi Y, Nakamura Y, Lee M, Oikawa T, Noda K, Kuwahara Y, Kawasaki K. Compensatory bone formation in young and old rats during tooth movement. Eur J Orthod. 2003;25(1):1–7. doi: 10.1093/ejo/25.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Boyan BD, Schwartz Z, Lohmann CH, Sylvia VL, Cochran DL, Dean DD, Puzas JE. Pretreatment of bone with osteoclasts affects phenotypic expression of osteoblast-like cells. J Orthop Res. 2003;21(4):638–647. doi: 10.1016/S0736-0266(02)00261-9. [DOI] [PubMed] [Google Scholar]
- 31.Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17(2):137–146. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- 32.Boyan BD, Lossdorfer S, Wang L, Zhao G, Lohmann CH, Cochran DL, Schwartz Z. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur Cell Mater. 2003;6:22–27. doi: 10.22203/ecm.v006a03. [DOI] [PubMed] [Google Scholar]
- 33.Zhao G, Zinger O, Schwartz Z, Wieland M, Landolt D, Boyan BD. Osteoblast-like cells are sensitive to submicron-scale surface structure. Clin Oral Implants Res. 2006;17(3):258–264. doi: 10.1111/j.1600-0501.2005.01195.x. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz Z, Lohmann CH, Vocke AK, Sylvia VL, Cochran DL, Dean DD, Boyan BD. Osteoblast response to titanium surface roughness and 1alpha,25-(OH)(2)D(3) is mediated through the mitogen-activated protein kinase (MAPK) pathway. J Biomed Mater Res. 2001;56(3):417–426. doi: 10.1002/1097-4636(20010905)56:3<417::aid-jbm1111>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 35.Keselowsky BG, Wang L, Schwartz Z, Garcia AJ, Boyan BD. Integrin alpha(5) controls osteoblastic proliferation and differentiation responses to titanium substrates presenting different roughness characteristics in a roughness independent manner. J Biomed Mater Res A. 2007;80(3):700–710. doi: 10.1002/jbm.a.30898. [DOI] [PubMed] [Google Scholar]
- 36.Olivares-Navarrete R, Sutha K, Hyzy SL, Hutton DL, Schwartz Z, McDevitt T, Boyan BD. Osteogenic Differentiation of Stem Cells Alters Vitamin D Receptor Expression. Stem Cells Dev. 2012 doi: 10.1089/scd.2011.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raines AL, Sunwoo M, Gertzman AA, Thacker K, Guldberg RE, Schwartz Z, Boyan BD. Hyaluronic acid stimulates neovascularization during the regeneration of bone marrow after ablation. J Biomed Mater Res A. 2011;96(3):575–583. doi: 10.1002/jbm.a.33012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz Z, Doukarsky-Marx T, Nasatzky E, Goultschin J, Ranly DM, Greenspan DC, Sela J, Boyan BD. Differential effects of bone graft substitutes on regeneration of bone marrow. Clin Oral Implants Res. 2008;19(12):1233–1245. doi: 10.1111/j.1600-0501.2008.01582.x. [DOI] [PubMed] [Google Scholar]