Abstract
Heart failure (HF) is a common disease that continues to be associated with high morbidity and mortality warranting novel therapeutic strategies. Cyclic guanosine monophosphate (cGMP) is the second messenger of several important signaling pathways based on distinct guanylate cyclases (GCs) in the cardiovascular system. Both the nitric oxide/soluble GC (NO/sGC) as well as the natriuretic peptide/GC-A (NP/GC-A) systems are disordered in HF, providing a rationale for their therapeutic augmentation. Soluble GC activation with conventional nitrovasodilators has been used for more than a century but is associated with cGMP-independent actions and the development of tolerance, actions which novel NO-independent sGC activators now in clinical development lack. Activation of GC-A by administration of naturally occurring or designer natriuretic peptides is an emerging field, as is the inhibition of enzymes that degrade endogenous NPs. Finally, inhibition of cGMP-degrading phosphodiesterases, particularly phosphodiesterase 5 provides an additional strategy to augment cGMP-signaling.
1. Heart Failure
Cardiovascular disease in its various forms is the leading cause of death in the USA. Notwithstanding the different etiologies, a common final stage is the syndrome of heart failure (HF), in which the heart fails to meet the metabolic demands of the body. Heart failure has achieved almost epidemic proportions in terms of increasing prevalence (> 5 million in the USA), high incidence (about 550,000 per year), and being the leading cause for hospitalizations (>1 million in 2004) of the elderly (Rosamond et al. 2008). While the widely used New York Heart Association classification provides a measure of current functional status, the new ACC/AHA classification of HF into stages A–D reflects that HF is in most cases a progressive disorder. Stage A represents the presence of risk factors for the development of HF (e.g. hypertension, diabetes mellitus) without structural cardiac disease. In stage B structural cardiac changes (e.g. hypertrophy) are present that are strongly associated with the development of HF. In stage C patients have current or prior symptoms of HF with underlying structural heart disease, and in stage D patients have advanced structural heart disease and refractory HF symptoms at rest (Hunt et al. 2005). With more people surviving into older age, improved treatments for myocardial infarction, and better prevention of sudden cardiac death, the incidence and prevalence of HF is likely to increase further. While pharmacologic (e.g. angiotensin converting enzyme (ACE) inhibitors, beta adrenergic receptor blockers, aldosterone receptor antagonists, nitrates in combination with hydralazine) and device-based (e.g. ventricular assist devices, implantable cardioverter-defibrillators, cardiac resynchronization therapy) treatment modalities have improved patient outcomes, morbidity and mortality remain substantial. Thus, there is a need for novel treatment strategies.
Hallmarks of HF include functional and structural changes in the heart, endothelial and vascular dysfunction with vasoconstriction, sodium and water retention by the kidney, and neurohumoral activation. With regard to new HF treatments, several important points should be noted. Heart failure patients frequently have significant comorbidities and represent an unstable patient population with substantial short-term mortality. Given the heterogeneity of HF individualized treatment approaches are required. Especially renal dysfunction has emerged as an important determinant of outcome and therapeutic challenge, as in the case of the “cardiorenal syndrome” and diuretic resistance (Liang et al. 2008). Indeed, the requirement to maintain a sufficient renal perfusion pressure is an important limitation to the dosing of vasodilating drugs. In addition, drugs with efficacy in some stages of HF may be detrimental in others, while improvement in symptoms or hemodynamic function in the short-term may turn out to be harmful in the long term. Also, efficacy of a drug observed when given as monotherapy may be reduced or absent when given on top of standard therapy. The ultimate test for medical interventions that appear rational and promising in preclinical and early clinical studies remains the randomized, controlled clinical trial with appropriate endpoints.
2. Cyclic Guanosine Monophosphate
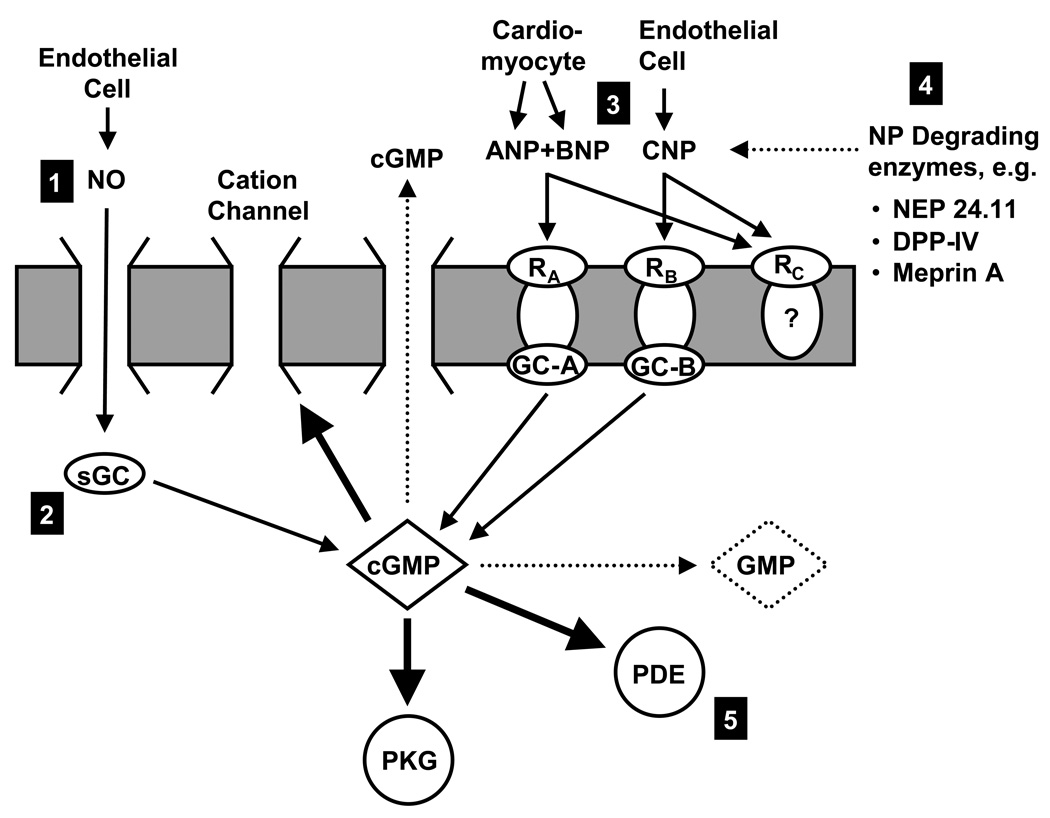
3’, 5’-cyclic guanosine monophosphate (cGMP) is the second messenger of a variety of signaling systems that use one of several distinct guanylate cyclases (GCs; E.C. 4.6.1.2). GCs are enzymes that convert guanosine 5’-triphosphate (GTP) to cGMP. To date, one cytosolic (soluble) and seven particulate GCs have been identified. Of special importance in the cardiovascular system and illustrated in Figure 1 are
soluble GC (also called nitric oxide (NO)-sensitive GC) with its endogenous ligand NO,
GC-A (also called natriuretic peptide A receptor (NPR-A)) with its endogenous ligands ANP and BNP
GC-B (also called NPR-B) with its endogenous ligand CNP.
Figure 1.
Simplified schematic of guanylate cyclase (GC) pathways. Cyclic GMP is the second messenger of several distinct signaling pathways. Nitric oxide is produced by endothelial cells and binds to soluble GC in the target cell. ANP and BNP, derived primarily from cardiomyocytes, stimulate GC-A (also called NP receptor A), while CNP, secreted by endothelial cells, stimulates GC-B (also called NP receptor B). Cyclic GMP modulates the activity of cGMP-dependent protein kinase G, cGMP-regulated PDEs, and cGMP-regulated cation channels. The cGMP signal is terminated by a variety of PDEs that hydrolyze cGMP to GMP, or by extrusion into the extracellular space. The NPs are degraded by a variety of peptidases. Cyclic GMP signaling can be augmented by (1) the use of NO-mimetics such as nitrovasodilators, (2) by direct sGC stimulators, (3) by administration of exogenous NPs, (4) by inhibiting NP degrading enzymes, and (5) by inhibiting the activity of cGMP-hydrolyzing PDEs. ANP, atrial natriuretic peptide, BNP, B-type natriuretic peptide, cGMP, cyclic guanosine monophosphate, GMP, guanosine monophosphate, GC, guanylate cyclase, DPP4, dipeptidyl peptidase IV, NEP, neutral endopeptidase, NO, nitric oxide, PDE, phosphodiesterase, PKG, protein kinase G, RA, natriuretic peptide receptor A, sGC, soluble guanylate cyclase.
Other GCs are GC-C (ligands: guanylin and uroguanylin, heat-stable enterotoxins) and GC-D, -E, -F, and -G for which the endogenous ligands still need to be identified.
Cyclic GMP affects the activity of a variety of effector molecules such as cGMP-dependent protein kinase (PKG), cGMP-regulated PDEs, and cGMP-regulated cation channels. Conversely, cGMP itself is a substrate of several PDEs, which hydrolyze cGMP to GMP and thus terminate the cGMP signal (Bender and Beavo 2006, Conti and Beavo 2007). Of note, by modulating PDEs that catabolize cAMP, cGMP can affect cAMP based signaling systems, such as catecholamines (Zaccolo and Movsesian 2007).
Despite having a common second messenger, activating different cGMP-dependent pathways will not necessarily result in similar actions. Receptors can differ both in their tissue and their intracellular distribution. Within the cell it has been shown that GC-A stimulation as compared to sGC stimulation leads to increases in distinct cGMP pools that are compartmentalized by the activity of PDEs (Castro et al. 2006, Piggott et al. 2006, Fischmeister et al. 2006, Takimoto et al. 2007, Hart et al. 2001). These findings are consistent with the observation of distinct actions profiles: e.g. ANP, BNP and NO have vasodilating actions, but only ANP and BNP are natriuretic. Regarding renal function, intrarenal administration of the NO synthase inhibitor NG-monomethyl-L-arginine (L-NMMA) in experimental acute HF reduced renal blood flow without affecting GFR and sodium excretion, whereas natriuretic peptide receptor blockade with HS-142-1 did not affect renal blood flow but decreased GFR and sodium excretion (Martin et al. 2007). It should also be noted that there can be reciprocal regulation of different cGMP pathways; e.g. in vascular smooth muscle cells activation of GC-A leads to an attenuated response to sGC and vice versa (Hussain et al. 2001, Madhani et al. 2006).
The remainder of this review will discuss specific strategies to augment cGMP signaling pathways in HF.
3. The NO-sGC/cGMP Pathway
Soluble GC is a heterodimeric heme enzyme consisting of an α- and β-subunit and a prosthetic heme group with a ferrous iron. Binding of NO, which is produced by one of three nitric oxide synthases, to the heme iron changes the conformation of the enzyme and increases its catalytic activity dramatically. Signaling via the NO/sGC/cGMP pathway has been shown to be attenuated in a variety of cardiovascular disease states. Part of this is due to scavenging of NO preventing activation of sGC. Indeed, the HF syndrome is associated with markedly increased oxidative stress in vascular and renal tissues contributing to endothelial dysfunction, which is adversely associated with patient outcome. More recent studies have demonstrated that not only can NO be scavenged but also the heme moiety of sGC can be oxidized or missing, rendering the enzyme insensitive to NO (Stasch et al. 2006, Munzel et al. 2007). This endothelial or vascular dysfunction provides a rationale for the therapeutic enhancement of the NO/sGC/cGMP pathway.
3.1 Conventional Nitrovasodilators
Soluble GC stimulation with nitrovasodilators has been employed for more than a century in cardiovascular disease (Brunton 1867). However, long-term administration of nitrovasodilators is associated with the development of tolerance and cGMP-independent actions, which may have beneficial but also detrimental actions. The latter include oxidative stress, mitochondrial toxicity, endothelial dysfunction, and protein nitrosation (Munzel et al. 1995, Caramori et al. 1998, Warnholtz et al. 2002, Sydow et al. 2004, Hess et al. 2005, Heck et al. 2005, Stasch, et al. 2006, Thomas et al. 2007). These side effects can at least in part be prevented by hydralazine, which has potent antioxidant properties. Indeed, clinical trials in which patients received a combination of a nitrate (isosorbide dinitrate) and hydralazine demonstrated improved outcomes compared to placebo before the introduction of ACE inhibitors (Cohn et al. 1986) and more recently in African Americans even on top of therapy with ACE inhibitors and beta blockers (Taylor et al. 2004).
3.2 NO-independent stimulator and activators of sGC
Ko et al. (1994) reported hat the molecule YC-1 activates sGC directly, i.e. independently of NO, which stimulated the search for similar compounds. Stasch et al. (2001, 2002) described two new classes of direct sGC stimulators, one NO-independent but heme-dependent, the other NO- and heme-independent. The former class only stimulates sGC if the heme iron is in the ferrous state (Fe2+), whereas the latter class activates the enzyme preferentially if the heme iron is oxidized (Fe3+) or the heme is absent.
3.2.1 NO-independent but Heme-Dependent sGC Stimulators
The first orally available NO-independent but heme dependent sGC stimulator to be described was BAY 41–2272, a pyrazolopyridine. It binds to a regulatory site on the alpha subunit of sGC and activates sGC synergistically with NO and can thus be considered a “NO sensitizer”. In experimental studies BAY 41–2272 lowered blood pressure, relaxed blood vessels that had been made tolerant to nitrovasodilators, and inhibited platelet aggregation. A related compound, BAY 41–8543, prolonged binding of NO to sGC 220 fold (Schmidt et al. 2003). In a canine model of HF induced by rapid right ventricular pacing, BAY 41–2272 decreased mean arterial pressure, pulmonary capillary wedge pressure and systemic and renal vascular resistances and increased cardiac output and renal blood flow (Boerrigter et al. 2003). The hemodynamic actions were similar to nitroglycerin with the important exception of right atrial pressure, which decreased with nitroglycerin but was unchanged with BAY 41–2272. This difference could be due to the fact that BAY 41–2272 acts synergistically with NO, which may be more abundant in arteries than veins. In contrast, nitroglycerin is bioactivated preferentially in the veins. An at least theoretical problem with the synergism is the potential for a “steal phenomenon”, i.e. BAY 41–2272 may be most efficacious in areas with higher endogenous levels of NO, thus potentially diverting blood away from diseased and ischemic areas with low endogenous NO. Also, venodilation, indicated by the decrease in right atrial pressure seen with nitroglycerin, would be desirable in most patients with HF, as it reduces preload and chamber dimensions, and thus wall stress and myocardial oxygen demand. It should be noted that actions of BAY 41–2272 other than stimulating sGC have been reported, e.g. inhibition of PDE5 (Mullershausen et al. 2004, Bischoff and Stasch 2004). While this would make BAY 41–2272 a less specific drug, it would not necessarily make it a worse therapeutic as is discussed in more detail later in this review.
The NO-independent but heme-dependent sGC stimulator in the most advanced stage of clinical development is BAY 63–2521 (riociguat), which is currently in clinical trials for the treatment of pulmonary arterial hypertension (Ghofrani et al. 2007). Other NO-independent but heme-dependent sGC stimulators are CFM-1571 and A-350619 (Selwood et al. 2001, Miller et al. 2003, Evgenov et al. 2006).
3.2.2 NO-and Heme-Independent sGC Activators
The first reported NO- and heme-independent sGC activator was BAY 58–2667, an amino dicarboxylic acid that binds to regulatory sites on the α- and β-subunit of sGC (Stasch et al. 2002). BAY 58–2667 potently activates sGC because it can compete with and replace the heme moiety, which in the non-nitrosylated state inhibits enzyme activity (Schmidt et al. 2004). Indeed, BAY 58–2667 activates preferentially the oxidized and heme-free enzyme, which makes BAY 58–2667 an interesting pharmacologic tool. Stasch et al. (2006) reported that in various experimental cardiovascular disease conditions the potency of nitrovasodilators was reduced, whereas it was increased for BAY 58–2667, strongly suggesting an increased prevalence of oxidized or heme-free sGC. These findings provide evidence for a potential additional pathophysiologic mechanism contributing to endothelial and vascular smooth muscle cell dysfunction in cardiovascular disease states. They also suggest that BAY 58–2667 may induce the opposite of a “steal phenomenon” by being most efficacious in diseased vessels.
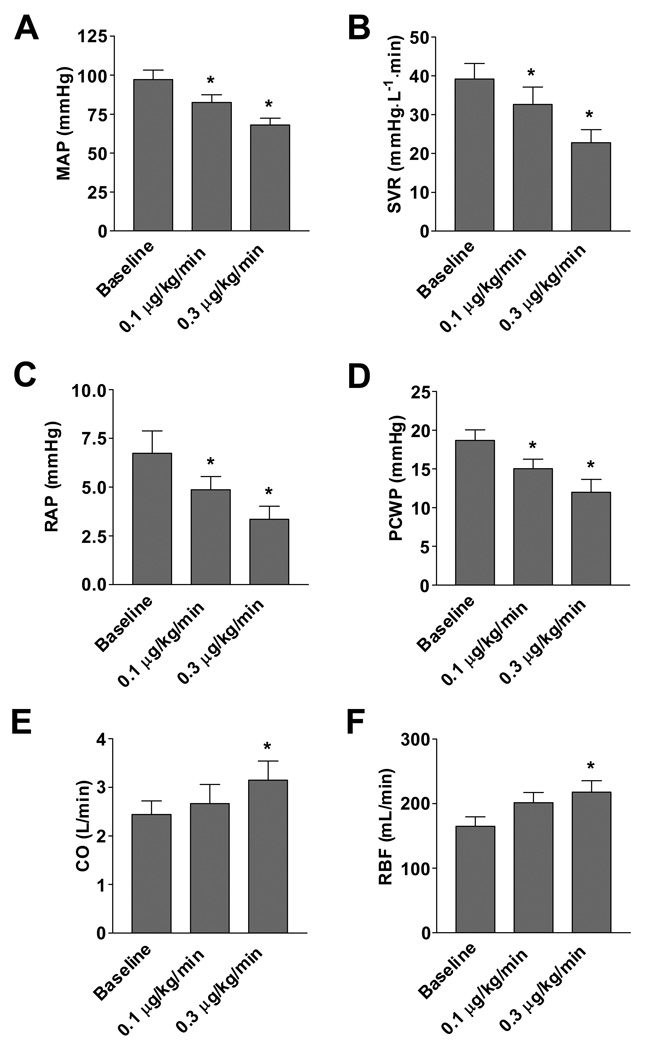
In vivo, BAY 58–2667 has an additive effect with NO and lowered blood pressure in rats and healthy canines (Stasch, et al. 2002). In experimental pacing-induced HF, BAY 58–2667 had hemodynamic actions similar to nitroglycerin with reductions in mean arterial and pulmonary capillary wedge pressure, systemic and renal vascular resistance, and increases in cardiac output and renal blood flow (Boerrigter et al. 2007) (Figure 2). Importantly, unlike BAY 41–2272, BAY 58–2667 also decreased right atrial pressure. GFR and urinary sodium excretion were preserved despite the hypotensive actions. There was also no activation of RAAS, whereas ANP and BNP decreased, consistent with cardiac unloading.
Figure 2.
Effect of BAY 58–2667 administration on (A) mean arterial pressure (MAP), (B) systemic vascular resistance, (C) right atrial pressure (RAP), (D) pulmonary capillary wedge pressure (PCWP), (E) cardiac output (CO), and (F) renal blood flow (RBF). * indicates p<0.05 compared to baseline. Taken from Boerrigter et al. (2007).
As BAY 58–2667 possesses impressive hemodynamic properties in experimental HF and preserves renal function, an unblinded, uncontrolled proof-of-concept study was recently undertaken and completed. Specifically, Lapp et al. (2007) assessed the effect of BAY 58–2667 on hemodynamic and symptomatic status in patients admitted to the hospital with acute decompensated HF, NYHA class III/IV symptoms, PCWP≥18 mmHg, parenteral pharmacotherapy, and invasive hemodynamic monitoring. Based upon an initial dose-finding part of the study (n=17), a starting dose of 100 µg/h was chosen for the proof-of-concept second part of the study (n=33), in which BAY 58–2667 was infused for a total of six hours. Hemodynamic function was assessed at baseline, after 2, 4, and 6 hours of drug infusion, and 2 hours after the end of infusion. Dose adjustments could be made at 2 and 4 hours; the maximal dose of 400 µg/h was administered to sixteen of 30 patients who completed the study. BAY 58–2667 significantly reduced PCWP, right atrial pressure, systemic and pulmonary vascular resistance, while cardiac output increased. A PCWP reduction of ≥4 mmHg was achieved in 53%, 83%, and 90% after 2, 4, and 6 hours, respectively, which was associated with an improvement in symptomatic status as assessed by a dyspnea score. This study shows that also in human HF there exists a pool of sGC that can be activated by BAY 58–2667 with subsequent favorable cardiac pre- and afterload reduction, warranting further studies. Other NO- and heme-independent sGC activators are HMR1766 (ataciguat) and S3448 (Schindler et al. 2006).
When these preclinical and clinical studies are taken together, the results are encouraging and provide evidence for therapeutic potential. As recently stated by Munzel et al. (2007), “these agents could revolutionize the treatment of not only HF but also other conditions, such as coronary artery disease, systemic hypertension, and pulmonary hypertension.”
4. The Natriuretic Peptide Pathways
The natriuretic peptides ANP and BNP are secreted by the heart in conditions of cardiac overload and via GC-A stimulate the production of cGMP. They have natriuretic, diuretic, vasodilating, renin- and aldosterone suppressing actions that help to unload the heart. In addition, they have antihypertrophic and antifibrotic properties (Tsuruda et al. 2002, Holtwick et al. 2003) as well as lusitropic (Lainchbury et al. 2000). CNP of endothelial origin functions via activation of GC-B and serves as a more local autocrine/paracrine peptide in endothelial cell/vascular smooth muscle cell control (Stingo et al. 1992). As discussed above, the intracellular cGMP signal of the NPs is terminated by PDEs.
NPs are inactivated in several ways: enzymatic degradation, endocytosis after binding to the natriuretic peptide clearance receptor (NPR-C), or renal excretion. Of note, ANP, BNP, and CNP bind to NPR-C, the main function of which was initially believed to be clearance of NPs; however, other functions have emerged (Villar et al. 2007, Huntley et al. 2006). Importantly, in more advanced HF stages the response to endogenous and exogenous NPs is blunted, which can at least partially be overcome by administration of exogenous NPs, providing a rationale for pharmacologically augmenting the NP system. This is possible in various ways, e.g. administration of exogenous NPs or prolonging the bioactivity of endogenous NPs by inhibiting their degradation.
4.1 Guanylate Cyclase A Activators
4.1.1 B-Type Natriuretic Peptide
BNP has been reported to have vasodilating, natriuretic, diuretic, lusitropic, anti-hypertrophic, and antifibrotic properties, all of which could be beneficial in HF (Yoshimura et al. 1991, Lainchbury, et al. 2000, Tsuruda, et al. 2002, Tamura et al. 2000). The response to exogenous BNP is blunted, suggesting resistance to BNP and providing a rationale for exogenous supplementation despite high endogenous levels. In canine tachypacing-induced HF BNP augmented the diuretic and natriuretic response to furosemide, increased GFR, and prevented an increase in aldosterone induced by furosemide alone (Cataliotti et al. 2004).
Nesiritide, a recombinant form of human BNP, was approved for the treatment of acute decompensated HF in the US in 2001. In the VMAC trial, nesiritide was administered as a bolus (2 µg/kg) followed by a continuous infusion (0.01 to 0.03 µg/kg/min) in patients with acute decompensated HF; in a subset of patients the dose could further be increased in steps of 0.005 µg/kg/min preceded by a 1 µg/kg bolus up to a maximum of 0.03 µg/kg/min (VMAC-Investigators 2002). Nesiritide led to a larger decrease in pulmonary capillary wedge pressure at 3 and 24 hours as compared to placebo and nitroglycerin, although symptomatic status was not improved compared to nitroglycerin. Subsequent meta-analyses of publicly available studies suggested that nesiritide was associated with an increase in serum creatinine and mortality (Sackner-Bernstein et al. 2005, Sackner-Bernstein et al. 2005). The mechanism for these findings is unclear but possibly the nesiritide dose administered, particularly the bolus, may have contributed to hypotension, which due to BNP’s half-life would be more prolonged as compared to e.g. nitroglycerin. Subsequent small studies reported no indication for detrimental or beneficial renal effects of nesiritide in acute decompensated HF (Wang et al. 2004, Witteles et al. 2007, Owan et al. 2008, Burnett and Korinek 2008). Of note, despite the fact that administration of a bolus was left at the discretion of the physician in the latter study, patients with nesiritide had significantly lower blood pressures, which may have offset some potentially beneficial actions by reducing renal perfusion pressure. The manufacturer-sponsored ongoing “Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure” (ASCEND-HF) with rehospitalization due to HF and all-cause mortality as a primary outcome measure may be able to provide definitive clarification. Using an innovative but more invasive approach, intrarenal administration of nesiritide with a bifurcated renal catheter in experimental canine HF resulted in a larger natriuresis and increase in glomerular filtration rate as compared to systemic nesiritide (Chen et al. 2006). Importantly, intrarenal unlike systemic administration did not lower systemic arterial blood pressure.
Nesiritide has also been evaluated in settings other than acute decompensated HF. The FUSION-II trial evaluated once or twice weekly outpatient infusions of nesiritide for 12 weeks in patients with chronic HF on standard therapy. No increased renal dysfunction or mortality, but also no benefit was observed (Cleland et al. 2007). One reason for the lack of efficacy could be that only intermittent administration of nesiritide was used. Feasibility of chronic subcutaneous administration has been demonstrated in animals and in humans (Chen et al. 2000, Chen et al. 2004) and studies to further explore the efficacy of chronic nesiritide are ongoing. In addition, peptide modification to increase the half-life of BNP or to allow oral delivery have been reported (Cataliotti et al. 2005).
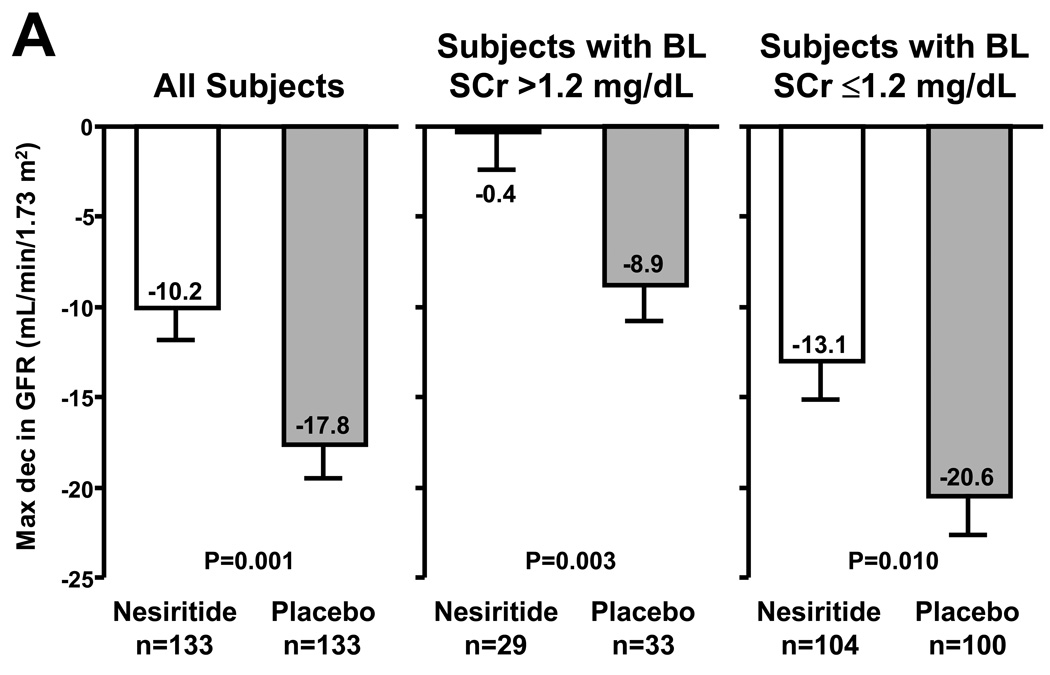
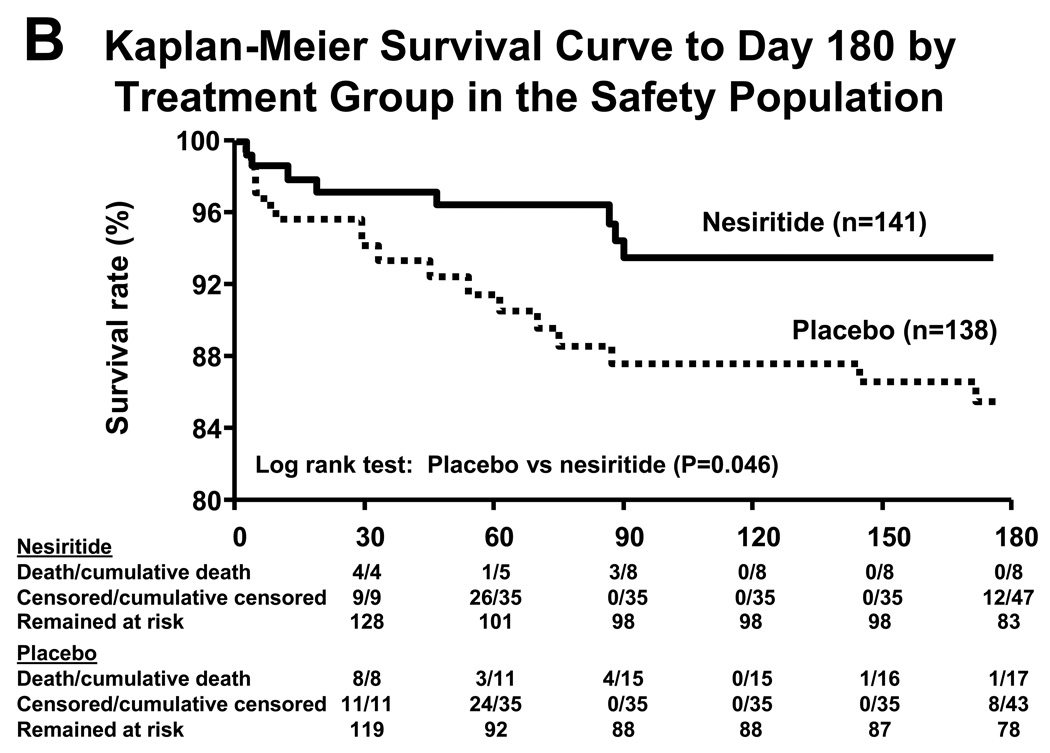
In the NAPA trial, nesiritide (0.01 µg/kg/min; no bolus; n=141) or placebo (n=138) was given to patients with a left ventricular ejection fraction ≤40% undergoing bypass surgery with anticipated cardiopulmonary bypass (Mentzer et al. 2007) (Figure 3). Patients randomized to nesiritide had a smaller rise in serum creatinine, larger urine output, shorter hospital stay, and a lower 180-day mortality. Similarly, in a study of patients with reduced renal function undergoing cardiac bypass surgery randomization to nesiritide as compared to control improved post-operative renal function (Chen et al. 2007). Another possible indication where studies are pursued is nesiritide as a treatment to ameliorate remodeling post myocardial infarction.
Figure 3.
Adjusted mean maximum decrease in glomerular filtration rate (GFR) from baseline through hospital discharge or by study day 14, whichever came first, using an analysis of covariance model. Lines above the bars indicate standard error of the mean (A). Kaplan-Meier survival curve to day 180 by treatment group in the ‘safety population’, which is a subset of the total study population added during the study to assess long-term safety (B). Taken from Mentzer et al. (2007); reprinted with permission from Elsevier.
4.1.2 Atrial Natriuretic Peptide
ANP (carperitide) has been approved for the treatment of HF in Japan since 1995 (Suwa et al. 2005). However, no double-blind placebo controlled studies are available evaluating its efficacy in HF. Most recently, in patients with acute myocardial infarction, carperitide as an adjunct to reperfusion therapy reduced infarct size as estimated by creatine kinase and increased left ventricular ejection fraction (Kitakaze et al. 2007). Similarly, in a study in patients with first anterior myocardial infarction, carperitide decreased LV end diastolic volume and increased LV ejection fraction fraction compared to infusion of isosorbide dinitrate (Kasama et al. 2007).
Urodilatin (ularitide) is a splice-variant of the ANP precursor with four additional N-terminal amino acids. It is secreted into the urine and does not appear in the circulation. In a phase II trial, 24-hour infusion of ularitide in patients with decompensated HF (n=221) dose-dependently decreased cardiac filling pressures and systemic arterial pressure and improved symptomatic status with no adverse effects on renal function observed during the first three days after study drug administration (Mitrovic et al. 2006). The most common adverse effect was hypotension, which required at least temporary interruption of drug administration in 5.0, 9.4, and 12.7% in the groups randomized to 7.5, 15, and 30 ng/kg/min of ularitide, respectively.
4.1.3 Dendroaspis Natriuretic Peptide
There are also other sources of naturally occurring NPs. Snake venom has been the source of several peptides that have been useful in the development of cardiovascular drugs, e.g. the ACE inhibitor captopril and the glycoprotein IIb/IIIa antagonist eptifibatide. In 1992, Schweitz et al. reported the in vitro biological actions of a newly discovered peptide Dendroaspis natriuretic peptide (DNP), which was isolated from the venom of the green mamba. DNP binds to GC-A and the natriuretic peptide clearance receptor, but not to GC-B (Schweitz et al. 1992, Johns et al. 2007). We reported that DNP in vivo was potently natriuretic and diuretic and possessed cardiac unloading actions but with significant hypotensive properties (Lisy et al. 1999, Lisy et al. 2001). These in vivo actions of DNP are consistent with GC-A activation as such effects closely mimic the properties of ANP and BNP but not CNP. Of note, DNP has a higher affinity for the GC-A receptor as compared to ANP and BNP (Singh et al. 2006, Johns, et al. 2007). As a non-endogenous peptide, there is however the potential of immunogenicity.
4.2 Guanylate Cyclase B Activators: C-Type Natriuretic Peptide
Another member of the endogenous NP family is CNP, which acts via GC-B (Tawaragi et al. 1991). CNP is a 22-amino acid (AA) peptide that shares structural homology with the other members of the NP family, all of which function via well-characterized particulate GC receptors as discussed above and the second messenger cGMP. While possessing structural similarity, CNP is genetically distinct from ANP and BNP. Also, unlike ANP or BNP, CNP lacks a carboxy-terminal amino acid extension, which may explain in part its lack of natriuretic properties (Clavell et al. 1993, Hunt et al. 1994). Since its discovery in 1990, we have learned that CNP is principally an endothelial cell derived peptide (Stingo et al 1992). In isolated venous and arterial rings, CNP activates GC-B receptors in veins while ANP and BNP activate GC-A receptors in both arteries and veins which is consistent with the less hypotensive actions of CNP as compared to ANP and BNP (Wei et al. 1993, Igaki et al. 1998, La Villa et al. 1998).
Studies have also demonstrated that CNP possesses more potent anti-proliferative and collagen suppressing properties in cardiac fibroblasts (CFs) as compared to ANP and BNP (Horio et al. 2003). Relevant to such antifibrotic properties, studies have reported that 14 days of continuous infusion of CNP in rodents with acute myocardial infarction (AMI) markedly attenuates ventricular dilation, cardiac fibrosis, and cardiomyocyte hypertrophy (Soeki et al. 2005). The chronic infusion of CNP was without any hypotensive actions.
In clear contrast to ANP and BNP, CNP lacks significant natriuretic and diuretic actions when infused into humans. This may explain its lack of utility in sodium and water retaining sydromes such as HF despite its attractive venodilating and antifibrotic properties. Interestingly, in HF GC-A is downregulated and GC-B is relatively more abundant than GC-A (Dickey et al. 2007, Bryan et al. 2007) which provides the rationale for GC-B agonist-based therapies in HF.
4.3 Designer Natriuretic Peptides
Given the complexity and diversity of HF, it should come as no surprise that currently available NPs are not efficacious or ideal for every patient. Indeed, as discussed in the section on BNP (4.1.1) hypotensive actions or specific renal resistance in some patients may completely offset any renal enhancing actions. Thus, attempts are made to design and develop NPs with an improved profile of action. Aims of modifying the peptide sequence could be to change the affinity to different receptors and the susceptibility to enzymatic degradation.
A key structural feature of DNP is that it possesses the longest C-terminus of the known natriuretic peptides consisting of 15 amino acids (AA) as compared to 5 AA for ANP, 6 AA for BNP, and none for CNP. Indeed, the long C-terminus of DNP may render DNP highly resistant to degradation by neprilysin (NEP; EC 3.4.24.11; also called neutral endopeptidase, CD10) contributing to potent natriuretic and diuretic actions (Chen et al. 2000). Further, the lack of a C-terminus for CNP may explain the observation that of the three known endogenous natriuretic peptides CNP is the most susceptible to degradation by NEP, which could limit its renal actions as NEP is strongly expressed in the kidney (Kenny et al. 1988).
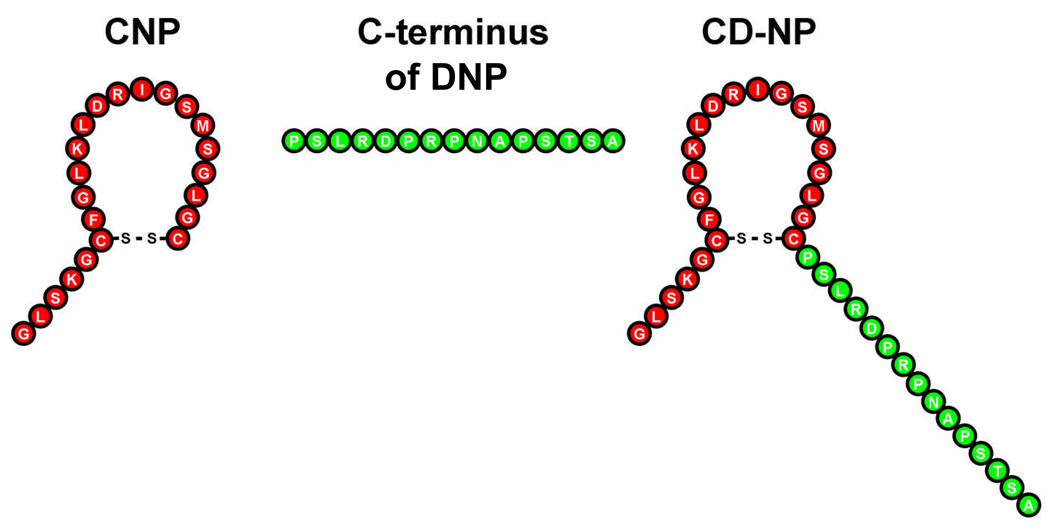
Based upon exploratory studies of the 15-AA C-terminus of DNP, we found that fusion of the 15 AA C-terminus of DNP into the C-terminus position of the core 22-AA ring structure of CNP resulted in a synthetic chimeric peptide that in vivo possessed the cardiac unloading actions of CNP with minimal hypotensive properties together with the additional renal effects of natriuresis and diuresis (Lisy et al. 2008) (Figure 4). We also observed that this chimera called CD-NP retained properties of CNP in vitro in activating cGMP in cardiac fibroblasts and inhibiting cell proliferation. Thus, this chimeric natriuretic peptide possesses potentially beneficial efficacy and safety for the treatment of cardiorenal disease states such as HF and AMI. A first in human study was recently completed in normal human subjects and demonstrated activation of plasma and urinary cGMP, natriuresis, aldosterone suppression with minimal actions on arterial pressure (Lee et al. 2008).
Figure 4.
CD-NP is a chimeric peptide consisting of the amino terminus and ring structure of C-type natriuretic peptide and the carboxy terminus of Dendroaspis natriuretic peptide.
4.4 Inhibitors of Natriuretic Peptide Degradation
An alternative way to augment the NP pathways is to inhibit enzymes involved in the degradation of endogenous NPs. However, as these enzymes are likely to have other substrates, this approach is a less specific strategy and a potential advantage of augmenting the NPs may be offset by reduced degradation of undesired endogenous peptides. Enzymes reported to be involved in NP degradation are NEP, dipeptidyl peptidase IV (DPP-IV; EC 3.4.14.5; also called CD26), and meprin A (EC 3.4.14) (Kenny and Stephenson 1988, Brandt et al. 2006, Pankow et al. 2007). NEP inhibitors have been tested in clinical trials but were not superior to placebo (Northridge et al. 1999, Cleland and Swedberg 1998). Omapatrilat is a vasopeptidase inhibitor that simultaneously inhibits ACE and NEP. In the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE, n=5770), omapatrilat demonstrated equal efficacy as enalapril regarding death or hospitalization for HF (Packer et al. 2002). Interestingly, had the end point criteria of the Studies of Left Ventricular Dysfunction (SOLVD) trial been employed, a statistically significant improvement with omapatrilat would have been found. However, in an echocardiographic OVERTURE substudy, no differences in LV remodeling and function was observed between randomization groups (Solomon et al. 2005). Of note, given that omapatrilat is a more potent and longer lasting inhibitor of ACE as compared to NEP, the once daily administration of omapatrilat may not have been sufficient to persistently enhance the NP system and demonstrate superiority as compared to ACE inhibition alone in this HF study population. In the Exercise and Symptoms Study of Heart Failure (IMPRESS, n=573), omapatrilat significantly reduced the composite end point of death, admission or discontinuation of study treatment for worsening HF compared to the ACE inhibitor lisinopril (Rouleau et al. 2000). The more favorable results in the IMPRESS trial may be due to the fact that patients enrolled in this trial had on average less severe HF.
A potential problem of NEP inhibition is that it could also inhibit the degradation of endothelin, a potent vasoconstrictor and also a NEP substrate. More upstream from NEP in the processing of BNP is DPP-IV which was reported to cleave BNP 1–32 to BNP 3–32 in vitro (Brandt, et al. 2006). In healthy canines, synthetic BNP 3–32 as compared to BNP 1–32 had no vasodilating actions and had reduced natriuretic actions (Boerrigter et al. 2007). No reports are available regarding the effect of DPP-IV inhibition on endogenous and exogenous BNP levels in vivo, which may be important to know particularly because the first DPP-IV inhibitor, sitagliptin, has recently been approved for the treatment of type II diabetes mellitus. Meprin A cleaves BNP 1–32 to BNP 8–32, which then also becomes a substrate of NEP (Pankow, et al. 2007). BNP 8–32 in healthy canines compared to BNP 1–32 had similar hemodynamic but reduced natriuretic actions (Boerrigter et al. 2008). Based upon these results one could speculate that enzymatic degradation of the NPs not necessarily only reduces their bioactivity, but it may also modify their profile of actions.
5. Phosphodiesterase Inhibition
The cGMP signal is effectively terminated by the action of specific phosphodiesterases that hydrolyze cGMP to GMP. Inhibition of the isoenzyme PDE5A (e.g. with sildenafil, vardenafil, tadalafil) reduces the vascular tone particularly in the corpus cavernosum and the pulmonary vasculature, leading to its application in erectile dysfunction and pulmonary arterial hypertension. In a murine model of pressure overload due to aortic banding, chronic sildenafil (100 mg/kg body weight) attenuated cardiac hypertrophy, dilation, and fibrosis, and improved cardiac function (Takimoto et al. 2005). Indeed, cardiac hypertrophy was not only prevented but, once established, could be reversed with sildenafil. This was associated with decreased activation of hypertrophic factors such as calcineurin, the mitogen-activated kinase ERK1/2, Akt, and PI3Kα. However, a different group using the same animal model reported that sildenafil (154±5 mg/kg body weight/day ) did not prevent hypertrophy and actually decreased ejection fraction (Eder et al. 2007). The reason for this discrepancy is unclear but may include the sildenafil dose and the degree of aortic banding. In canine pacing-induced HF, chronic PDE5 inhibition with sildenafil reduced systemic vascular resistance and increased cardiac output but did not affect cardiac filling pressures or renal function (Chen et al. 2006). Of note, this model is not associated with the development of cardiac hypertrophy. Sildenafil post-myocardial infarction in mice reduced infarct size and apoptosis, improved left ventricular function 7 and 28 days post-MI, reduced cardiac hypertrophy, and improved survival (Salloum et al. 2008). It would be interesting to know whether short-term administration for only a few days post-MI would be sufficient to improve remodeling or whether chronic therapy is required.
In a small human study (n=34) in systolic HF patients with secondary pulmonary hypertension (mean pulmonary artery pressure >25 mmHg), those randomized to sildenafil (25–75 mg orally 3 times daily for 12 weeks) demonstrated reduced pulmonary vascular resistance at rest and during exercise compared to placebo (Lewis et al. 2007). Peak cardiac output and maximal oxygen uptake during exercise also increased, while six-minute walk distance and symptomatic status improved. No significant change in mean arterial pressure, systemic vascular resistance, pulmonary capillary wedge pressure, or heart rate was observed. Of note, the sample size was small and slightly more than half the patients were in NYHA class II. In another study, male HF patients (n=46; NYHA class II or III) were randomized to sildenafil (50 mg thrice daily) or placebo and reevaluated after 3 and 6 months (Guazzi et al. 2007). Sildenafil led to sustained reduction of pulmonary artery systolic pressure, improved flow-mediated dilation of the brachial artery, improved peak oxygen uptake exercise ventilation, and improved symptomatic status. No serious adverse events with sildenafil were obvious in either trial. These studies suggest that at a minimum PDE5 inhibition can improve functional status in HF patients. It remains to be determined what its effect on mortality is and how patients with more advanced disease respond.
6. Multivalency Strategies
Taking the individual strategies to augment cGMP systems one step further, one could combine two or more strategies (Figure 1). Little is known about simultaneous activation of sGC and GC-A. As both pathways differ in their tissue and cellular targets, augmenting both may be a beneficial strategy. In canine pacing-induced HF, combination of BNP and the NO- and heme-independent sGC activator BAY 58–2667 resulted in vasodilation, increased cardiac output and renal blood flow, and natriuresis, a profile not achieved by either compound alone (Boerrigter et al. 2007). In the NAPA trial which showed beneficial effects of perioperative nesiritide infusion during cardiac surgery in patients with impaired LV function, more than half the patients in the nesiritide arm also received a nitrovasodilator (Mentzer, et al. 2007). Chen et al reported that in canine pacing-induced HF after chronic PDE5 inhibition (see section 5) (Chen, et al. 2006) the response to BNP was augmented. Interestingly, chronic PDE5 inhibition led to significantly increased plasma cGMP levels despite lower BNP levels, which would be consistent with a “leaking” sGC-dependent cGMP pool.
Currently, medication with nitrates is considered a contraindication for PDE5-inhibition, due to the potential of excessive hypotension; however, it may be worthwhile to formally test the cardiorenal actions of this strategy with correspondingly lower sGC stimulator doses. This may be especially be the case in the cardiorenal syndrome that is characterized in a model of overt HF to be resistant to ANP in association with reduction in GFR and renal blood flow together with increases in glomerular PDE activities (Supaporn et al. 1996).
7. Conclusion and Future Directions
Cyclic GMP is a second messenger crucially involved in important signaling pathways in cardiovascular disease, including heart failure. Potent drugs are now available to augment these signaling systems with conventional sGC stimulators, novel NO-independent sGC stimulators, GC-A and GC-B agonists, and PDE inhibitors. Given some of the already available promising preclinical and clinical data, it is likely that the cGMP systems will remain attractive areas of research and drug development. Indeed, in strategies to treat heart failure, the focus has been on antagonizing endogenous neurohumoral systems. Cyclic GMP research has opened a new direction in heart failure therapeutics by providing us with an exciting way to promote signaling pathways that possesses robust properties of cardiorenal protection warranting further basic and clinical research.
Acknowledgments
This research was supported by grants POI HL076611, HL-36634 (JCB, Jr), and HL07111 (GB) from the National Institute of Health, and by the Mayo and Marriott Foundations.
BIBLIOGRAPHY
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- Bischoff E, Stasch JP. Effects of the sGC stimulator BAY 41-2272 are not mediated by phosphodiesterase 5 inhibition. Circulation. 2004;110:e320–e321. doi: 10.1161/01.CIR.0000142209.28862.12. author reply e320–321. [DOI] [PubMed] [Google Scholar]
- Boerrigter G, Costello-Boerrigter L, Burnett JC. B-Type Natriuretic Peptide 8–32 (BNP 8–32), Which is Produced From Mature BNP 1–32 by the Metalloprotease Meprin A, has Reduced Bioactivity. J Am Coll Cardiol. 2008;51 doi: 10.1152/ajpregu.00059.2009. ABSTRACT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerrigter G, Costello-Boerrigter LC, Cataliotti A, Lapp H, Stasch JP, Burnett JC., Jr Targeting heme-oxidized soluble guanylate cyclase in experimental heart failure. Hypertension. 2007;49:1128–1133. doi: 10.1161/HYPERTENSIONAHA.106.083832. [DOI] [PubMed] [Google Scholar]
- Boerrigter G, Costello-Boerrigter LC, Cataliotti A, Tsuruda T, Harty GJ, Lapp H, et al. Cardiorenal and humoral properties of a novel direct soluble guanylate cyclase stimulator BAY 41–2272 in experimental congestive heart failure. Circulation. 2003;107:686–689. doi: 10.1161/01.cir.0000055737.15443.f8. [DOI] [PubMed] [Google Scholar]
- Boerrigter G, Costello-Boerrigter LC, Harty GJ, Lapp H, Burnett JC., Jr Des-serine-proline brain natriuretic peptide 3–32 in cardiorenal regulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R897–R901. doi: 10.1152/ajpregu.00569.2006. [DOI] [PubMed] [Google Scholar]
- Boerrigter G, Costello-Boerrigter LC, Lapp H, Stasch JP, Burnett JCJ. Beneficial Actions of Co-Targeting Particulate and Soluble Guanylate Cyclase Dependent cGMP Pools in Experimental Heart Failure. Circulation. 2007;116:2494. II-550. [Google Scholar]
- Brandt I, Lambeir AM, Ketelslegers JM, Vanderheyden M, Scharpe S, De Meester I. Dipeptidyl-peptidase IV converts intact B-type natriuretic peptide into its des-SerPro form. Clin Chem. 2006;52:82–87. doi: 10.1373/clinchem.2005.057638. [DOI] [PubMed] [Google Scholar]
- Brunton T. Use of nitrite of amyl in angina pectoris. Lancet. 1867;2:97–98. [Google Scholar]
- Bryan PM, Xu X, Dickey DM, Chen Y, Potter LR. Renal hyporesponsiveness to atrial natriuretic peptide in congestive heart failure results from reduced atrial natriuretic peptide receptor concentrations. Am J Physiol Renal Physiol. 2007;292:F1636–F1644. doi: 10.1152/ajprenal.00418.2006. [DOI] [PubMed] [Google Scholar]
- Burnett JC, Jr, Korinek J. The Tumultous Journey of Nesiritide: Past, Present, and Future. Circ Heart Fail. 2008;1:6–8. doi: 10.1161/CIRCHEARTFAILURE.108.776294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramori PR, Adelman AG, Azevedo ER, Newton GE, Parker AB, Parker JD. Therapy with nitroglycerin increases coronary vasoconstriction in response to acetylcholine. J Am Coll Cardiol. 1998;32:1969–1974. doi: 10.1016/s0735-1097(98)00456-2. [DOI] [PubMed] [Google Scholar]
- Castro LR, Verde I, Cooper DM, Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation. 2006;113:2221–2228. doi: 10.1161/CIRCULATIONAHA.105.599241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataliotti A, Boerrigter G, Costello-Boerrigter LC, Schirger JA, Tsuruda T, Heublein DM, et al. Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide-induced aldosterone activation in experimental heart failure. Circulation. 2004;109:1680–1685. doi: 10.1161/01.CIR.0000124064.00494.21. [DOI] [PubMed] [Google Scholar]
- Cataliotti A, Schirger JA, Martin FL, Chen HH, McKie PM, Boerrigter G, et al. Oral human brain natriuretic peptide activates cyclic guanosine 3′,5′-monophosphate and decreases mean arterial pressure. Circulation. 2005;112:836–840. doi: 10.1161/CIRCULATIONAHA.105.538520. [DOI] [PubMed] [Google Scholar]
- Chen HH, Grantham JA, Schirger JA, Jougasaki M, Redfield MM, Burnett JC., Jr Subcutaneous administration of brain natriuretic peptide in experimental heart failure. J Am Coll Cardiol. 2000;36:1706–1712. doi: 10.1016/s0735-1097(00)00911-6. [DOI] [PubMed] [Google Scholar]
- Chen HH, Huntley BK, Schirger JA, Cataliotti A, Burnett JC., Jr Maximizing the renal cyclic 3′-5′-guanosine monophosphate system with type V phosphodiesterase inhibition and exogenous natriuretic peptide: a novel strategy to improve renal function in experimental overt heart failure. J Am Soc Nephrol. 2006;17:2742–2747. doi: 10.1681/ASN.2006020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Redfield MM, Nordstrom LJ, Horton DP, Burnett JC., Jr Subcutaneous administration of the cardiac hormone BNP in symptomatic human heart failure. J Card Fail. 2004;10:115–119. doi: 10.1016/j.cardfail.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Chen HH, Schirger JA, Cataliotti A, Martin FL, Burnett JC. Intra-Renal Infusion of BNP: A Novel Strategy to Overcome Renal Resistance to BNP in Severe Experimental Heart Failure. J Am Coll Cardiol. 2006;47:73A. [Google Scholar]
- Chen HH, Sundt TM, Cook DJ, Heublein DM, Burnett JC., Jr Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary-bypass surgery: a double-blind placebo-controlled pilot study. Circulation. 2007;116:I134–I138. doi: 10.1161/CIRCULATIONAHA.106.697250. [DOI] [PubMed] [Google Scholar]
- Clavell AL, Stingo AJ, Wei CM, Heublein DM, Burnett JC., Jr C-type natriuretic peptide: a selective cardiovascular peptide. Am J Physiol. 1993;264:R290–R295. doi: 10.1152/ajpregu.1993.264.2.R290. [DOI] [PubMed] [Google Scholar]
- Cleland JG, Coletta AP, Clark AL. Clinical trials update from the American College of Cardiology 2007: ALPHA, EVEREST, FUSION II, VALIDD, PARR-2, REMODEL, SPICE, COURAGE, COACH, REMADHE, pro-BNP for the evaluation of dyspnoea and THIS-diet. Eur J Heart Fail. 2007;9:740–745. doi: 10.1016/j.ejheart.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Cleland JG, Swedberg K. Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. The International Ecadotril Multi-centre Dose-ranging Study Investigators. Lancet. 1998;351:1657–1658. doi: 10.1016/s0140-6736(05)77712-6. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;314:1547–1552. doi: 10.1056/NEJM198606123142404. [DOI] [PubMed] [Google Scholar]
- Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- Dickey DM, Flora DR, Bryan PM, Xu X, Chen Y, Potter LR. Differential regulation of membrane guanylyl cyclases in congestive heart failure: natriuretic peptide receptor (NPR)-B, Not NPR-A, is the predominant natriuretic peptide receptor in the failing heart. Endocrinology. 2007;148:3518–3522. doi: 10.1210/en.2007-0081. [DOI] [PubMed] [Google Scholar]
- Eder E, van Eickels M, Frantz S, Volker K, Baba HMK. Chronic inhibition of cyclic GMP phosphodiesterase 5A may promote pressure overload-induced chamber dilatation in mice. BMC Pharmacology. 2007;7(Suppl. 1):17. [Google Scholar]
- Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, et al. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res. 2006;99:816–828. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- Ghofrani H, Weimann G, Frey R, Voswinckel R, Thamm M, Bolkow D, et al. BAY 63–2521, an oral soluble guanylate cyclase stimulator, has a favourable safety profile, improves cardiopulmonary haemodynamics and has therapeutic potential in pulmonary hypertension. BMC Pharmacology. 2007;7:S8. [Google Scholar]
- Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol. 2007;50:2136–2144. doi: 10.1016/j.jacc.2007.07.078. [DOI] [PubMed] [Google Scholar]
- Hart CY, Hahn EL, Meyer DM, Burnett JC, Jr, Redfield MM. Differential effects of natriuretic peptides and NO on LV function in heart failure and normal dogs. Am J Physiol Heart Circ Physiol. 2001;281:H146–H154. doi: 10.1152/ajpheart.2001.281.1.H146. [DOI] [PubMed] [Google Scholar]
- Heck DE, Kagan VE, Shvedova AA, Laskin JD. An epigrammatic (abridged) recounting of the myriad tales of astonishing deeds and dire consequences pertaining to nitric oxide and reactive oxygen species in mitochondria with an ancillary missive concerning the origins of apoptosis. Toxicology. 2005;208:259–271. doi: 10.1016/j.tox.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, et al. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest. 2003;111:1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T, Tokudome T, Maki T, Yoshihara F, Suga S, Nishikimi T, et al. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology. 2003;144:2279–2284. doi: 10.1210/en.2003-0128. [DOI] [PubMed] [Google Scholar]
- Hunt PJ, Richards AM, Espiner EA, Nicholls MG, Yandle TG. Bioactivity and metabolism of C-type natriuretic peptide in normal man. J Clin Endocrinol Metab. 1994;78:1428–1435. doi: 10.1210/jcem.78.6.8200946. [DOI] [PubMed] [Google Scholar]
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- Huntley BK, Sandberg SM, Noser JA, Cataliotti A, Redfield MM, Matsuda Y, et al. BNP-induced activation of cGMP in human cardiac fibroblasts: interactions with fibronectin and natriuretic peptide receptors. J Cell Physiol. 2006;209:943–949. doi: 10.1002/jcp.20793. [DOI] [PubMed] [Google Scholar]
- Hussain MB, MacAllister RJ, Hobbs AJ. Reciprocal regulation of cGMP-mediated vasorelaxation by soluble and particulate guanylate cyclases. Am J Physiol Heart Circ Physiol. 2001;280:H1151–H1159. doi: 10.1152/ajpheart.2001.280.3.H1151. [DOI] [PubMed] [Google Scholar]
- Igaki T, Itoh H, Suga SI, Hama N, Ogawa Y, Komatsu Y, et al. Effects of intravenously administered C-type natriuretic peptide in humans: comparison with atrial natriuretic peptide. Hypertens Res. 1998;21:7–13. doi: 10.1291/hypres.21.7. [DOI] [PubMed] [Google Scholar]
- Johns DG, Ao Z, Heidrich BJ, Hunsberger GE, Graham T, Payne L, et al. Dendroaspis natriuretic peptide binds to the natriuretic peptide clearance receptor. Biochem Biophys Res Commun. 2007;358:145–149. doi: 10.1016/j.bbrc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- Kasama S, Toyama T, Hatori T, Sumino H, Kumakura H, Takayama Y, et al. Effects of intravenous atrial natriuretic peptide on cardiac sympathetic nerve activity and left ventricular remodeling in patients with first anterior acute myocardial infarction. J Am Coll Cardiol. 2007;49:667–674. doi: 10.1016/j.jacc.2006.09.048. [DOI] [PubMed] [Google Scholar]
- Kenny AJ, Stephenson SL. Role of endopeptidase-24.11 in the inactivation of atrial natriuretic peptide. FEBS Lett. 1988;232:1–8. doi: 10.1016/0014-5793(88)80375-2. [DOI] [PubMed] [Google Scholar]
- Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- La Villa G, Mannelli M, Lazzeri C, Vecchiarino S, de Feo ML, Tosti Guerra C, et al. Different effects of atrial and C-type natriuretic peptide on the urinary excretion of endothelin-1 in man. Clin Sci (Lond) 1998;95:595–602. doi: 10.1042/cs0950595. [DOI] [PubMed] [Google Scholar]
- Lainchbury JG, Burnett JC, Jr, Meyer D, Redfield MM. Effects of natriuretic peptides on load and myocardial function in normal and heart failure dogs. Am J Physiol Heart Circ Physiol. 2000;278:H33–H40. doi: 10.1152/ajpheart.2000.278.1.H33. [DOI] [PubMed] [Google Scholar]
- Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- Liang KV, Williams AW, Greene EL, Redfield MM. Acute decompensated heart failure and the cardiorenal syndrome. Crit Care Med. 2008;36:S75–S88. doi: 10.1097/01.CCM.0000296270.41256.5C. [DOI] [PubMed] [Google Scholar]
- Lisy O, Jougasaki M, Heublein DM, Schirger JA, Chen HH, Wennberg PW, et al. Renal actions of synthetic dendroaspis natriuretic peptide. Kidney Int. 1999;56:502–508. doi: 10.1046/j.1523-1755.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- Lisy O, Lainchbury JG, Leskinen H, Burnett JC., Jr Therapeutic actions of a new synthetic vasoactive and natriuretic peptide, dendroaspis natriuretic peptide, in experimental severe congestive heart failure. Hypertension. 2001;37:1089–1094. doi: 10.1161/01.hyp.37.4.1089. [DOI] [PubMed] [Google Scholar]
- Madhani M, Okorie M, Hobbs AJ, MacAllister RJ. Reciprocal regulation of human soluble and particulate guanylate cyclases in vivo. Br J Pharmacol. 2006;149:797–801. doi: 10.1038/sj.bjp.0706920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FL, Supaporn T, Chen HH, Sandberg SM, Matsuda Y, Jougasaki M, et al. Distinct roles for renal particulate and soluble guanylyl cyclases in preserving renal function in experimental acute heart failure. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1580–R1585. doi: 10.1152/ajpregu.00284.2007. [DOI] [PubMed] [Google Scholar]
- Mentzer RM, Jr, Oz MC, Sladen RN, Graeve AH, Hebeler RF, Jr, Luber JM, Jr, et al. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery:the NAPA Trial. J Am Coll Cardiol. 2007;49:716–726. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Miller LN, Nakane M, Hsieh GC, Chang R, Kolasa T, Moreland RB, et al. A-350619: a novel activator of soluble guanylyl cyclase. Life Sci. 2003;72:1015–1025. doi: 10.1016/s0024-3205(02)02361-5. [DOI] [PubMed] [Google Scholar]
- Mitrovic V, Seferovic PM, Simeunovic D, Ristic AD, Miric M, Moiseyev VS, et al. Haemodynamic and clinical effects of ularitide in decompensated heart failure. Eur Heart J. 2006;27:2823–2832. doi: 10.1093/eurheartj/ehl337. [DOI] [PubMed] [Google Scholar]
- Mullershausen F, Russwurm M, Friebe A, Koesling D. Inhibition of phosphodiesterase type 5 by the activator of nitric oxide-sensitive guanylyl cyclase BAY 41–2272. Circulation. 2004;109:1711–1713. doi: 10.1161/01.CIR.0000126286.47618.BD. [DOI] [PubMed] [Google Scholar]
- Munzel T, Genth-Zotz S, Hink U. Targeting heme-oxidized soluble guanylate cyclase: solution for all cardiorenal problems in heart failure? Hypertension. 2007;49:974–976. doi: 10.1161/HYPERTENSIONAHA.106.085456. [DOI] [PubMed] [Google Scholar]
- Munzel T, Sayegh H, Freeman BA, Tarpey MM, Harrison DG. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J Clin Invest. 1995;95:187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northridge DB, Currie PF, Newby DE, McMurray JJ, Ford M, Boon NA, et al. Placebo-controlled comparison of candoxatril, an orally active neutral endopeptidase inhibitor, and captopril in patients with chronic heart failure. Eur J Heart Fail. 1999;1:67–72. doi: 10.1016/S1388-9842(98)00003-8. [DOI] [PubMed] [Google Scholar]
- Owan TE, Chen HH, Frantz RP, Karon BL, Miller WL, Rodeheffer RJ, et al. The effects of nesiritide on renal function and diuretic responsiveness in acutely decompensated heart failure patients with renal dysfunction. J Card Fail. 2008;14:267–275. doi: 10.1016/j.cardfail.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) Circulation. 2002;106:920–926. doi: 10.1161/01.cir.0000029801.86489.50. [DOI] [PubMed] [Google Scholar]
- Pankow K, Wang Y, Gembardt F, Krause E, Sun X, Krause G, et al. Successive action of meprin A and neprilysin catabolizes B-type natriuretic peptide. Circ Res. 2007;101:875–882. doi: 10.1161/CIRCRESAHA.107.153585. [DOI] [PubMed] [Google Scholar]
- Piggott LA, Hassell KA, Berkova Z, Morris AP, Silberbach M, Rich TC. Natriuretic peptides and nitric oxide stimulate cGMP synthesis in different cellular compartments. J Gen Physiol. 2006;128:3–14. doi: 10.1085/jgp.200509403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Rouleau JL, Pfeffer MA, Stewart DJ, Isaac D, Sestier F, Kerut EK, et al. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet. 2000;356:615–620. doi: 10.1016/s0140-6736(00)02602-7. [DOI] [PubMed] [Google Scholar]
- Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- Salloum FN, Abbate A, Das A, Houser JE, Mudrick CA, Qureshi IZ, et al. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol. 2008;294:H1398–H1406. doi: 10.1152/ajpheart.91438.2007. [DOI] [PubMed] [Google Scholar]
- Schindler U, Strobel H, Schonafinger K, Linz W, Lohn M, Martorana PA, et al. Biochemistry and pharmacology of novel anthranilic acid derivatives activating heme-oxidized soluble guanylyl cyclase. Mol Pharmacol. 2006;69:1260–1268. doi: 10.1124/mol.105.018747. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Schramm M, Schroder H, Stasch JP. Mechanisms of nitric oxide independent activation of soluble guanylyl cyclase. Eur J Pharmacol. 2003;468:167–174. doi: 10.1016/s0014-2999(03)01674-1. [DOI] [PubMed] [Google Scholar]
- Schmidt PM, Schramm M, Schroder H, Wunder F, Stasch JP. Identification of residues crucially involved in the binding of the heme moiety of soluble guanylate cyclase. J Biol Chem. 2004;279:3025–3032. doi: 10.1074/jbc.M310141200. [DOI] [PubMed] [Google Scholar]
- Schweitz H, Vigne P, Moinier D, Frelin C, Lazdunski M. A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps) J Biol Chem. 1992;267:13928–13932. [PubMed] [Google Scholar]
- Selwood DL, Brummell DG, Budworth J, Burtin GE, Campbell RO, Chana SS, et al. Synthesis and biological evaluation of novel pyrazoles and indazoles as activators of the nitric oxide receptor, soluble guanylate cyclase. J Med Chem. 2001;44:78–93. doi: 10.1021/jm001034k. [DOI] [PubMed] [Google Scholar]
- Singh G, Maguire JJ, Kuc RE, Skepper JN, Fidock M, Davenport AP. Characterization of the snake venom ligand [125I]-DNP binding to natriuretic peptide receptor-A in human artery and potent DNP mediated vasodilatation. Br J Pharmacol. 2006;149:838–844. doi: 10.1038/sj.bjp.0706924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeki T, Kishimoto I, Okumura H, Tokudome T, Horio T, Mori K, et al. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J Am Coll Cardiol. 2005;45:608–616. doi: 10.1016/j.jacc.2004.10.067. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Skali H, Bourgoun M, Fang J, Ghali JK, Martelet M, et al. Effect of angiotensin-converting enzyme or vasopeptidase inhibition on ventricular size and function in patients with heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) echocardiographic study. Am Heart J. 2005;150:257–262. doi: 10.1016/j.ahj.2004.09.056. [DOI] [PubMed] [Google Scholar]
- Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, et al. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol. 2002;136:773–783. doi: 10.1038/sj.bjp.0704778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, H SA, Meurer S, et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest. 2006;116:2552–2561. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingo AJ, Clavell AL, Aarhus LL, Burnett JC., Jr Cardiovascular and renal actions of C-type natriuretic peptide. Am J Physiol. 1992;262:H308–H312. doi: 10.1152/ajpheart.1992.262.1.H308. [DOI] [PubMed] [Google Scholar]
- Supaporn T, Sandberg SM, Borgeson DD, Heublein DM, Luchner A, Wei CM, et al. Blunted cGMP response to agonists and enhanced glomerular cyclic 3′,5′-nucleotide phosphodiesterase activities in experimental congestive heart failure. Kidney Int. 1996;50:1718–1725. doi: 10.1038/ki.1996.491. [DOI] [PubMed] [Google Scholar]
- Suwa M, Seino Y, Nomachi Y, Matsuki S, Funahashi K. Multicenter prospective investigation on efficacy and safety of carperitide for acute heart failure in the 'real world' of therapy. Circ J. 2005;69:283–290. doi: 10.1253/circj.69.283. [DOI] [PubMed] [Google Scholar]
- Sydow K, Daiber A, Oelze M, Chen Z, August M, Wendt M, et al. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J Clin Invest. 2004;113:482–489. doi: 10.1172/JCI19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto E, Belardi D, Tocchetti CG, Vahebi S, Cormaci G, Ketner EA, et al. Compartmentalization of cardiac beta-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation. 2007;115:2159–2167. doi: 10.1161/CIRCULATIONAHA.106.643536. [DOI] [PubMed] [Google Scholar]
- Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci U S A. 2000;97:4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawaragi Y, Fuchimura K, Tanaka S, Minamino N, Kangawa K, Matsuo H. Gene and precursor structures of human C-type natriuretic peptide. Biochem Biophys Res Commun. 1991;175:645–651. doi: 10.1016/0006-291x(91)91614-i. [DOI] [PubMed] [Google Scholar]
- Taylor AL, Ziesche S, Yancy C, Carson P, D'Agostino R, Jr, Ferdinand K, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- Thomas GR, DiFabio JM, Gori T, Parker JD. Once daily therapy with isosorbide-5-mononitrate causes endothelial dysfunction in humans: evidence of a free-radical-mediated mechanism. J Am Coll Cardiol. 2007;49:1289–1295. doi: 10.1016/j.jacc.2006.10.074. [DOI] [PubMed] [Google Scholar]
- Tsuruda T, Boerrigter G, Huntley BK, Noser JA, Cataliotti A, Costello-Boerrigter LC, et al. Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res. 2002;91:1127–1134. doi: 10.1161/01.res.0000046234.73401.70. [DOI] [PubMed] [Google Scholar]
- Villar IC, Panayiotou CM, Sheraz A, Madhani M, Scotland RS, Nobles M, et al. Definitive role for natriuretic peptide receptor-C in mediating the vasorelaxant activity of C-type natriuretic peptide and endothelium-derived hyperpolarising factor. Cardiovasc Res. 2007;74:515–525. doi: 10.1016/j.cardiores.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VMAC-Investigators. Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- Wang DJ, Dowling TC, Meadows D, Ayala T, Marshall J, Minshall S, et al. Nesiritide does not improve renal function in patients with chronic heart failure and worsening serum creatinine. Circulation. 2004;110:1620–1625. doi: 10.1161/01.CIR.0000141829.04031.25. [DOI] [PubMed] [Google Scholar]
- Warnholtz A, Mollnau H, Heitzer T, Kontush A, Moller-Bertram T, Lavall D, et al. Adverse effects of nitroglycerin treatment on endothelial function, vascular nitrotyrosine levels and cGMP-dependent protein kinase activity in hyperlipidemic Watanabe rabbits. J Am Coll Cardiol. 2002;40:1356–1363. doi: 10.1016/s0735-1097(02)02133-2. [DOI] [PubMed] [Google Scholar]
- Wei CM, Aarhus LL, Miller VM, Burnett JC., Jr Action of C-type natriuretic peptide in isolated canine arteries and veins. Am J Physiol. 1993;264:H71–H73. doi: 10.1152/ajpheart.1993.264.1.H71. [DOI] [PubMed] [Google Scholar]
- Witteles RM, Kao D, Christopherson D, Matsuda K, Vagelos RH, Schreiber D, et al. Impact of nesiritide on renal function in patients with acute decompensated heart failure and pre-existing renal dysfunction a randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol. 2007;50:1835–1840. doi: 10.1016/j.jacc.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Yasue H, Morita E, Sakaino N, Jougasaki M, Kurose M, et al. Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure. Circulation. 1991;84:1581–1588. doi: 10.1161/01.cir.84.4.1581. [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Movsesian MA. cAMP and cGMP signaling cross-talk: role of phosphodiesterases and implications for cardiac pathophysiology. Circ Res. 2007;100:1569–1578. doi: 10.1161/CIRCRESAHA.106.144501. [DOI] [PubMed] [Google Scholar]