Abstract
Objective
To examine the hypothesis that speckle myocardial imaging (SMI) modalities, including longitudinal, radial and circumferential systolic (s) and diastolic (d) myocardial velocity imaging, displacement (D), strain rate (SR) and strain (S), as well as left ventricular (LV) rotation/torsion are sensitive for detecting early myocardial dysfunction in isolated LV non-compaction (iLVNC).
Design and results
Twenty patients with iLVNC diagnosed by cardiac magnetic resonance (15) or echocardiography (5) were included. Patients were divided into two groups: ejection fraction (EF)>50% (n=10) and EF≤50% (n=10). Standard measures of systolic and diastolic function including pulsed wave tissue Doppler Imaging (PWTDI) were obtained. Longitudinal, radial and circumferential SMI, and LV rotation/torsion were compared with values for 20 age/sex-matched controls. EF, PWTDI E′, E/E′ and all of the SMI modalities were significantly abnormal for patients with EF≤50% compared with controls. In contrast, EF and PWTDI E′, E/E′ were not significantly different between controls and patients with iLVNC (EF>50%). However, SMI-derived longitudinal sS, sSR, sD and radial sS, as well as LV rotation/torsion values, were all reduced in iLVNC (EF>50%) compared with controls. Measurements with the highest discriminating power between iLVNC (EF>50%) and controls were longitudinal sS mean of the six apical segments (area under the curve (AUC)=0.94), sS global average (AUC=0.94), LV rotation apical mean (AUC=0.94); LV torsion (AUC=0.93) LV torsion rate (AUC=0.94).
Conclusions
LV SMI values are reduced in patients with iLVNC, even those with normal EF and PWTDI. The most accurate SMI modalities to discriminate between patients and controls are longitudinal sS mean of the six apical segments, LV apical rotation or LV torsion rate.
INTRODUCTION
Isolated left ventricular non-compaction (iLVNC) is a rare congenital cardiomyopathy resulting from arrest in normal endomyocardial embryogenesis and consisting of multiple trabeculations and deep intertrabecular recesses of the myocardium.1 Although iLVNC is included in the 2006 WHO classification of cardiomyopathies, its aetiology, pathogenesis, diagnosis and management have to be further defined.
The purpose of this study was twofold: (a) to determine the potential role of speckle myocardial imaging (SMI) for identifying LV dysfunction in patients with iLVNC who had no evidence on two-dimensional (2-D) imaging or standard Doppler imaging of cardiac impairment, compared with age and sex comparable controls; (b) to establish which of the SMI modalities is the most accurate for detection of early LV dysfunction in patients with iLVNC.
METHODS
Study population
This study was approved by the Institutional Review Board of the Mayo Clinic. Twenty consecutive patients with a confirmed diagnosis of iLVNC were enrolled and compared with 20 age- and sex-matched subjects, free of cardiovascular disease, who served as controls. Subjects with iLVNC were prospectively selected from patients undergoing evaluation in the cardiovascular division at the Mayo Clinic, Rochester Minnesota, from July 2006 through April 2009. The diagnosis of iLVNC was made by cardiac magnetic resonance (CMR) imaging (n = 15) or by standard echocardiography (n = 5). CMR diagnostic criteria defined LV non-compaction as myocardium with more than three trabeculations in one imaging plane, apically from the insertion of the papillary muscles, with a non-compacted to compacted diastolic ratio of ≥2.3:1.2 Segmental analysis was evaluated using a standard 17-segment cardiac model as defined by the American Heart Association/American College of Cardiology for standardised myocardial segmentation. The severity of LV non-compaction was taken to indicate the ratio of non-compacted to compacted myocardium for a given myocardial segment.
The diagnosis of iLVNC by transthoracic echocardiography was made in accordance with suggested criteria.1 Concurrence of two independent experienced echocardiographers (CJB and HMC) was required to confirm the diagnosis in the five patients diagnosed in this way. Echocardiographic diagnostic criteria included:
▶ Hypertrabeculation of the left ventricle with an apical X-to-Y ratio of 0.33, where X represents the distance between the epicardial surface and the trough of the recess and Y represents the distance between the epicardial surface and the peak of the trabeculations. To measure the magnitude of the myocardial non-compaction, X-to-Y ratios were measured at the level of the apex, papillary muscles and mitral valve.
▶ Colour Doppler evidence that the deep intertrabecular recesses were in communication with the ventricular cavity.
Exclusion criteria included age <18 years, history of moderate or greater systemic or pulmonary hypertension, significant valvular heart disease, clinical or electrocardiographic evidence of ischaemic heart disease and evidence of concomitant complex congenital heart disease.
Controls (group I) were selected from patients undergoing clinically indicated echocardiography who had no cardiac symptoms and no history of hypertension or cardiac disease. They were referred to the echocardiography laboratory for atypical chest pain (n = 6), low-intensity systolic murmur at physical examination (n = 4), or to define risk of imminent non-cardiac surgery (n = 10).
Patients with iLVNC were divided in two groups based on the ejection fraction (EF): patients with an EF >50% (n = 10) were labelled as group II; patients with EF ≤50% (n = 10) were labelled as group III.
Standard echocardiography
All ultrasound examinations were performed with a commercially available echocardiographic instrument (Vivid 7; GE Healthcare, Milwaukee, Wisconsin, USA). The thickness of the ventricular septum and LV posterior wall, the end-systolic and end-diastolic LV diameters were determined from M-mode or 2-D imaging, and LV mass and EF were calculated as previously described.3 Pulsed wave Doppler imaging of mitral inflow and LV outflow was performed as previously described.3 Pulsed wave tissue Doppler imaging (PWTDI) was measured placing the sample volume on the medial mitral annulus in the apical four-chamber view. Offline analysis of standard echocardiographic variables was performed with the use of dedicated software (ProSolv CV analyser V.3.0); three consecutive beats were measured and averaged for each measurement.
Speckle myocardial imaging
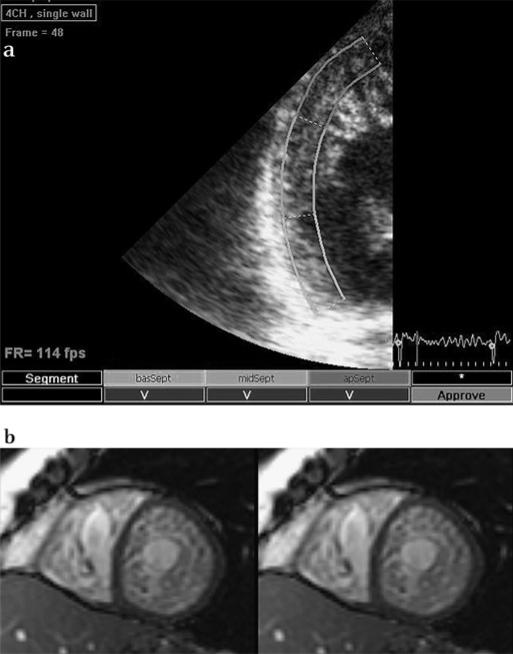
For SMI, apical four-chamber, long-axis and two-chamber views were acquired, as well as a parasternal short-axis view at the basal, mid- and apical LV level, as previously described (figure 1a).4 In addition, narrow sector views were acquired for each LV wall from apical views. 2-D recordings were collected with a frame rate ranging from 60 (full apical views) to 160 (narrow sector views) frames/s during brief breath hold. Three consecutive cardiac cycles were recorded as 2-D cine loops and the acquired raw data were saved on magneto optical disks or DVDs for offline analysis.
Figure 1.
a) Speckle myocardial imaging: speckle tracking of the lateral wall (apical four-chamber, narrow view) in a patient with overt isolated left ventricular non-compaction. The image was analysed offline with the help of a specific software (Echopac BTW09, GE Vingmend Ultrasound Medical Systems); tracking quality for each segment is reported at the bottom of the figure. (b) Cardiac magnetic resonance: short-axis cardiovascular magnetic resonance of a steady state free precession image illustrating severe myocardial trabeculation with deep intertrabecular recesses. Biventricular myocardial involvement with features consistent with non-compaction can be appreciated.
Longitudinal systolic peak values were determined for myocardial velocity (sMV), displacement (sD) strain rate (sSR) and strain (sS). Longitudinal early diastolic peak values were determined for myocardial velocity (dMV-E) and strain rate (dSR-E). Radial and circumferential systolic and early diastolic peak values were measured for sSR, dSR-E and sS. For longitudinal SMI, analysis was performed considering a model of 18 LV segments individually and also combining them in clusters according to two different criteria. The first criterion was determined by LV level (basal, middle and apical). The SMI values (sMV, dMV-E, sD, sSR, dSR-E, and sS) were averaged for the six basal segments (basal mean), for the six middle segments (middle mean) and for the six apical segments (apical mean). The second criterion was determined by LV wall. The SMI values were averaged for the anterolateral, inferoseptal, posterior, anteroseptal, inferior and anterior walls.
The averaged LV rotation and rotational velocity profile from six segments of short-axis views at the basal and apical levels were used for the calculation of LV torsion as previously described.5 After procurements of LV rotation at the two short-axis levels, LV torsion was calculated as the net difference between LV rotation angles obtained from basal (clockwise) and apical (counterclockwise) short-axis planes at the same time point—that is, LV torsion (degree) = (apical LV rotation – basal LV rotation).
In the current version of the software used for the measurement of SMI values (Echopac BT09, GE Vingmend Ultrasound Medical Systems, Milwaukee, Wisconsin, USA), the tracking quality for each segment was automatically evaluated and summarised in the tracking table as V (valid tracking) and X (non-acceptable tracking).
Cardiac magnetic resonance
ECG-gated CMR was performed on a 1.5 T system (Twin speed EXCITE, GE Healthcare, Waukesha, Wisconsin, USA, figure 1b) and consisted of steady-state free precession (SSFP) cine imaging using an eight-element, phased-array, cardiac coil for signal reception. Left ventricular function was obtained with cine images using an SSFP technique (TR/TE, 3.5/1.4; matrix, 192 × 192; field of view, 34×34 cm; slice thickness, 8 mm) obtained in two-chamber, four-chamber and short-axis planes.
Figure 2.
Comparison of longitudinal systolic strain (sS) of the left ventricle at the basal, mid and apical level, for each group. p Values were calculated using repeated-measures analysis of variance and denote differences across three segments from the base, mid and apex, and differences as a result of the interaction of group and level. *p<0.001 comparing the base, mid and apex within the same group.
Assessment of the CMRs was performed by one observer (MM) by offline measurements of the compacted and non-compacted myocardium in each segment of the 17-segment model.
Follow-up
At the end of the follow-up period (31 October 2009) all medical records were reviewed. In addition, study patients were contacted by phone to inquire about clinical status or echocardiographic evaluations not performed at our institution. In particular, information on New York Heart Association (NYHA) class, EF and estimated LV filling pressures (as E/E' ratio) was collected if present. The vital status of each patient was confirmed by a contacted relative, review of medical records, or query of the Social Security Death Index. Owing to limited follow-up time, no formal statistical inference was performed in order to avoid type II errors in the analysis.
Statistical analysis
Statistical analyses were performed with a commercially available software program (STATA V.10.1; STATA Institute, Cary, Texas, USA). Pairwise comparisons between groups were made by Wilcoxon rank sum test, Fisher exact test or exact inference for ordered contingency tables. For measurements of the longitudinal sS at the basal, middle, and apical segments, considering each group separately, a repeated-measures analysis of variance (ANOVA) containing group and segment effects was carried out and the group-segment interaction was fitted. Differences across the three segments within each group were compared using ANOVA for repeated measures. A p value <0.05 for repeated-measures ANOVA was considered statistically significant.
Diagnostic accuracy of the SMI modalities (longitudinal, radial, circumferential and rotation/torsion), including global mean values, mean value for clusters and measurements for individual LV segments, was compared by the receiver operating characteristics (ROC) curves for patients with iLVNC and EF >50% vs controls. Formal comparisons of the areas under the ROC curves (AUC) were performed as well, using the method of Delong and Delong6 for comparing AUC between two tests performed on the same subjects.
Intraobserver and interobserver variability
To examine intraobserver variability (repeatability), a sample of 10 echocardiographic examinations was randomly selected for masked review by the same investigator. To examine interobserver variability a co-investigator (GV) blinded to the clinical information and to the results of the first investigator examined 10 randomly selected echocardiographic examinations. Intraclass correlation coefficients (ICCs) for the same observer and different observers were calculated using previously described formulae7 for single segments and for the global mean of each Doppler myocardial imaging modality. Data are expressed as mean value±SD, or count (percentage). A difference was considered statistically significant when the p value was <0.05.
RESULTS
Demographic standard echocardiography and CMR data
Twenty patients comprised the control group (group I), 10 patients had iLVNC with EF >50% (group II) and 10 patients had iLVNC with EF ≤50% (group III), with mean±SD EF of 64±4, 58±4 and 34±14%, respectively (p<0.001 between group III compared with I and II, table 1).
Table 1.
Clinical, ECG, standard echocardiographic and cardiac magnetic resonance variables for patients (groups II and III) and controls (group I)
| Variable (mean±SD) |
Controls Group I (n=20) |
Non-compaction Ejection fraction > 50% Group II (n=10) |
Non-compaction Ejection fraction ≤ 50% Group III (n=10) |
|---|---|---|---|
| Age (years) | 41±15 | 35±13 | 49±13 |
| Men (%) | 13 (65) | 7 (70) | 4 (40) |
| NYHA III/IV (%) | 0 (0) | 0 (0) | 3 (30)*,† |
| Heart rate (bpm) | 70±13 | 63±9 | 63±11 |
| Atrial fibrillation (%) | 0 (0) | 0 (0) | 1 (10) |
| PM/ICD (%) | 0 (0) | 2 (20) | 5 (50)*,† |
| Ratio of non-compacted to compacted | N/A | 2.39±0.6 | 2.40±0.5 |
| Left ventricular wall thickness (mm) | 10±1.2 | 10±1.3 | 10±1.5 |
| Left ventricular mass index (g/m2) | 82±17 | 108±55 | 156±48*,‡ |
| LVEDD (mm) | 47.1±4.9 | 46.1±3.6 | 57.4±8.9§,¶ |
| LVESD (mm) | 28.3±5.2 | 30.1±3.6 | 45.1±12.1* ¶ |
| E-wave velocity (m/s) | 0.8±0.2 | 0.8±0.3 | 0.6±0.2§ |
| E-wave deceleration time (ms) | 193.5±36.2 | 189.1±36.2 | 209±44.5 |
| A-wave velocity (m/s) | 0.6±0.1 | 0.5±0.1 | 0.6±0.4 |
| E/A | 1.5±0.4 | 1.8±0.4 | 1.3±0.6 |
| Ejection fraction (%) | 64±4.3 | 58±4.4 | 34±13.7*,† |
| Stroke volume (ml) | 93±26 | 84±8 | 78±17 |
| Cardiac index (l/m2/min) | 3.4±0.7 | 2.8±0.3 | 2.6±0.5§ |
| Medial mitral annulus S’ velocity (m/s) | 0.08±0.01 | 0.09±0.04 | 0.07±0.04 |
| Medial mitral annulus E’ velocity (m/s) | 0.08±0.02 | 0.10±0.03 | 0.06±0.03§,‡ |
| Medial mitral annulus A’ velocity (m/s) | 0.08±0.01 | 0.07±0.03 | 0.07±0.02** |
| E/E’ | 8.4±2.2 | 9.1±4.7 | 13.8±7.5** |
Descriptions are with mean±SD or count (percentage). Comparisons are made using the pairwise Wilcoxon rank sum test, or Fisher exact test.
p<0.001 vs controls.
p<0.001 vs non-compaction. EF >50%.
p<0.05 vs non-compacted EF>50%.
p<0.01 vs controls.
p<0.01 vs non-compaction. EF >50%.
p<0.05 vs controls.
EF, ejection fraction; LVEDD, left ventricle end-diastolic diameter; LVESD, left ventricle end-systolic diameter; NYHA, New York Heart Association; PM/ICD, pacemaker/intracardiac device.
Age and gender distribution were not different between patients and controls (table 1). Among patients, there were two familial iLVNC pairs (father–son and brother–sister, total four patients) evaluated, all of whom had a family history of sudden cardiac death. Two patients with iLVNC had a family history of dilated cardiomyopathy. Among patients with EF ≤50% (group III), three subjects were in functional class III/IV, whereas no patients in groups I and II were symptomatic. All patients with decreased EF were receiving appropriate medical treatment including ACE inhibitors and β blockers. Six patients had undergone prophylactic implantation of an automatic internal cardioverter-defibrillator, four in group III and two in group II, with no reported therapeutic shocks. One patient from group III died of complications related to primary pulmonary fibrosis. Although LV wall thickness was not different among the three groups, LV mass index and LV end-systolic and end-diastolic diameters were all increased in group III compared with group II and controls and not different between group II and controls (table 1).
Although E-wave velocity was reduced in group III compared with the other groups, all other measures of trans-mitral flow (Awave and E/A) were not different among the groups. PWTDI E’ and S’ velocities were lower and E/E’ were higher in group III patients than in the other groups, and not different between groups II and group l (table 1).
Cardiac magnetic resonance
The presence of hypertrabeculation was assessed for each of 17 LV segments, and involved some middle LV segments and all apical segments, with basal segments spared. For the 15 patients referred for CMR, the ratio of compacted to non-compacted segments was not different between groups II and III. Anterior and lateral walls were the most involved (considering all patients together, these walls had 17 and 23 non-compacted segments, respectively).
Longitudinal SMI
Data on longitudinal systolic and diastolic SMI measures are reported in tables 2 and 3, respectively. Longitudinal sMV measures were abnormally reduced in group III patients compared with the other groups; the clusters of LV segments by wall or by level, as well as the global mean of all 18 segments, were not different between group II and group I. Longitudinal sD measures, as well as sSR and sS values, including most of the clusters of segments and the global mean of 18 LV segments, were reduced in group III compared with both groups; they were also significantly reduced in group II compared with group I.
Table 2.
Longitudinal systolic speckle myocardial imaging modalities and measures for patients (groups II and III) and controls (group I)
| Variable (mean±SD) |
Controls Group I (n=20) |
Non-compaction Ejection fraction >50% Group II (n=10) |
Non-compaction Ejection fraction ≥50% Group III (n=10) |
|---|---|---|---|
| Tissue velocity imaging (cm/s) | |||
| Basal mean | 6.8±1.1 | 6.2±0.9 | 4.8±1.6*,† |
| Middle mean | 5.0±1.1 | 4.6±0.9 | 3.8±1.3‡ |
| Apex mean | 3.1±0.9 | 2.8±0.4 | 2.6±0.8 |
| Lateral | 5.5±1.8 | 4.9±1.4 | 4.0±1.6‡ |
| Inferoseptum | 4.6±1 | 4.3±0.9 | 3.4±1.2* |
| Posterior | 6.2±1.2 | 5.5±0.8 | 3.9±1.7*,† |
| Anteroseptum | 3.7±1.2 | 3.6±1.2 | 3.2±0.9 |
| Inferior | 5.2±1.3 | 4.8±0.6 | 3.8±1.6‡ |
| Anterior | 4.5±1.4 | 4.2±1.1 | 4.0±1.7 |
| Global average | 5.0±0.9 | 4.5±0.6 | 3.7±1.2*,† |
| Displacement (mm) | |||
| Basal mean | 15.7±1.4 | 13±2.1§ | 9.5±3.6§,† |
| Middle mean | 10.7±1.1 | 8.6±1.5§ | 6.5±2.3§,† |
| Apex mean | 5.3±1.0 | 4.3±1.0 | 3.4±1.2§ |
| Lateral | 11.0±3.0 | 8.0±1.9 | 6.0±1.4§,† |
| Inferoseptum | 10.6±2.2 | 10.0±3.1 | 7.2±2.9§,† |
| Posterior | 11.6±1.9 | 9.8±1.3* | 6.1±3.3§,† |
| Anteroseptum | 9.2±2.4 | 7.4±2.2 | 5.9±2.3* |
| Inferior | 11.3±2.1 | 9.4±1.8* | 7.1±3.8§ |
| Anterior | 9.6±1.8 | 7.2±2.2§ | 5.9±2.7* |
| Global average | 10.6±1.0 | 8.6±1.4§ | 6.5±2.3§,† |
| Strain rate imaging (1/s) | |||
| Basal mean | −1.4±0.2 | −1.3±0.2 | −1.060.3§,† |
| Middle mean | −1.3±0.1 | −1.1±0.2‡ | −0.960.3§ |
| Apex mean | −1.4±0.2 | −1.1±0.2§ | −1.0±0.3§ |
| Lateral | −1.3±0.2 | −1.2±0.3 | −1.0±0.4 |
| Inferoseptum | −1.2±0.2 | −1.0±0.2§ | −0.9±0.2§ |
| Posterior | −1.4±0.2 | −1.2±0.3‡ | −1.0±0.2§ |
| Anteroseptum | −1.3±0.3 | −1.2±0.3 | −1.0±0.4* |
| Inferior | −1.4±0.2 | −1.2±0.3 | −1.0±0.4§ |
| Anterior | −1.4±0.3 | −1.2±0.3‡ | −1.0±0.2§ |
| Global average | −1.3±0.2 | −1.2±0.2‡ | −1.0±0.3§ |
| Strain imaging (%) | |||
| Basal mean | −21.4±1.8 | −18.0±3.4* | −14.1±4.7§,† |
| Middle mean | −21.8±1.7 | −17.4±3.6§ | −13.7±4.9§,† |
| Apex mean | −22.6±2.2 | −17.0±3.7§ | −13.6±4.1§,† |
| Lateral | −21.1±2.5 | −16.0±5.0* | −15±5.5.0§ |
| Inferoseptum | −22±2.3 | −17.5±2.9§ | −12.7±4.0§,¶ |
| Posterior | −21.8±2.2 | −17.8±5.2‡ | −14.7±4.9§ |
| Anteroseptum | −21.4±3 | −18.3±2.7‡ | −13.5±7.0§,+ |
| Inferior | −22.8±3.4 | −18.8±5.6‡ | −14.1±5.8§ |
| Anterior | −21.9±2.8 | −16.2±3.4§ | −12.6±3.6§ |
| Global average | −21.9±1.7 | −17.4±3.3§ | −13.8±4.5§,† |
Single-segment measures have been summarised in clusters by level and by wall, and the global mean of 18 left ventricular segments is reported as well. Comparisons are made using the pairwise Wilcoxon rank sum test.
p<0.01 vs controls.
p<0.05 vs non-compaction. EF >50%.
p<0.05 vs controls.
p<0.001 vs controls.
p<0.01 vs non-compaction. EF >50%.
Table 3.
Longitudinal diastolic speckle myocardial imaging modalities (dMV-E and dSR-E) and measures for patients (groups II and III) and controls (group I).
| Variable (mean±SD) |
Controls Group I (n=20) |
Non-compaction Ejection fraction >50% Group II (n=10) |
Non-compaction Ejection fraction <50% Group III (n=10) |
|---|---|---|---|
| Tissue velocity (cm/s) | |||
| Basal meanE | −9.0±1.9 | −7.5±2.1 | −4.6±2.2*,† |
| Middle meanE | −6.9±2.3 | −5.2±1.7‡ | −3.5±1.2*,† |
| Apex meanE | −4.4±1.5 | −3.3±1.0‡ | −2.5±0.9*,† |
| Lateral E | −7.3±2.1 | −5.5±2.4 | −3.9±1.8* |
| Inferoseptum E | −6.2±1.9 | −5±1.6 | −3.4±1.3* |
| Posterior E | −8.6±2.5 | −6.7±2.4 | −3.7±1.7*,§ |
| Anteroseptum E | −5.8±1.7 | −4.2±1.2‡ | −3.1±1.0*,† |
| Inferior E | −6.7±2.0 | −5.9±1.7 | −3.4±1.7*,† |
| Anterior E | −6.3±2.2 | −4.6±1.6‡ | −3.6±1.7* |
| Global average E | −6.8±1.8 | −5.3±1.5 | −3.5±1.4*,† |
| Strain rate (cm/s) | |||
| Basal meanE | 2.0±0.4 | 1.9±0.5 | 1.3±0.5*,† |
| Middle meanE | 1.7±0.3 | 1.4±0.4 | 1.0±0.4*,† |
| Apex meanE | 1.9±0.4 | 1.6±0.4‡ | 1.1±0.4*,† |
| Lateral E | 1.9±0.5 | 1.6±0.4 | 1.5±0.5‡ |
| Inferoseptum E | 1.8±0.4 | 1.7±0.3 | 1.0±0.4*,† |
| Posterior E | 1.8±0.3 | 1.7±0.6 | 1.4±0.5‡ |
| Anteroseptum E | 1.8±0.4 | 1.6±0.3 | 1.0±0.6*,† |
| Inferior E | 1.9±0.4 | 1.8±0.8 | 1.0±0.5*,† |
| Anterior E | 2.0±0.5 | 1.5±0.6 | 1.0±0.4* |
| Global average E | 1.9±0.3 | 1.6±0.4 | 1.1±0.4*,† |
Single-segment measures have been summarised in clusters by level and by wall, and the global mean of 18 left ventricular segments is reported as well. Comparisons are made using the pairwise Wilcoxon rank sum test.
p<0.001 vs controls.
p<0.05 vs non-compaction. EF >50%.
p<0.05 vs controls.
p<0.01 vs non-compaction. EF >50%.
Repeated measures ANOVA inspecting longitudinal sS of the basal, middle and apical LV segments to depict differences across LV level in the same group or a group-level interaction, showed significant differences in group I (p<0.0009) of sS across the basal, middle and apical segments, with the apical sS, on average, more negative than the basal or middle sS. The analysis showed no differences in sS across LV levels in either group II (p = 0.40) or group III (p = 0.63), although the trend was reversed in patients compared with group I, with the middle and apical sS measures lower (ie, less negative) than basal sS (figure 2). Finally, the group-level interaction was significant (p<0.0001), showing that being in a specific group (I, II or III), significantly influenced longitudinal sS values at each LV level.
Longitudinal dMV-E and dSR-E values at all LV levels and most LV walls, as well as the global mean of 18 LV segments, were reduced in group III compared with either group II or group I. dMV-E measures were also significantly reduced in group II compared with group I considering the mean of the six middle segments, mean of the six apical segments and the anterior and anteroseptal walls. When compared with group I, patients in group II had a significant reduction of dSR-E values of the mean of the six apical segments and of the LV anterior wall.
Radial and circumferential SMI, LV rotation/torsion
Radial and circumferential sSR, dSR-E and sS were all reduced in group III compared with the other groups, whereas only radial sS was significantly reduced in group II compared with group I (table 4).
Table 4.
Radial, circumferential speckle myocardial imaging modalities and left ventricular rotation/torsion measures for patients (groups II and III) and controls (group I)
| Variable (mean±SD) |
Controls Group I (n=20) |
Non-compaction Ejection Fraction > 50% Group II (n=10) |
Non-compaction Ejection Fraction ≥ 50% Group III (n=10) |
|---|---|---|---|
| Radial sSR (1/s) | 2.0±0.6 | 1.7±0.4 | 1.2±0.4*,† |
| Radial dSR-E (1/s) | −2.4±0.7 | −2.1±1 | −1.4±0.9‡ |
| Radial sS (%) | 51.9±12.8 | 32.7±9.3ss | 19±12.1*,† |
| Circumferential sSR (1/s) | −1.5±0.4 | −1.4±0.2 | −0.9±0.3§,† |
| Circumferential dSR-E (1/s) | 1.9±0.4 | 1.8±0.3 | 1.2±0.3§,¶ |
| Circumferential sS (%) | −20.9±2.8 | −18.5±3.2 | −12±5.6§,† |
| Rotation basal mean (degrees) | −7.6±3.4 | −6.0±1.7 | −3.9±2.5‡ |
| Rotation apical mean (degrees) | 22.2±12.8 | 5.0±4.5* | 2.3±3.3* |
| Torsion (degrees) | 28.3±12.3 | 9.6±6.4* | 3.9±4.5*,† |
| Torsion rate (degrees/s) | 171.7±49.8 | 71.2±29.4* | 67.7±43.3* |
| Non-torsion rate IVRT (degrees/s) | 181.4±56.1 | 101.7±48.7§ | 59.5±66§,† |
| Non-torsion rate late (degrees/s) | 99.4±62.5 | 42.6±21.7§ | 36.9±11.9§ |
Comparisons are made using the Wilcoxon rank sum test.
p<0.001 vs controls.
p<0.05 vs non-compaction. EF >50%.
p<0.05 vs controls.
p<0.01 vs controls.
<0.01 vs non-compaction. EF >50%.
dMV-E, early diastolic peak values for myocardial velocity; dSR-E, early diastolic peak values for strain rate; IVRT, isovolumic relaxation time; sS, systolic peak values for strain; sSR, systolic peak values for strain rate.
The LV rotation of the six basal segments was reduced in group III compared with the other groups, whereas it was not different between groups II and I. LV rotation of the six apical segments as well as LV torsion rate and LV non-torsion rate at late diastole, were reduced in both group III and group II compared with group I but were not significantly different between group III and group II. LV torsion and the LV non-torsion rate at early diastole were reduced in group III compared with either group II or group I and in group II compared with group I as well (table 4).
ROC analysis
ROC analysis demonstrated that SMI measurement for any single LV segment is not sufficient to accurately discriminate between group II and the controls. Calculation of longitudinal systolic SMI, and specifically the mean of the segments in a level, as opposed to the mean of segments in walls, tended to be the clustering technique providing better discrimination (figure 3a-c). Considering the global and level means of longitudinal, radial and circumferential sMV, dMV-E, sD, sSR, dSR-E and sS, as well as LV rotation/torsion measures, the longitudinal sS global mean (cut-off value = −19.0%, sensitivity = 90%, specificity = 100%), sS mean of the six apical segments (cut-off value = −20.0%, sensitivity = 90%, specificity = 90%), the LV rotation of the six apical segments (cut-off value = 8.0 degrees, sensitivity = 100%, specificity = 90%), LV torsion (cut-off value = 22.0 degrees, sensitivity = 87%, specificity = 89%), and LV torsion rate (cut-off value = 110.0 degrees/s, sensitivity = 93%, specificity = 89%), were the most accurate for discrimination between group II and group I, and their diagnostic accuracies were not significantly different (p = 0.77). Longitudinal sD global mean (cut-off value = 10.0 mm, sensitivity = 75%, specificity = 80%) had high accuracy as well.
Figure 3.
Receiver operating characteristic (ROC) analysis for the speckle myocardial imaging (SMI) modalities, comparison between patients with isolated left ventricular non-compaction and ejection fraction >50% (group II) vs controls (group I). sS glob mean, mean of the sS values for 18 left ventricular (LV) segments; sS apical mean, mean of the sS values for six LV apical segments; sSR apical mean, mean of the sSR values for six LV apical segments; sD global mean, mean of the sD values for 18 LV segments; sD middle mean, mean of the sD values for six LV middle segments; Radial sS mean, radial sS mean of the six LV segments at level of the papillary muscles in short-axis (SAX) view; Circumferential sS mean, circumferential sS mean of the six LV segments at level of the papillary muscles in SAX view; Rotation apical mean, mean of the LV rotation for the six LV apical segments; Torsion: LV torsion (computed as (apical LV rotation – basal LV rotation)); Torsion rate, LV torsion rate (degrees/s). (a) SMI longitudinal measures; areas under the curve (AUCs) group II vs group I: sS global mean=0.90 (95% CI 0.82 to 1.00); sS apical mean=0.93 (95% CI 0.82 to 1.00); sS middle mean=0.94 (95% CI 0.72 to 1.00); sSR apical mean=0.80 (95% CI 0.63 to 0.98); sD global mean=0.87 (95% CI 0.74 to 1.00); sD middle mean=0.87 (95% CI 0.74 to 1.00). (b) SMI radial, circumferential, and LV rotation/torsion measures; AUCs group II vs group I: Radial sS mean=0.82 (95% CI 0.61 to 1.00); Circumferential sS mean=0.60 (0.26 to 0.93); LV rotation apical mean=0.94 (95% CI 0.83 to 1.00); LV torsion=0.93 (95% CI 0.81 to 1.00); LV torsion rate=0.94 (95% CI 0.84 to 1.00). (c) SMI best modalities; AUCs group II vs group I: sS apical mean, sS global mean, LV rotation apical mean, LV torsion, LV torsion rate. p Value for the ROC curves comparison=0.57.
Clinical follow-up
At a median follow-up time of 21 months (interquartile range 11–27) six patients underwent both clinical re-evaluation and standard echocardiography. Of these, three had EF ≤50% at baseline and three had EF >50%. NYHA class was stable in all patients but one, where it improved from NYHA II to I with optimal medical treatment for heart failure. This patient had significant improvement in EF (25–49%) and E/E’ (12.5–8.6). There was an EF reduction in three patients (range of EF reduction = 5–10%): two of them had EF ≤50 at baseline, one patient had EF >50%.
During the same follow-up period two patients died. The first death was due to idiopathic pulmonary fibrosis. At baseline this patient had an NYHA class = I, EF = 40%, with longitudinal sS LV global average = 17% and sSR LV global average = 1.3 1/s. The second death was due to LV systolic dysfunction in a patient with NYHA class = IV, EF = 15%, sS global average of the LV = 4.5% along with sSR global average of the LV = 0.5 1/s.
Intra- and inter-reader reproducibility
The ICC for intrareader reproducibility was computed for the global sMV (ICC = 0.97), global sD average (ICC = 0.95), global sSR average (ICC =0.93), global sS average (ICC=0.98), radial sS (ICC = 0.92), circumferential sS (0.89), LV torsion (ICC = 0.91) and LV torsion rate (ICC = 0.90).
The intraclass correlation coefficient (ICC) for inter-reader reproducibility was computed for the global sMV (ICC = 0.95), global sD average (ICC = 0.87), global sSR average (ICC = 0.88), global sS average (ICC = 0.94), radial sS (ICC = 0.90), circumferential sS (0.85), LV torsion (ICC = 0.86) and LV torsion rate (ICC = 0.88).
DISCUSSION
To the best of our knowledge, this is the first investigation to obtain SMI measurements of all 18 LV segments in patients with iLVNC with normal and abnormal EF, and to compare these measurements with SMI measurements from healthy subjects. The main findings of the current investigation are as follows: (a) LV function, as assessed by different SMI modalities, is impaired in patients with iLVNC compared with controls, even in the group of patients with normal EF, as well as normal PWTDI E’ velocity and E/E’ ratio; (b) the measurements with the highest diagnostic accuracy to discriminate between patients with iLVNC and normal EF and healthy subjects are the longitudinal sS global mean of 18 LV segments and the longitudinal sS mean of the six apicalsegments; (c) consistent with peak SMI values, LV rotation/torsion values are abnormally reduced in patients with iLVNC and normal EF compared with controls. Of these, the LV rotation mean of the six apical segments, LV torsion and torsion rate have the highest diagnostic accuracy, comparable to the longitudinal sS global average or the mean of the six apical segments.
Lofiego et al have already reported that LV function in patients with asymptomatic iLVNC was not accompanied by less extensive non-compaction, suggesting that non-compaction is probably not the cause of LV dysfunction but rather a marker of an underlying diffuse cardiomyopathy.8 We have confirmed this finding showing the ratio of compacted over non-compacted segments to be no different between groups II and III. Murphy and coworkers have shown that there is a long preclinical phase of disease during which patients have no symptoms or are paucisymptomatic.9 Therefore, it becomes critical to study whether or not there is early LV myocardial impairment in iLVNC, for diagnostic as well as potential prognostic purposes.
McMahon et al have reported that conventional PWTDI values are reduced in patients with iLVNC and low EF,10 while Tufekcioglu and coworkers have shown abnormal systolic PWTDI values in patients with iLVNC and normal EF.11 In contrast, we found that PWTDI S’ velocity was not reduced in patients with iLVNC and EF >50% compared with controls. Perhaps this was due to the different LV areas at which PWTDI was measured (at the medial mitral annulus in our study, at the mid-posterior wall in the previous work). We believe our results provide clear evidence of significant early LV systolic and diastolic impairment inpatients with iLVNC and normal EF (and normal conventional PWTDI values), assessed by multiple SMI modalities, which goes beyond the apical anatomical location, thus, providing proof that iLVNC, as currently defined, is accompanied by an underlying myopathic process that extends beyond the non-compacted tissue.
Consistent with our previous observations in another diffuse cadiomyopathy,12 longitudinal sMV and dMV-E, dSR-E and circumferential SMI values, although reduced in patients with iLVNC and low EF (group III), were not useful to discriminate between patients with iLVNC patients and EF >50% (group II) and controls (group I). Indeed, they were neither reduced in group II nor had sufficient sensitivity for clinical purposes (as detected by ROC analysis). On the other hand, other longitudinal SMI measurements, such as sD, sSR and sS, were significantly reduced already in group II as compared with group I, and the reduction was greater in group III. It is interesting to note the higher sensitivity of sD compared with sMVI or dMV-E for the early detection of LV impairment in group II: diagnostic accuracy of longitudinal sD, specifically of the global average of 18 LV segments, which was higher than longitudinal sSR and radial sS (although the difference was not significant) and close to the accuracy reached by longitudinal sS. Among different longitudinal SMI modalities, longitudinal sS showed the highest accuracy in discriminating between group II and group I. In particular the global sS average of 18 LV segments or the mean of the six apical segments had very similar AUCs by ROC analysis, demonstrating that both measurements are equally useful for early detection of LV dysfunction in patients with iLVNC and normal EF.
The observation that apical sS values are more negative in healthy subjects than the mid- and basal sS values is consistent with classic studies reporting that the LV apex has a greater component of radially/circumferentially, rather than longitudinally, oriented myocardial fibres than other LV levels.13,14 Although the observation that LV function as assessed by longitudinal SMI is most impaired at the apical and middle levels was not surprising, considering the location of hypertrabeculations and the previously published studies on pathology in iLVNC,15 it is noteworthy that longitudinal sD and sS (but not longitudinal sSR or radial SMI values) showed that LV basal segments were also impaired in group II. This observation supports the heretofore mentioned hypothesis that iLVNC is not a localised disease limited to the areas of ‘spongy’ myocardium, but rather, a diffuse cardiomyopathy affecting LV function globally and significantly earlier than symptoms of heart failure occur. This early impairment is independent of the number of non-compacted segments (indeed group II and group III had a comparable ratio of non-compacted to compacted segments).
Diagnostic usefulness of LV rotation/torsion has been already shown,16 specifically in diastolic dysfunction.5 A recent study employing a canine model of congestive heart failure showed the important relationship between LV apical rotation and global LV systolic function.17 Our study population had an impressive reduction of LV rotation of the six apical segments (and therefore of LV torsion) as well as of the torsion and non-torsion rates, that was already present in group II. Moreover, LV rotation of the six apical segments, LV torsion and LV torsion rate had the highest diagnostic accuracy for discriminating between group II and group I, with AUCs obtained by ROC curves, comparable to those defined using longitudinal sS values. This suggests that impairment of the LV apex in iLVNC is significant and involves longitudinal as well as radial/circumferential function, making these measurements of the greatest diagnostic usefulness.
Limitations
This study is limited by a small sample of patients with iLVNC. However, considering the rarity of this cardiomyopathy and the statistically significant results obtained, sample size was sufficient to test our hypothesis and fulfil the aims of the study.
Unfortunately, sample size was not sufficient to obtain a meaningful outcomes analysis. The follow-up period was also probably insufficient, considering the long preclinical phase reported by Murphy et al.9 Nevertheless, the fact that SMI values were abnormally low in the only patient where heart failure was the cause of death, may support the potential role that this new technique might have in defining risk stratification in patients with iLVNC.
CONCLUSIONS
In this study, we observed that abnormalities of different SMI modalitiesd—in particular, longitudinal sD, sS and sSR, as well as LV rotation/torsion measures, are present in patients with iLVNC, even in those asymptomatic patients with preserved EF and normal conventional PWTDI measurements. These findings provide evidence of an ongoing, subclinical myopathic process related to the morphological presence of iLVNC. Longitudinal sS and LV rotation/torsion were the most accurate SMI modalities to differentiate between these patients and controls, and thus, may serve as the physiological diagnostic complement to the current morphology-based diagnosis. Furthermore, SMI assessment may have important prognostic implications that have not yet been proved. We believe SMI analysis should be performed in all patients with iLVNC referred for echocardiography and should, at a minimum, include calculation of the mean sS or LV rotation of the six apical segments, as well as LV torsion and torsion rate. In the event that sS curves are not satisfactory for these segments, or LV rotation/torsion is not measurable, measurement of the sD global average of the 18 LV segments is a suitable second option. Further studies in larger populations are warranted.
Acknowledgments
Funding This study was supported in part by a grant from the American Heart Association Heartland Affiliate (grant no 0620073Z) and by the research award of the American Society of Echocardiography (research award 2008–9, Diego Bellavia).
Footnotes
DB and HIM contributed equally to this study.
Competing interests None.
Ethics approval This study was conducted with the approval of the Institutional Review Board, Mayo Clinic and Foundation, Rochester, Minnesota, USA.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Chin TK, Perloff JK, Williams RG, et al. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82:507–13. doi: 10.1161/01.cir.82.2.507. [DOI] [PubMed] [Google Scholar]
- 2.Petersen SE, Selvanayagam JB, Wiesmann F, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46:101–5. doi: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 3.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Leitman M, Lysyansky P, Sidenko S, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021–9. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Park SJ, Miyazaki C, Bruce CJ, et al. Left ventricular torsion by two-dimensional speckle tracking echocardiography in patients with diastolic dysfunction and normal ejection fraction. J Am Soc Echocardiogr. 2008;21:1129–37. doi: 10.1016/j.echo.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Mehta CR, Patel NR, Tsiatis AA. Exact significance testing for ordered categorical data. Biometrics. 1984;40:819–25. [PubMed] [Google Scholar]
- 7.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 8.Lofiego C, Biagini E, Pasquale F, et al. Wide spectrum of presentation and variable outcomes of isolated left ventricular non-compaction. Heart. 2007;93:65–71. doi: 10.1136/hrt.2006.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy RT, Thaman R, Blanes JG, et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur Heart J. 2005;26:187–92. doi: 10.1093/eurheartj/ehi025. [DOI] [PubMed] [Google Scholar]
- 10.McMahon CJ, Pignatelli RH, Nagueh SF, et al. Left ventricular non-compaction cardiomyopathy in children: characterisation of clinical status using tissue Doppler-derived indices of left ventricular diastolic relaxation. Heart. 2007;93:676–81. doi: 10.1136/hrt.2006.093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tufekcioglu O, Aras D, Yildiz A, et al. Myocardial contraction properties along the long and short axes of the left ventricle in isolated left ventricular non-compaction: pulsed tissue Doppler echocardiography. Eur J Echocardiogr. 2008;9:344–50. doi: 10.1016/j.euje.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Bellavia D, Pellikka PA, Abraham TP, et al. Evidence of impaired left ventricular systolic function by Doppler myocardial imaging in patients with systemic amyloidosis and no evidence of cardiac involvement by standard two-dimensional and Doppler echocardiography. Am J Cardiol. 2008;101:1039–45. doi: 10.1016/j.amjcard.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 13.Kowalski M, Kukulski T, Jamal F, et al. Can natural strain and strain rate quantify regional myocardial deformation? A study in healthy subjects. Ultrasound Med Biol. 2001;27:1087–97. doi: 10.1016/s0301-5629(01)00388-x. [DOI] [PubMed] [Google Scholar]
- 14.Andersen NH, Poulsen SH. Evaluation of the longitudinal contraction of the left ventricle in normal subjects by Doppler tissue tracking and strain rate. J Am Soc Echocardiogr. 2003;16:716–23. doi: 10.1016/S0894-7317(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 15.Burke A, Mont E, Kutys R, et al. Left ventricular noncompaction: a pathological study of 14 cases. Hum Pathol. 2005;36:403–11. doi: 10.1016/j.humpath.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Sengupta PP, Krishnamoorthy VK, Korinek J, et al. Left ventricular form and function revisited: applied translational science to cardiovascular ultrasound imaging. J Am Soc Echocardiogr. 2007;20:539–51. doi: 10.1016/j.echo.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Nagueh SF, Mathuria NS, et al. Left ventricular twist mechanics in a canine model of reversible congestive heart failure: a pilot study. J Am Soc Echocardiogr. 2009;22:95–8. doi: 10.1016/j.echo.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]