Abstract
Although CD4+ T cells are known to contribute to the pathology of atopic dermatitis (AD) and psoriasis, the role of CD8+ T cells in these diseases remains poorly characterized. The aim of this study was to characterize the cytokine production of T cells from AD and psoriasis skin. We found that CD4+ T cells isolated from AD skin were largely Th2-biased, in agreement with prior reports. However, we also observed large numbers of CD8+ T cells producing IL-13, IFN-γ and IL-22. We observed increased numbers of CD8+ T cells isolated from AD skin, and immunohistochemistry studies confirmed the presence of CD8+ T cells in the dermis and epidermis of AD skin lesions. Surprisingly, T cell cytokine production was similar in the lesional and non-lesional skin of patients with AD. T cells from psoriatic lesional skin predominantly produced IFN-γ, IL-17 and IL-22, in agreement with prior studies. However, in addition to Th17 cells, we observed high percentages of CD8+ T cells that produced both IL-22 and IL-17 in psoriatic skin lesions. Our findings demonstrate that CD8+ T cells are a significant and previously unappreciated source of inflammatory cytokine production in both AD and psoriasis.
INTRODUCTION
Atopic dermatitis (AD) and psoriasis are the two most common chronic inflammatory skin diseases. In both diseases, dense infiltrates consisting mainly of T cells are found within lesional skin and it is well established that T cells play an important role in the pathogenesis of both diseases. AD was previously considered a prototypical Th2 disease, with predominance of IL-4, IL-5 and IL-13 producing CD4+ T cells. This hypothesis was modified after it was found that in the chronic stages of AD, and late phase of the atopy patch test, there was substantially more IFN-γ production, leading to a mixed Th1/Th2 profile (Thepen et al., 1996). Psoriasis is a very distinct disorder from AD, with recent evidence suggesting a critical role for pathogenic Th17 and Th22 cells, with some attendant production of IFN-γ (Guttman-Yassky et al., 2011). Although AD has classically been considered a disease induced by Th2 CD4+ T cells, more recent findings have suggested a pathogenic role for CD8+ T cells (Hennino et al., 2007; Hennino et al., 2011). CD8+ T cells isolated from AD skin lesions were shown to be potent producers of inflammatory cytokines, including IL-22 (Nograles et al., 2009).
In this study, we conducted a comprehensive analysis of T cells isolated from short-term explant cultures of both lesional and non-lesional skin in patients with AD and psoriasis. This approach allowed comprehensive analysis of T cell cytokine production without the need for an enzymatic digestion or T cell cloning.
RESULTS
CD4+ T cells from the lesional skin of AD patients are Th2 polarized and Th1 and Th17 T cells are frequent in the skin lesions of psoriasis
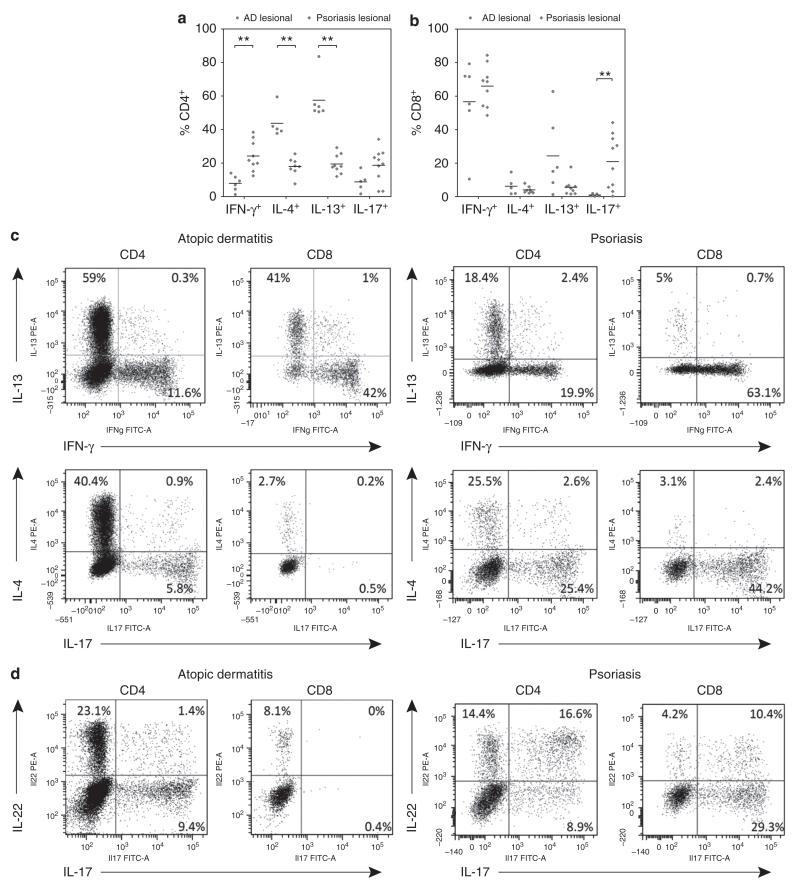
The cytokine production profiles of T cells isolated from lesional AD and psoriasis skin were analyzed by intracellular cytokine staining and flow cytometry analysis. CD4+ T cells from both disorders showed distinct cytokine production profiles (Figure 1a). Lesional CD4+ T cells from AD patients showed decreased percentages of IFN-gamma expressing cells compared to CD4+ T cells isolated from lesional psoriatic skin (8.1% versus 21.6%; P<0.01). The percentages of CD4+ IL-4 expressing T cells were increased in AD compared to psoriasis (40.4% versus 18.1%; P<0.01). Expression of IFN-gamma and IL-13 was largely mutually exclusive (Figure 1c; representative FACS plots, upper panels). As expected, high percentages of CD4+ IL-17 expressing T cells (20.8%) were isolated from lesional psoriasis skin.
FIGURE 1.
T cells from AD and psoriatic skin have distinct cytokine production profiles.
(a) CD4+ T cells isolated from lesional skin of AD patients showed significantly increased expression of IL-4 and IL-13 (Th2), whereas CD4+ T cells isolated from lesional psoriasis skin showed increased expression of interferon-gamma (Th1). (b) CD8+ T cells isolated from lesional psoriasis skin demonstrated increased expression of IL-17 compared to CD8+ cells isolated from lesional AD skin, whereas CD8+ T cells from AD skin showed a trend towards increased percentages of IL-13 producing cells (P=0.088). Representative FACS plots: (c) expression of IL-13 and IFN-γ is mutually exclusive (upper plots), as is expression of IL-17 and IL-4 (lower plots); (d) expression of IL-22 and IL-17 in both CD4+ and CD8+ subsets. Whereas expression of IL-17 and IL-22 was largely mutually exclusive in AD patients, psoriasis patients showed IL-17+IL-22+ cells in both CD4+ and CD8+ T cell populations. Sample numbers may vary between groups (as a result of small numbers of cells isolated from specific samples). Horizontal bars represent median percentages for each group. ** P<0.01
CD8+ T cells are a potent source of inflammatory cytokines in both AD and psoriasis
High percentages of CD8+ T cells isolated from the skin lesions of both AD and psoriasis produced IFN-gamma (Figure 1b; 63.5% and 68.7%, respectively). Increased numbers of IL-17 producing CD8+ T cells were observed in psoriatic skin lesions as compared to AD skin (22.8% versus 0.5%; P<0.01) and high percentages of IL-13 producing CD8+ T cells were observed in lesional AD skin (16.3%). Secretion of IL-13 and IFN-gamma by CD8+ T cells isolated from skin biopsies was confirmed by ELISA after magnetic assisted cell sorting for CD8+ T cells (Figure S2). Levels of IL-13 and IFN-gamma secreted by the CD8+ T cell population were found to be in the same magnitude as in the CD8− population (mainly CD4+ T cells).
IL-22 is produced by CD4+ and CD8+ T cells in both AD and psoriasis
The expression of IL-22 was investigated in a subset of patients. IL-22 was produced by CD4+ T cells isolated from lesional skin of both AD (n=4) and psoriasis (n=4) patients (11.2% versus 18.9%). In addition, high percentages of CD8+ T cells isolated from both AD (n=4) and psoriasis (n=4) skin also produced IL-22 (30.3% versus 18.7%) (Figure 1d; representative FACS plots; and Figure S1). We found that production of IL-17 and IL-22 by T cells in AD was largely mutually exclusive (Figure 1d, left panels), in agreement with prior studies (Eyerich et al., 2009). However, in psoriasis patients, a relatively large population of CD8+ T cells produced both IL-22 and IL-17 (Figure 1d, right panels).
CD8+ T cells are abundant in the skin of patients with AD
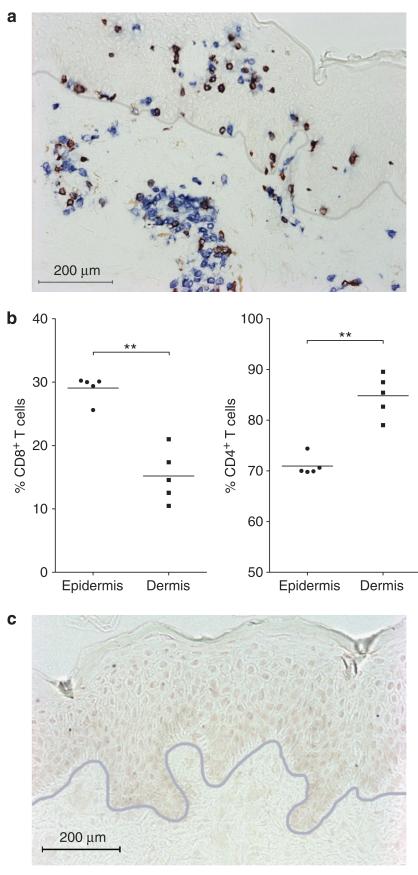
Our studies demonstrated significant cytokine production by CD8+ T cells in AD skin lesions. To confirm the presence of CD8+ T cells in AD skin in vivo, biopsies taken from lesional AD skin (n=5) were stained using antibodies directed against CD3 and CD8. CD8+ T cells were abundant in lesional AD skin (Figure 2a). In the dermis, CD8+ T cells were found mainly in the perivascular infiltrates together with CD4+ T cells. In order to quantify the numbers of CD4+ T cells, slides were stained for CD3 and CD4 (a double-label staining was used to exclude non-T-cells expressing CD4, i.e. monocytes) and cell numbers were determined. We calculated that the percentage of CD8+ T cells was significantly increased in the epidermis compared to the dermis of lesional AD skin (29.1% ± 0.9% versus 15.2% ± 1.7%; P<0.01; Figure 2b).
FIGURE 2.
CD8+ T cells are abundant in AD skin.
(a) Representative immunohistochemical staining showing abundant CD8+ T cells in the dermis and epidermis of lesional AD skin (CD3 = blue, CD8 = red/brown). The gray line represents the dermo-epidermal junction. (b) The percentage of CD8+ T cells was significantly increased in the epidermis compared to the dermis of lesional AD skin. (c) Isotype control staining. Scale bar = 200 μm. Horizontal bars represents mean percentages. ** P<0.01
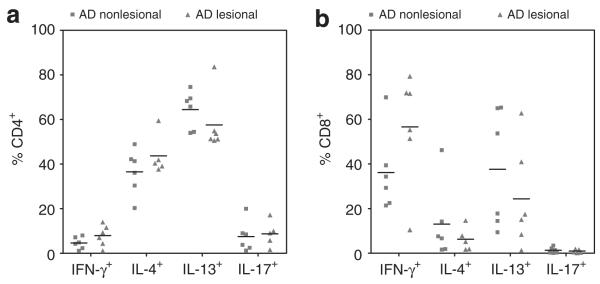
T cells from lesional and non-lesional skin of patients with AD had similar cytokine production profiles
Relative frequencies of different T-cell subsets (Th1, Th2 and Th17) between lesional and non-lesional AD skin were determined. Both lesional and non-lesional CD4+ skin T cells were found to express a Th2 profile, with high percentages of IL-4 and IL-13 expressing CD4+ T cells and relatively low expression of IFN-gamma (Figure 3a). No significant differences were found between lesional and non-lesional AD skin. CD8+ T cells from lesional AD skin showed a trend towards lower expression of IFN-gamma (63.5% versus 31.8%; P=0.094) in lesional compared to non-lesional AD skin (Figure 3b).
FIGURE 3.
CD4+ T cells from both lesional and non-lesional AD skin had robust production of Th2 cytokines. (a) High numbers of CD4+ T cells isolated from lesional and non-lesional AD skin were found to produce IL-4 and IL-13. Strikingly, no significant differences were found between cells isolated from lesional and non-lesional AD skin. (b) High percentages CD8+ T cells from both lesional and non-lesional AD skin were found to express IFN-gamma or IL-13. Expression of IL-13 and interferon-gamma was mutually exclusive (Figure 1c). Sample numbers may vary between groups. Horizontal bars represent median percentages for each group.
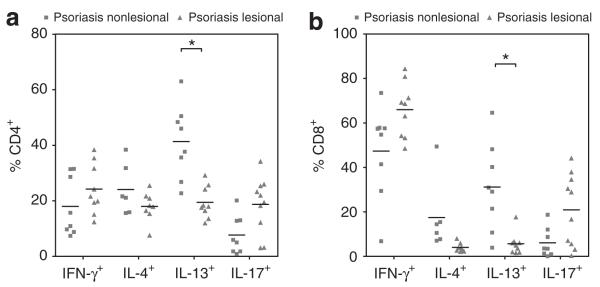
IL-13 production is markedly reduced in lesional as compared to non-lesional skin in psoriasis
We compared the production of Th1, Th2 and Th17 cytokines by CD4+ and CD8+ T cells in lesional versus non-lesional psoriatic skin. IL-13 production by both CD4+ and CD8+ T cells was significantly decreased in lesional vs. non-lesional skin (CD4+: 18.0% versus 41.9%: P<0.05; CD8+: 5.0% versus 30.0%: P<0.05) (Figure 4a). Although not statistically significant, IL-4 expression was decreased in CD4+ T cells isolated from lesional compared to non-lesional psoriatic skin (18.1% versus 21.4%: NS), whereas IFN-gamma was increased in lesional compared to non-lesional skin (21.6% versus 13.3%: NS).
FIGURE 4.
IL-13 production is markedly reduced in lesional as compared to non-lesional skin in psoriasis. (a) CD4+ and (b) CD8+ T cells isolated from lesional psoriasis skin expressed significantly less IL-13 compared to non-lesional skin. Lesional CD4+ T cells from psoriasis patients showed a trend towards higher expression of IL-17 compared to non-lesional skin (P=0.15).
Sample numbers may vary between groups. Horizontal bars represent median percentages for each group. * P<0.05
DISCUSSION
In this report we describe a comprehensive analysis of T cells isolated from the lesional and non-lesional skin of patients with AD and psoriasis. Although CD4+ T cells are well known to play an important role in the pathogenesis of AD and psoriasis, we show that also CD8+ T cells produce key cytokines in both diseases and confirm the presence of CD8+ T cells in the epidermis and dermis of AD skin. We found decreased expression of IL-17 by CD8+ T cells from AD compared to psoriasis and show that IL-22 is produced by both CD4+ and CD8+ cells in AD and psoriasis. IL-17 and IL-22 producing T cells have recently been identified as key players in the pathogenesis of psoriasis (Guttman-Yassky et al., 2011). IL-17 producing T cells play a critical role in protecting the skin from extracellular pathogens and cytokines produced by these cells lead to upregulation of antimicrobial peptide (AMP) production by keratinocytes (Liang et al., 2006). Several of these AMPs are microbicidal for S. aureus and the reduced production of AMP in AD patients may underlie their susceptibility to colonization and infection with this pathogen (Midorikawa et al., 2003; Ong et al., 2002). As expected, we observed increased percentages of CD4+ and CD8+ T cells expressing IL-17 in psoriatic skin lesions as compared to AD, in agreement with prior studies (Nograles et al., 2009). We observed production of IL-17 by 21% of the CD8+ T cells in psoriatic skin lesions suggesting they may play an important role in the pathogenesis of psoriasis. Indeed, a population of CD8+ T cells was found to remain resident in the previously lesional skin of healed psoriatic skin lesions (Suarez-Farinas et al., 2011a). We should note that one prior study failed to observe CD8+ IL17+ T cells in psoriatic skin lesions (Nograles et al., 2009). However, this study did not include epidermal T cells in the analysis and IL-17 producing CD8+ T cells have been reported to localize predominantly to the epidermis in psoriasis (Kryczek et al., 2008).
Th17 T cells were recently found to express the IL-13 receptor and IL-13 has been reported to be a negative regulator of IL-17 production in these cells (Newcomb et al., 2011). We find enhanced production of IL-17 and reduced production of IL-13 in psoriatic lesional skin T cells and conversely, enhanced production of IL-13 and reduced production of IL-17 in AD skin, in agreement with a reciprocal relationship between these two cytokines. Previous studies at the level of mRNA and protein expression have demonstrated increased production of IL-13 in AD lesional skin (Hamid et al., 1996; Tazawa et al., 2004). We report that both CD4+ and CD8 T+ cells are significant sources of IL-13 production in AD skin. IL-13 has several biologic activities that may contribute to its pathogenicity in AD skin lesions. IL-13 enhances IgE production and also induces expression of the chemokine MDC (CCL22) in human primary keratinocytes and activates matrix metalloproteinase-9, activities that may facilitate migration of leukocytes into the epidermis (Purwar et al., 2006; Purwar et al., 2007). Recently, IL-13 and IL-4 have been shown to decrease the expression of filaggrin and human beta-defensin-3 in vitro, suggesting they may contribute to decreased barrier function of the skin (Howell et al., 2007; Howell et al., 2008). Expression of IL-13 in the skin of IL-13 transgenic mice induces a pruritic dermatitis that resembles AD (Zheng et al., 2009). Furthermore, IL-13 is a key effector cytokine in ulcerative colitis that is known to disrupt epithelial tight junctions (Heller et al., 2005). Disruption of epithelial tight junctions in AD has been gaining more attention recently as a critical biologic event in this disease (De Benedetto A. et al., 2011). In conclusion, IL-13 may contribute to barrier dysfunction and chronic inflammation by several mechanisms and may therefore play an important role in the pathogenesis of AD. Our observation that both CD4+ and CD8 T+ cells are important sources of IL-13 production suggests that therapies that selectively target T-cell activation may be capable of reducing IL-13 production in AD skin lesions.
In the skin, IL-22 induces keratinocyte proliferation and epidermal hyperplasia and it was recently shown that the frequency of IL-22 expressing T cells in AD skin correlated with disease severity (Boniface et al., 2005; Nograles et al., 2009). However, the exact role of IL-22 in AD remains largely uncharacterized (Guttman-Yassky et al., 2011). We observed production of IL-22 by substantial percentages of CD4+ and CD8+ T cells isolated from the skin of patients with both psoriasis and AD (n=4, see Figures 1d and S3), with no statistically significant difference in the number of the cells between the two diseases. This is in contrast to a prior study that showed only modest production of IL-22 production by CD8+ T cells in psoriasis but impressive production IL-22 by CD8+ T cells in AD (Nograles et al., 2009). But again, this study did not analyze epidermal T cells which may explain the differences, since the majority of CD8+ T cells in psoriatic skin lesions are found within the epidermis (Conrad et al., 2007).
We observed a large population of T cells in psoriasis that produced both IL-17 and IL-22, whereas production of these two cytokines in AD skin was largely mutually exclusive (Figure 1d). This finding is similar to that observed in T cell clones from psoriasis and AD skin (Eyerich et al., 2009). The infiltration of IL-22 producing CD8+ T cells into the epidermis in AD and psoriasis may result in epidermal acanthosis, which is a histopathological feature of both diseases.
Previous studies utilizing immunohistochemistry and in situ hybridization of AD skin and skin from atopy patch testing suggested that acute AD was a largely Th2 driven process but that a switch occurred in the chronic phase of AD to a more Th1 driven process (Thepen et al., 1996; Grewe et al., 1995). In support of this contention, IFN-γ was expressed at higher levels than IL-4 in chronic AD lesions (Grewe et al., 1994). We observed large numbers of IFN-γ producing CD8+ T cells in chronic AD skin lesions (Tc1 cells, mean = 56.7% of total CD8+ T cells) but few IFN-γ producing CD4+ T cells. In addition, CD8+ T cells in AD skin were also potent producers of IL-13 (Tc2 cells). Our studies suggest that CD8+ T cells contribute in a substantial manner to pathogenic cytokine production in AD skin lesions and that classic models of AD that focus primarily on the pathogenic role of CD4+ T cells may be incomplete.
In the current study, T cells isolated from skin biopsies were cultured ex vivo for 3 weeks. This may result in phenotypical changes by differential survival characteristics of T cell subsets. However, we found no significant changes in the percentages of CD4+ and CD8+ T cells over time (Figure S5). In addition, we found no differences in cytokine expression profiles of T cells during the first 3 weeks of culture (data not shown). Our isolation/culture methods may however have induced changes in the cytokine production profiles of the isolated T cells compared to baseline.
T cells isolated from normal skin were also found to express a variety of cytokines, including IFN-γ, IL-4, and IL-13 (Figure S1). However, the interpretation of these data is difficult, because the absolute numbers of T cells present in both lesional and nonlesional AD skin, and lesional psoriasis skin are highly increased compared to normal skin. So, although normal skin T cells may be able to produce different cytokines, the absolute numbers of cytokine producing T cells present in normal skin are very small compared to inflamed skin. In addition, T cells in normal skin are supposed to be in a more quiescent condition compared to activated T cells present in the skin of patients with inflammatory skin conditions, suggesting that although there is a potential to produce cytokines, no cytokines will be secreted under normal circumstances. Unfortunately, it is currently impossible to investigate the amounts of secreted cytokines from a specific cell type directly in skin samples.
Although CD8+ T cells are known to contribute to the pathogenesis of psoriasis (Nestle et al., 2009), the role of these cells in AD is underappreciated. CD8+ T cells are known to be increased in lesional AD skin; one prior study showed that up to 33% of T cells in lesional AD skin were CD8+ (Akdis et al., 1999). An influx of CD8+ T cells in the early stages of the atopy patch test reaction has been reported, suggesting CD8+ T cells may play a role in the initiation of AD lesions (Thepen et al., 1996; Holm et al., 2004). In a mouse model of AD, CD8+ T cell influx is an early and essential event in the induction of eczematous skin lesions (Hennino et al., 2007; Hennino et al., 2011).
We find robust production of inflammatory cytokines, including IFN-γ, by CD8+ T cells in AD skin. IFN-γ production by T cells has been shown to increase the susceptible of keratinocytes to Fas/FasL-induced apoptosis (Trautmann et al., 2000). IFN-γ producing CD8+ T cells invading the epidermis may therefore contribute to barrier disruption and epithelial dysfunction. In psoriasis, migration of CD8+ T cells into the epidermis was associated with expression of VLA-1 (Conrad et al., 2007). In a subset of AD patients (n=4), we also found increased expression of VLA-1 on CD8+ T cells compared to CD4+ T cells isolated from AD skin (Figure S4). This suggests that also in AD, CD8+ T cells may have an increased tropism for the epidermis and this may also explain the increased percentages of CD8+ T cells in the epidermis compared to the dermis of lesional AD skin (Figure 2b). Furthermore, this emphasizes the importance of including T cells derived from the epidermis in the analysis when studying T cell mediated inflammatory skin conditions. Using immunohistochemical studies, we confirmed that significant numbers of CD8+ T cells are present in the dermis and epidermis of AD skin lesions (Figure 2a).
In addition to comparing lesional AD and psoriasis skin T cells, we also investigated differences between T cell cytokine production in lesional versus non-lesional skin. Clinically normal appearing skin in patients with AD has been reported to have histological abnormalities compared to normal skin, including increased numbers of infiltrating lymphocytes (Mihm, Jr. et al., 1976). Our results confirm that although there is no clinically apparent inflammation in non-lesional AD skin, T cells isolated from non-lesional skin show a T cell phenotype that is almost identical to AD lesional skin (Figure 2). This is in agreement with a recent study demonstrating increased expression of several inflammatory products in both lesional and non-lesional AD skin compared to normal skin (Suarez-Farinas et al., 2011b). In psoriasis however, there were marked differences in T cell cytokine production in lesional versus non-lesional skin. IL-13 producing T cells, both CD4+ and CD8+, were significantly reduced in lesional skin as compared to non-lesional psoriatic skin (Figure 4). This is in agreement with a previous study that demonstrated an eightfold decrease in IL-13 mRNA expression in non-lesional versus lesional psoriatic skin (van der Ploeg et al., 1997). Our findings suggest that IL-13 production may play a protective role in non-lesional psoriatic skin by down-regulating pathogenic IL-17 production.
Cytokine expression profiles of T cells isolated from AD and psoriasis biopsies were found not to correlate with disease severity measures, respectively SCORAD and PASI scores (data not shown). This may be the result of correlating the local phenomenon of cytokine production in a specific lesion/skin area to an overall measure for disease severity in relatively small patient populations.
In conclusion, we have shown that T cells isolated from the skin of patients with AD and psoriasis show distinct cytokine expression profiles (Figure 1). We demonstrate that CD8+ T cells are potent producers of cytokines, including IFN-γ, IL-13, IL-22 in both diseases, and also IL-17 in psoriasis. By invading the epidermis and producing IL-22 and IFN-γ, CD8+ T cells in AD may play an important role in epidermal thickening (acanthosis) and barrier disruption of the skin. The high production of IL-13 by both CD4+ and CD8+ T cells in AD skin may play an important role in barrier disruption by suppressing IL-17 production, decreasing filaggrin expression and disrupting tight junctions. Taken together, our results suggest that CD8+ T cells are an important but underappreciated source of inflammatory cytokines in both psoriasis and AD.
METHODS
Patients and samples
Patients with moderate to severe chronic AD were diagnosed according to the criteria of Hanifin and Rajka (Hanifin and Rajka, 1980). After written informed consent was obtained, skin biopsies (4 mm diameter) from chronic lesional and non-lesional skin were collected from six patients with AD (age 22-63 years; median, 36 years; mean modified SCORAD (SCORAD without the subjective signs): 28±8.7; mean serum CCL17 levels 1618±610 pg/mL) and eleven chronic plaque psoriasis patients (age 33-76 years; median, 59 years; mean PASI score 5.2±1.3). A therapeutic washout period of six weeks for oral medication and 1 week for topical treatment of the biopsy area was implemented before biopsies were collected. For immunohistochemistry, we used lesional biopsies from AD patients (n=5) that were diagnosed according to the criteria of Hanifin and Rajka. Healthy human skin samples were obtained as discarded material after cosmetic surgery. The protocols of this study were approved by the Institutional Review Board of the Utrecht University Medical Center (Utrecht, The Netherlands), adhering to the Declaration of Helsinki Principles.
Skin explant cultures
Explant cultures were established as previously described (Clark et al., 2006). Shortly, skin was cut into small fragments and placed on the surface of a matrix. Three-dimensional matrices (Statamatrix) were obtained from Cytomatrix (Australia). Matrices were incubated with 100 μg/mL rat tail collagen I (BD Biosciences, Bedford, MA) and placed into 1 well of a 24-well plate in 2 mL/well of Iscoves modified medium (Mediatech, Herndon, VA) with 20% heat-inactivated fetal bovine serum (FBS; Sigma, St Louis, MO), penicillin and streptomycin, and 3.5 μL/L β-mercaptoethanol. Cultures were fed 3 times a week by careful aspiration of 1 mL of culture medium and replacement with fresh medium. Cells were harvested at 3 weeks. IL-15 (10 ng/mL) (R&D systems, Minneapolis, MN)) and IL-2 (10 IU/mL) (a kind gift from Novartis Research Institute, Vienna, Austria) were added to the culture medium and refreshed with each feeding.
Flow cytometry studies
Flow cytometry analysis of T cells was performed using directly conjugated monoclonal antibodies (mAbs). CD3, CD4 and CD8 antibodies were obtained from BD Biosciences (San Diego, CA). Analysis of flow cytometry samples was performed on a Becton Dickinson FACSCanto instrument (San Jose, CA), and data were analyzed with FACSDiva software (BD Pharmingen). For intracellular cytokine analysis, T cells were stimulated with either control medium or 50 ng/ml PMA (ICN Biomedicals, Irvine, CA) and 750 ng/ml ionomycin (Invitrogen, Carlsbad, CA) for six hours; 10 μg/ml Brefeldin A (Calbiochem, San Diego, CA) was included in the culture medium for the last 5 hours of incubation. Cells were then stained for surface marker expression, fixed, permeabilized, stained with anti-cytokine antibodies (IL-4, IL-5, IL-13, IL-17, IL-22 and IFN-gamma antibodies were purchased from Biolegend, San Diego, CA) and examined by flow cytometry.
Cytokine secretion essays
T cells were isolated as described in the skin explant cultures section. Subsequently, CD8+ T cells were separated by positive selection using magnetic-activated cell sorting (MACS), according to the manufacturers’ recommendations (Miltenyi Biotech, Bergisch Gladbach, Germany). The CD8 negative fraction (mainly including CD4+ T cells) was used as a reference. Fifty thousand cells per well were stimulated for 24 hours with phorbol 12-myristate 13-acetate (PMA)/ionomycin. Secretion of IL-13 and IFN-gamma was determined in culture supernatants by ELISA, according to the manufacturers’ recommendations (Sanquin, Amsterdam, The Netherlands). The detection limit for IL-13 was 1.2 pg/mL, and 3.1 pg/mL for IFNg.
Immunohistochemistry
For immunohistochemistry we used lesional skin biopsies of AD patients (n=5) and normal skin (n=4) that were snap-frozen in liquid nitrogen, subsequently embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek, Torrance, CA) and stored at −80 degrees Celsius. Frozen 7 μm sections were cut and fixed in acetone with hydrogen peroxide for 10 minutes. Double-label immunohistochemical stainings (CD3, CD8; and CD3, CD4) were performed as follows: samples were incubated with 10% human and 10% normal horse serum in PBS for 20 minutes. Slides were then incubated for 1 hour with unlabeled primary antibodies: anti-CD8 mAb (Clone: T8) or anti-CD4 mAb (Clone: MT310) purchased from DAKO (Glostrup, Denmark). After washing, the slides were incubated with biotin-labeled horse-anti-mouse (Vector, Burlingame, CA) antibodies for 45 minutes. Subsequently samples were incubated with 10% normal mouse serum and 10% normal sheep serum for 10 minutes. Samples were then incubated with FITC-conjugated anti-CD3 mAb (BD Biosciences, San Jose, CA) for 45 minutes and subsequently washed and incubated with Alkaline Phosphatase-labeled sheep-anti-fluorescein antibodies (Roche, Indianapolis, IN) for 45 minutes. Afterwards, samples were incubated with Vecta Blue (Vector Laboratories, Burlingame, CA) for 8 minutes. Finally, slides were incubated with AEC-chromogen solution (ScyTek, Logan, UT) for 10 minutes.
Supplementary Material
Footnotes
Conflicts of interest
Dr. Thomas Kupper is scientific advisor of TremRx, a company that focuses on vaccine development
REFERENCES
- Akdis CA, Akdis M, Simon D, et al. T cells and T cell-derived cytokines as pathogenic factors in the nonallergic form of atopic dermatitis. J Invest Dermatol. 1999;113:628–634. doi: 10.1046/j.1523-1747.1999.00720.x. [DOI] [PubMed] [Google Scholar]
- Boniface K, Bernard FX, Garcia M, et al. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- Clark RA, Chong BF, Mirchandani N, et al. A novel method for the isolation of skin resident T cells from normal and diseased human skin. J Invest Dermatol. 2006;126:1059–1070. doi: 10.1038/sj.jid.5700199. [DOI] [PubMed] [Google Scholar]
- Conrad C, Boyman O, Tonel G, et al. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med. 2007;13:836–842. doi: 10.1038/nm1605. [DOI] [PubMed] [Google Scholar]
- De Benedetto A, Rafaels NM, McGirt LY, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127:773–786. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe M, Gyufko K, Schopf E, et al. Lesional expression of interferon-gamma in atopic eczema. Lancet. 1994;343:25–26. doi: 10.1016/s0140-6736(94)90879-6. [DOI] [PubMed] [Google Scholar]
- Grewe M, Walther S, Gyufko K, et al. Analysis of the cytokine pattern expressed in situ in inhalant allergen patch test reactions of atopic dermatitis patients. J Invest Dermatol. 1995;105:407–410. doi: 10.1111/1523-1747.ep12321078. [DOI] [PubMed] [Google Scholar]
- Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis-Part II: Immune cell subsets and therapeutic concepts. J Allergy Clin Immunol. 2011;127:1420–1432. doi: 10.1016/j.jaci.2011.01.054. [DOI] [PubMed] [Google Scholar]
- Hamid Q, Naseer T, Minshall EM, et al. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996;98:225–231. doi: 10.1016/s0091-6749(96)70246-4. [DOI] [PubMed] [Google Scholar]
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92:44–47. [Google Scholar]
- Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Hennino A, Jean-Decoster C, Giordano-Labadie F, et al. CD8(+) T cells are recruited early to allergen exposure sites in atopy patch test reactions in human atopic dermatitis. J Allergy Clin Immunol. 2011;127:1064–1067. doi: 10.1016/j.jaci.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Hennino A, Vocanson M, Toussaint Y, et al. Skin-infiltrating CD8+ T cells initiate atopic dermatitis lesions. J Immunol. 2007;178:5571–5577. doi: 10.4049/jimmunol.178.9.5571. [DOI] [PubMed] [Google Scholar]
- Holm L, Matuseviciene G, Scheynius A, et al. Atopy patch test with house dust mite allergen--an IgE-mediated reaction? Allergy. 2004; 59:874–882. doi: 10.1111/j.1398-9995.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- Howell MD, Fairchild HR, Kim BE, et al. Th2 Cytokines Act on S100/A11 to Downregulate Keratinocyte Differentiation. J Invest Dermatol. 2008;128:2248–2258. doi: 10.1038/jid.2008.74. [DOI] [PubMed] [Google Scholar]
- Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–155. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Bruce AT, Gudjonsson JE, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midorikawa K, Ouhara K, Komatsuzawa H, et al. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect Immun. 2003;71:3730–3739. doi: 10.1128/IAI.71.7.3730-3739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihm MC, Jr., Soter NA, Dvorak HF, et al. The structure of normal skin and the morphology of atopic eczema. J Invest Dermatol. 1976;67:305–312. doi: 10.1111/1523-1747.ep12514346. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- Newcomb DC, Boswell MG, Zhou W, et al. Human T(H)17 cells express a functional IL-13 receptor and IL-13 attenuates IL-17A production. J Allergy Clin Immunol. 2011;127:1006–1013. doi: 10.1016/j.jaci.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nograles KE, Zaba LC, Shemer A, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–1252. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Purwar R, Kraus M, Werfel T, et al. Modulation of Keratinocyte-Derived MMP-9 by IL-13: A Possible Role for the Pathogenesis of Epidermal Inflammation. J Invest Dermatol. 2007;128:59–66. doi: 10.1038/sj.jid.5700940. [DOI] [PubMed] [Google Scholar]
- Purwar R, Werfel T, Wittmann M. IL-13-stimulated human keratinocytes preferentially attract CD4+CCR4+ T cells: possible role in atopic dermatitis. J Invest Dermatol. 2006;126:1043–1051. doi: 10.1038/sj.jid.5700085. [DOI] [PubMed] [Google Scholar]
- Suarez-Farinas M, Fuentes-Duculan J, Lowes MA, et al. Resolved psoriasis lesions retain expression of a subset of disease-related genes. J Invest Dermatol. 2011a;131:391–400. doi: 10.1038/jid.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Farinas M, Tintle SJ, Shemer A, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011b;127:954–964. doi: 10.1016/j.jaci.2010.12.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazawa T, Sugiura H, Sugiura Y, et al. Relative importance of IL-4 and IL-13 in lesional skin of atopic dermatitis. Arch Dermatol Res. 2004;295:459–464. doi: 10.1007/s00403-004-0455-6. [DOI] [PubMed] [Google Scholar]
- Thepen T, Langeveld-Wildschut EG, Bihari IC, et al. Biphasic response against aeroallergen in atopic dermatitis showing a switch from an initial TH2 response to a TH1 response in situ: an immunocytochemical study. J Allergy Clin Immunol. 1996;97:828–837. doi: 10.1016/s0091-6749(96)80161-8. [DOI] [PubMed] [Google Scholar]
- Trautmann A, Akdis M, Kleemann D, et al. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest. 2000;106:25–35. doi: 10.1172/JCI9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg I, Jeddi TM, Matuseviciene G, et al. IL-13 over-expression in skin is not confined to IgE-mediated skin inflammation. Clin Exp Immunol. 1997;109:526–532. doi: 10.1046/j.1365-2249.1997.4671365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T, Oh MH, Oh SY, et al. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J Invest Dermatol. 2009;129:742–751. doi: 10.1038/jid.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.