Abstract
Objectives
We hypothesized an impaired renal endocrine and natriuretic response to volume expansion (VE) in humans with pre-clinical systolic dysfunction (PSD) and pre-clinical diastolic dysfunction (PDD). We further hypothesized that exogenous B-type natriuretic peptide (BNP) could rescue an impaired natriuretic response in PSD and PDD.
Background
Recent reports suggest that in early systolic heart failure (HF), there is an impaired natriuretic response to acute VE.
Methods
PSD was defined as left ventricular ejection fraction < 40% without HF symptoms. PDD was defined as ejection fraction > 50%, moderate to severe diastolic dysfunction by Doppler criteria, and no HF symptoms. A double-blinded, placebo-controlled, crossover study was employed to determine the renal response to VE (0.25 ml/kg/min of normal saline for 60 min) in the presence and absence of exogenous BNP. Twenty healthy control subjects, 20 PSD subjects, and 18 PDD subjects participated.
Results
In healthy control subjects, urinary cyclic guanosine monophosphate (cGMP) and natriuresis increased after VE. In contrast, among PSD and PDD subjects, there was a paradoxical decrease in urinary cGMP and attenuated natriuresis. Pre-treatment with subcutaneous BNP resulted in similar increases in both urinary cGMP and natriuresis among healthy normal, PSD, and PDD subjects.
Conclusions
In PSD and PDD, there is impaired renal cGMP activation, which contributes to impaired natriuresis in response to VE. Impaired activation of urinary cGMP and reduced natriuresis may contribute to volume overload and the progression of HF among PSD and PDD subjects. Importantly, the impaired renal excretory response to VE is rescued by exogenous BNP in PSD and PDD. (J Am Coll Cardiol 2011;58:2095–103) © 2011 by the American College of Cardiology Foundation
Keywords: B-type natriuretic peptide, cyclic guanosine monophosphate, diastolic dysfunction, heart failure, natriuretic peptide, pre-clinical, systolic dysfunction
The American Heart Association/American College of Cardiology define stage B heart failure (HF) as abnormal heart structure/function in the absence of HF symptoms (1). With the advancement of cardiac imaging and biomarkers, abnormal heart structure and function can be detected before the development of symptoms. Pre-clinical, or stage B, HF can represent either diastolic or systolic dysfunction, and both are at increased risk of adverse cardiac events and development of symptomatic HF.
Pre-clinical left ventricular systolic dysfunction (PSD) is increasingly common among the general population and is associated with markedly increased risk of progression to symptomatic HF and death, with a median survival of 7.1 years (2). Further studies suggest that, despite the absence of signs and symptoms of congestion in PSD, there is impairment of cardiorenal and neuroendocrine regulatory systems (3,4). Diastolic HF represents 40% to 50% of subjects with HF (5,6). Similar to PSD, pre-clinical diastolic dysfunction (PDD) is prevalent in the community (7–9). Importantly, we and others have established that subjects with PDD are at increased risk of HF and death similar to that for PSD and higher than for subjects with normal diastolic dysfunction (5).
It is well established that the natriuretic peptide (NP) family, including atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP), play an important role in cardiorenal homeostasis in PSD and HF (10). Specifically, NP receptor antagonism and genetic deletion of the natriuretic peptide receptor-A results in an impaired renal response to volume expansion (VE) and hypertension (11,12). These studies suggest that impairment of the natriuretic peptide system may be integral to the pathophysiologic changes in PSD. However, the integrated cardiorenal and humoral response to achieve sodium homeostasis remains poorly characterized in humans with PSD and is undefined in PDD.
Despite recent advances in the treatment of overt symptomatic HF, mortality and morbidity among patients with HF remains high (13). Further, therapies for delaying the progression to terminal HF are limited. The only recommended therapy for PSD is the use of an angiotensin-converting enzyme inhibitor (14). Reflecting the absence of proven therapies for diastolic HF, the recently published heart failure practice guidelines stress the need to develop and evaluate treatment strategies directed at patients with pre-clinical diastolic dysfunction (15).
Therefore, the broad objective of the current study was to define the integrated cardiorenal response to acute VE in humans with PSD, PDD, and normal cardiac function. We hypothesized that there is an impaired cardiorenal endocrine response to acute VE in PSD and PDD that is characterized by the lack of appropriate activation of urinary cyclic guanosine monophosphate excretion (UcGMPV) and urinary sodium excretion (UNaV). Further, we hypothesized that PSD subjects, PDD subjects, and normal control subjects would respond similarly to exogenous administration BNP, which is the most natriuretic of the natriuretic peptides.
Methods
Study design
We utilized a double-blind, placebo-controlled, crossover protocol to compare the cardiorenal and endocrine response to acute VE (0.9% normal saline, 0.25 ml/kg/min for 1 h) after either subcutaneous (SQ) placebo or BNP administration. This study was approved by the Mayo Foundation institutional review board, and was performed at the General Clinical Research Center at Mayo Clinic, Rochester, Minnesota.
Study population
Participants included 20 normal control subjects, 20 PSD subjects, and 18 PDD subjects. Inclusion criteria for each group are detailed in the following text, and baseline characteristics are reported in Table 1.
Table 1.
Baseline Characteristics of the Study Population
| Normal Controls (n = 20) |
Pre-Clinical Systolic Dysfunction (n = 20) |
Pre-Clinical Diastolic Dysfunction (n = 18) |
||||
|---|---|---|---|---|---|---|
| Age, yrs | 37 | 11 | 65 | 12*† | 72 | 7* |
| Female | 85 | 10*† | 50* | |||
| Body mass index, kg/m2 | 25 | 4 | 31 | 5* | 30 | 5* |
| Heart rate, beats/min | 68 | 12 | 68 | 9† | 60 | 13 |
| Blood pressure, mm Hg | ||||||
| Systolic | 116 | 14 | 119 | 12† | 133 | 15* |
| Diastolic | 74 | 9 | 71 | 8 | 75 | 9 |
| Hypertension | 0 | 35*† | 72* | |||
| Creatinine, mg/dl | 0.9 | 0.1 | 1.1 | 0.2* | 1.1 | 0.3* |
| Blood urea nitrogen, mg/dl | 11 | 5 | 21 | 10* | 23 | 6* |
| Hyperlipidemia | 0 | 75* | 56* | |||
| Diabetes mellitus | 0 | 20 | 11 | |||
| Coronary artery disease | 0 | 65 | 56* | |||
| Myocardial Infarction | 0 | 50 | 33* | |||
| Cardiovascular medications | ||||||
| ACEI or ARB | 0 | 75 | 61* | |||
| Nitrates | 0 | 10 | 6 | |||
| Beta-blocker | 0 | 90*† | 61* | |||
| Digoxin | 0 | 30*† | 0 | |||
| Thiazide diuretic | 0 | 5† | 28* | |||
| Loop diuretic | 0 | 25 | 6 | |||
| Aldosterone antagonist | 0 | 20 | 11 | |||
| Statin | 0 | 80*† | 50* | |||
| LV ejection fraction, % | 65 | 4 | 38 | 11*† | 63 | 6 |
| LA volume index, ml/m2 | 22 | 3 | 35 | 8* | 33 | 10* |
| E/e=, medial | 7.3 | 1.6 | 15.7 | 12.5* | 14.0 | 3.4* |
| RVSP, mm Hg | 24 | 3 | 35 | 9* | 33 | 10* |
| Atrial natriuretic peptide, pg/ml | 21 | 12 | 45 | 28* | 42 | 32* |
| BNP, pg/ml | 19 | 14 | 111 | 147* | 109 | 104* |
| Plasma cGMP, pmol/ml | 2.6 | 1.0 | 4.5 | 2.7* | 3.9 | 1.8* |
Values are mean SD or %.
p < 0.05 versus normal controls, and
p < 0.05 versus pre-clinical diastolic dysfunction as measured by t test for normally distributed continuous variables, the rank-sum test for continuous variables with a skewed distribution, and the chi-square test for independence for categorical variables.
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin-receptor blocker; BNP = B-type natriuretic peptide; cGMP = cyclic guanosine monophosphate; E/e= = E velocity/e= velocity; LA = left atrium; LV = left ventricle; RVSP = right ventricle systolic pressure.
NORMAL CONTROLS
Normal control subjects had an ejection fraction (EF) >50%, normal Doppler diastolic function; no clinical signs, symptoms, or history of cardiovascular or renal disease; and had never been treated with any cardiovascular medications.
PRE-CLINICAL SYSTOLIC DYSFUNCTION
The PSD subjects had an EF <40%; no clinical signs or symptoms of congestive HF; and a minimal distance on 6-min walk test of >450 m. If receiving cardiovascular medications, all doses were stable for at least 2 weeks before the study.
PRE-CLINICAL DIASTOLIC DYSFUNCTION
The PDD subjects had an EF >50%; moderate or severe diastolic dysfunction as assessed by Doppler echocardiography; no clinical signs or symptoms of congestive HF; and a minimal distance on 6-min walk test of >450 m. If receiving cardiovascular medications, all doses were stable for at least 2 weeks before the study.
Echocardiographic assessment
Echocardiographic images were obtained from standard acoustic windows according to the recommendations of the American Society of Echocardiography.
SYSTOLIC FUNCTION
Ventricular volumes (systolic and diastolic) were assessed by Biplane Simpsons (method of disks) analysis obtained from 2 orthogonal cuts of the left ventricle (LV), apical 4- and 2-chamber, as previously described (7). The EF was calculated from the volumes as calculated from the Biplane Simpsons analysis. Stroke volume was calculated as LV diastolic volume minus LV systolic volume.
ASSESSMENT OF LV DIASTOLIC FUNCTION AND LV FILLING PRESSURES
Pulsed-wave Doppler examination of mitral venous inflow (E) (before and with Valsalva maneuver) and pulmonary venous inflow as well as Doppler tissue imaging of the mitral annulus (e=) were performed. Diastolic function was categorized as previously described (7,16,17), according to the progression of diastolic dysfunction (DD): normal; grade 1, impaired relaxation without evidence of increased filling pressures; grade 1a, impaired relaxation with evidence of increased filling pressures (mild DD); grade 2, “pseudonormal” pattern (moderate DD); and grade 3/4, “reversible or fixed restrictive filling” pattern (severe DD). This diastolic function classification scheme has been validated against invasive measurement of diastolic function and filling pressures (17,18) and has been shown to have independent prognostic value in the general population (7).
Study protocol
Before initiation of the study, subjects were stabilized for 1 week on a no added salt diet (120 mEq sodium per day). On the active study day, subjects were given their usual dose of medications and were placed in the supine position for 60 min. Subjects were asked to empty their bladder spontaneously every 30 min (if subjects were unable to void every 30 min, a urinary catheter was placed). Adequate bladder emptying was insured by ultrasonography. Every 30 min throughout the study, subjects drank an amount of water equivalent to the sum of the blood losses and urinary flow. After the 60-min equilibration period, a 30-min baseline clearance was performed. Each clearance included collecting urine samples over 30 min for determination of UcGMPV and UNaV. Blood pressure was measured using an automatic blood pressure cuff, and heart rate was continuously monitored by electrocardiography. Venous blood samples were obtained for ANP, BNP, and cGMP assessment. Echocardiography was performed during the baseline clearance to determine systolic and diastolic function.
Immediately after the baseline clearance was performed, the subjects were randomly assigned to receive either SQ placebo or SQ BNP (Scios, Mountain View, California [for safety reasons, the first 10 patients received 5 J..g/kg and the subsequent patients received 10 J..g/kg]), which was administered in abdominal SQ tissue. The randomization schedule was provided by the Mayo Clinic Division of Biomedical Statistics and Informatics and implemented by the Mayo Clinic Pharmacy. The investigators did not know to which randomization arm subjects were assigned. Fifteen minutes after administration of either placebo or SQ BNP, an acute saline load was administered (normal saline 0.9% 0.25 ml/kg/min for 60 min). Two 30-min clearances were performed during the 60-min period of VE. Echocardiography was repeated immediately after the end of the saline infusion.
All subjects returned 2 weeks after the initial study for the crossover study.
Neurohormonal and electrolyte analysis
Plasma ANP (Phoenix Pharmaceuticals, Mountain View, California) and plasma/urine cGMP (PerkinElmer, Shelton, California) were measured by radioimmunoassay as previously described (19). Plasma BNP was measured by fluorescence immunoassay (Biosite Diagnostics) as described previously (20).
Statistical analysis
Continuous variables were presented as mean SD, and categorical variables as percentage. Comparisons between each of the 3 groups (normal control, PSD, and PDD) and within groups (baseline, 30 min, and 60 min) were made by t tests for normally distributed continuous variables, the rank-sum test for continuous variables with a skewed distribution, and the chi-square test for independence for categorical variables. For selected continuous variables, the overall change from baseline to after volume expansion between groups was compared using an analysis of variance. All p values are corrected for multiple comparisons when performed using a Bonferroni correction. For selected continuous variables, we ran additional comparisons adjusting for age, using an analysis of covariance. For these, the comparison was between the normal group and each of the other 2 groups (PSD and PDD). For all analyses, statistical significance was accepted as p < 0.05.
Results
Study population
Baseline characteristics of the normal control subjects (n = 20), PSD subjects (n = 20), and PDD subjects (n = 18) are shown in Table 1. The PSD and PDD subjects were older, more likely to be male, and more likely to have a higher creatinine, blood urea nitrogen, and body mass index compared to normal controls. The PSD subjects were more likely to be male and younger than PDD subjects but had similar creatinine, blood urea nitrogen, and body mass index measurements. Systolic (but not diastolic) blood pressure and presence of hypertension was higher in PDD subjects compared to normal control and PSD subjects. The presence of coronary artery disease and diabetes mellitus was similar between PSD and PDD subjects. Of note, by study design, normal control subjects lacked any cardiovascular comorbidities and took no cardiovascular medications. Mean LV EF was lower in PSD subjects (38%) than in normal subjects (65%) and PDD subjects (63%). Medial E velocity/e= velocity (E/e=) and right ventricular systolic pressure were significantly higher in both the PSD subjects and PDD subjects compared to normal control subjects, suggesting PSD subjects also had a component of diastolic dysfunction. ANP, BNP, and plasma cGMP were greater in the PSD and PDD subjects compared to normal control subjects, but not different between PSD and PDD subjects.
Response to VE, placebo pre-treatment
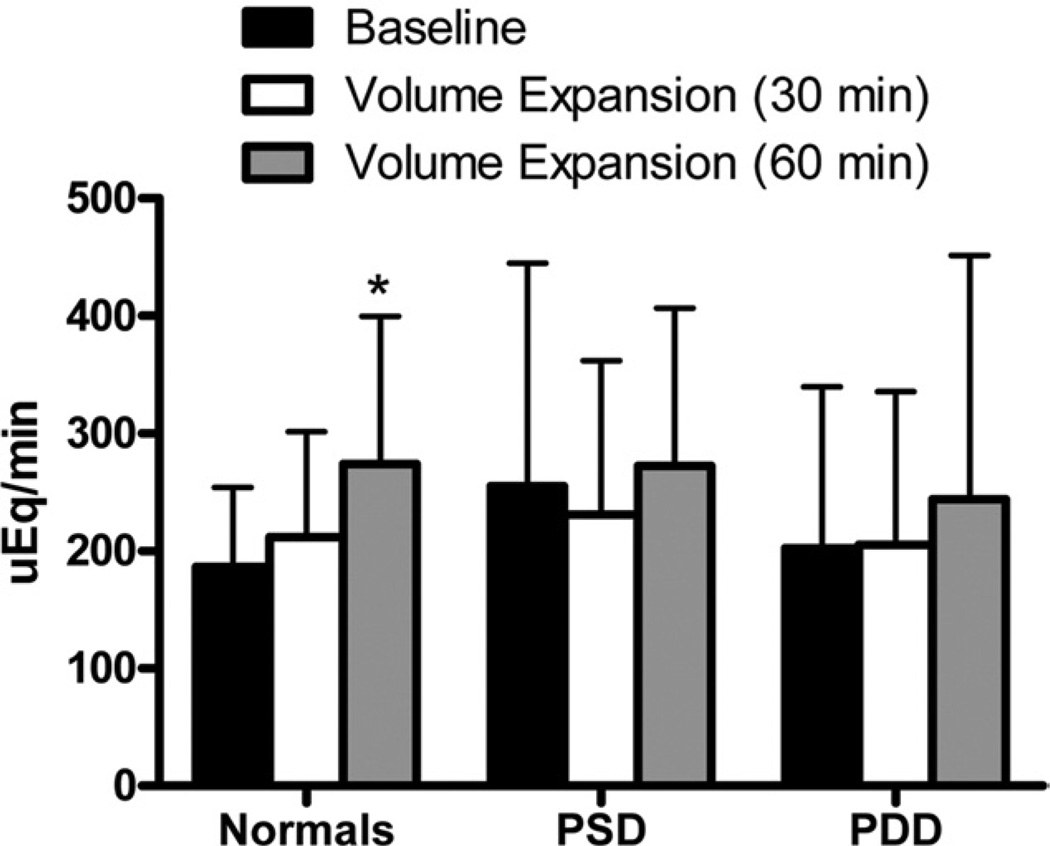
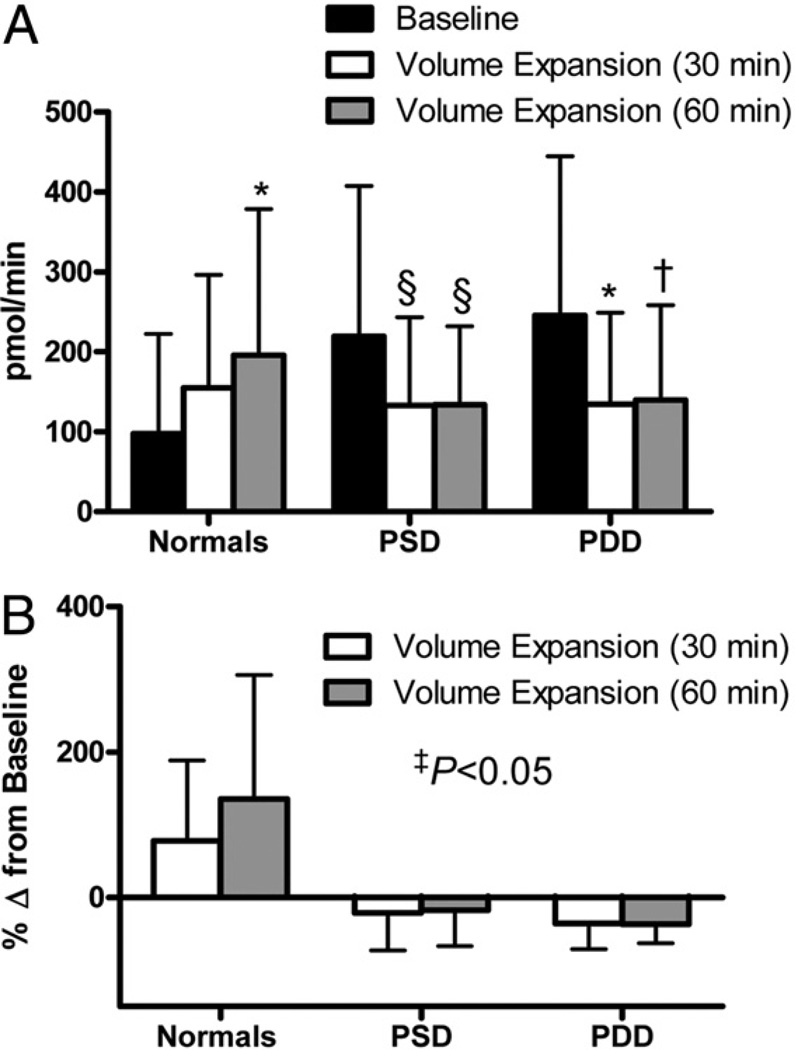
All subjects underwent intravascular VE (normal saline 0.9% 0.25 ml/ kg/min for 1 h). Assessment of renal function was performed at baseline (immediately before VE) and at 30 and 60 min after initiation of VE. Urinary sodium excretion is shown in Figure 1. Among normal control subjects there was a significant increase in UNaV at 60 min. In contrast, there was no significant increase in UNaV in PSD and PDD subjects after VE. The change in UNaV from baseline to 60 min after VE was not significantly different among the 3 groups (normal vs. PSD, p = 0.09; normal vs. PDD, p = 0.59; PSD vs. PDD, p = 0.26). Urinary cGMP (Fig. 2A), which is known to promote natriuresis, was significantly increased in normal control subjects at 60 min. In contrast, there was no increase in UcGMPV among subjects with PSD or PDD. Indeed, there was a strong trend toward reduced urinary cGMP in PSD subjects at 30 and 60 min (p = 0.08 for both timepoints). Similarly, there was a significant reduction and trend toward reduction (p = 0.06) of urinary cGMP in PDD subjects at 30 and 60 min, respectively. When we compare the percent change in urinary cGMP from baseline to after VE, there was a significant difference among the 3 groups (Fig. 2B). Further, the change in UcGMPV from baseline to 60 min after VE was significantly different among normal control subjects compared to PSD and PDD subjects (normal vs. PSD, p < 0.01; normal vs. PDD, p < 0.01; PSD vs. PDD, p = 0.27). Importantly, adjustment for age did not significantly alter the results for both UNaV and UcGMPV.
Figure 1. Urinary Sodium Excretion After Volume Expansion.
Urinary sodium excretion in normal control, pre-clinical systolic dysfunction (PSD), and pre-clinical diastolic dysfunction (PDD) subjects at baseline (black bars), 30 min after volume expansion (white bars), and 60 min after volume expansion (gray bars). *p < 0.05 versus baseline as measured by t test using a Bonferroni correction for multiple comparisons.
Figure 2. Urinary cGMP Excretion After Volume Expansion.
Urinary cyclic guanosine monophosphate (cGMP) excretion (A) and change in urinary cGMP excretion from baseline (B) in normal control subjects (normals), PSD subjects, and PDD subjects at baseline (black bars), 30 min after volume expansion (white bars), and 60 min after VE (gray bars). *p < 0.05, †p = 0.06, §p = 0.08 versus baseline as measured by t test using a Bonferroni correction for multiple comparisons. ‡The p value represents comparison of change from baseline to VE between groups as measured by 1-way analysis of variance. Abbreviations as in Figure 1.
Plasma ANP levels decreased nonsignificantly in PSD and PDD subjects after VE. In contrast, plasma ANP levels were stable in normal control subjects after VE. The percent changes in plasma ANP 60 min after VE compared to baseline were 9.1 53.9%, −10.8 40.5%, and −16.2 33.7% for normal control subjects, PSD subjects, and PDD subjects, respectively; there was trend toward a differential response among the 3 groups but it did not achieve statistical significance (p = 0.18). Plasma BNP and cGMP levels were not significantly altered in any of the 3 groups after VE (Table 2).
Table 2.
Echocardiographic and Humoral Assessment at Baseline and After Volume Expansion
| Normal (n = 20) |
PSD (n = 20) |
PDD (n = 18) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Volume | Expansion | Baseline | Volume | Expansion | Baseline | Volume | Expansion | ||||
| EF, % | 64.7 | 4.4 | 66.9 | 3.1 | 37.9 | 11.3 | 42.4 | 10.7 | 63.3 | 6.0 | 66.6 | 4.7 |
| SV, ml/beat | 85.9 | 14.2 | 93.3 | 13.5 | 93.7 | 18.9 | 99.3 | 18.7 | 100.2 | 23.3 | 105.4 | 26.0 |
| E/e=, medial | 7.3 | 1.6 | 7.2 | 1.3 | 15.7 | 12.5 | 16.7 | 12.7 | 14.0 | 3.4 | 14.7 | 4.3 |
| RVSP, mm Hg | 23.7 | 3.4 | 24.4 | 2.9 | 34.8 | 9.3 | 33.8 | 10.9 | 33.1 | 9.6 | 33.9 | 8.3 |
| BNP, pg/ml | 19 | 14 | 20 | 15 | 111 | 147 | 109 | 137 | 109 | 104 | 101 | 92 |
| Plasma cGMP, pmol/ml | 2.6 | 1.0 | 2.3 | 1.5 | 4.5 | 2.7 | 5.5 | 8.3 | 3.9 | 1.8 | 3.4 | 1.3 |
Values are mean SD. Echocardiographic, BNP, and cGMP assessment was performed at baseline (before volume expansion) and immediately after volume expansion. There were no significant differences in echocardiographic assessment, BNP, and cGMP from baseline to volume expansion in the normal control, PSD, or PDD groups as measured by t test for normally distributed continuous variables and by the rank-sum test for continuous variables with a skewed distribution.
EF = ejection fraction; PDD = pre-clinical diastolic dysfunction; PSD = pre-clinical systolic dysfunction; SV = stroke volume; other abbreviations as in Table 1.
Echocardiographic parameters at baseline and 60 min after the initiation of VE are shown in Table 2. Measures of systolic (EF and stroke volume) and diastolic (medial E/e= and right ventricular systolic pressure) function were not significantly altered after VE in normal control, PSD, or PDD subjects.
Volume expansion and BNP pre-treatment
Utilizing a double-blind, placebo-controlled study design, we assessed whether pre-treatment with SQ BNP attenuated the impaired natriuretic and urinary cGMP response observed in PSD and PDD subjects. With BNP pre-treatment, plasma BNP and cGMP significantly increased in all 3 groups (p < 0.05 vs. baseline at 30 and 60 min) (Table 3).
Table 3.
Echocardiographic and Humoral Assessment After Volume Expansion and Placebo or BNP Treatment
| Normal (n = 20) |
PSD (n = 20) |
PDD (n = 18) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volume Expansion and Placebo |
Volume Expansion and SQ BNP |
Volume Expansion and Placebo |
Volume Expansion and SQ BNP |
Volume Expansion and Placebo |
Volume Expansion and SQ BNP |
|||||||
| EF, % | 66.9 | 3.1 | 68.0 | 3.6 | 42.4 | 10.7 | 43.2 | 9.7 | 66.6 | 4.7 | 67.3 | 4.4 |
| SV, ml/beat | 93.3 | 13.5 | 85.5 | 13.0 | 99.3 | 18.7 | 95.6 | 21.1 | 105.4 | 26.0 | 102.4 | 25.4 |
| E/e=, medial | 7.2 | 1.3 | 7.2 | 1.5 | 16.7 | 12.7 | 11.9 | 1.3 | 14.7 | 4.3 | 12.2 | 4.7 |
| RVSP, mm Hg | 24.4 | 2.9 | 22.5 | 3.3 | 33.8 | 10.9 | 30.5 | 9.9 | 33.9 | 8.3 | 32.6 | 11.0 |
| BNP, pg/ml | 20 | 15 | 196 | 273* | 109 | 137 | 541 | 757* | 101 | 92 | 663 | 1,550* |
| Plasma cGMP, pmol/ml | 2.3 | 1.5 | 12.8 | 20.7* | 5.5 | 8.3 | 9.6 | 6.5* | 3.4 | 1.3 | 10.4 | 8.5* |
Values are mean SD. Echocardiographic, BNP, and cGMP assessment was performed immediately after volume expansion and placebo or SQ BNP pre-treatment.
p < 0.05 compared to volume expansion and placebo as measured by t test for normally distributed continuous variables and the rank-sum test for continuous variables with a skewed distribution.
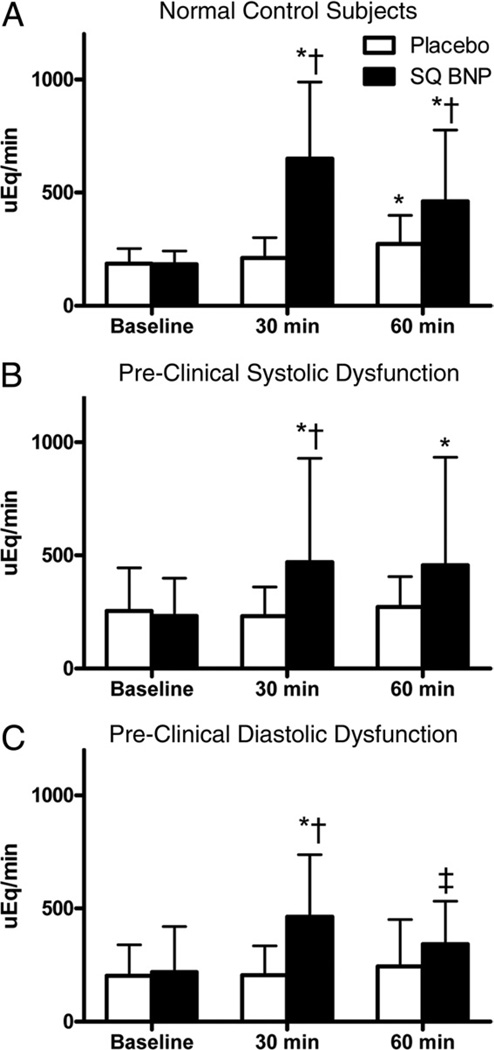
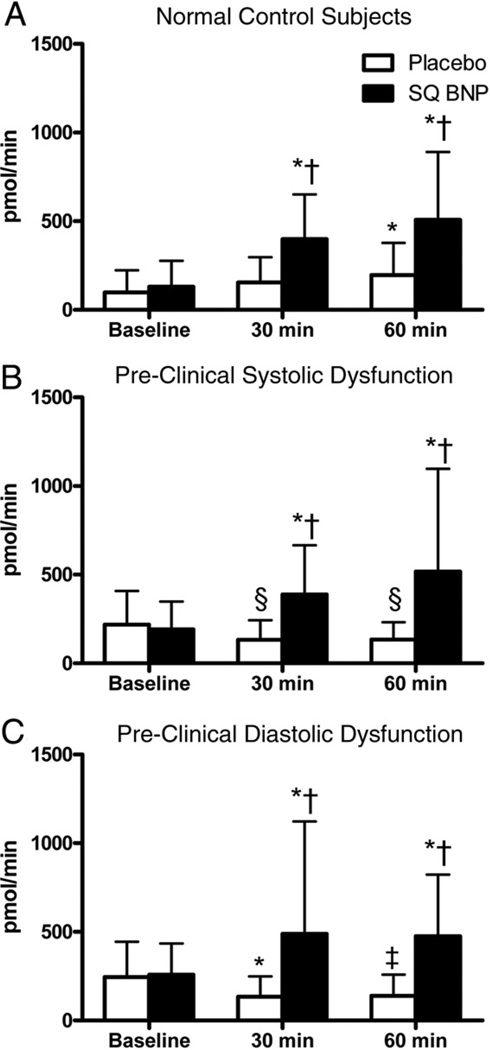
Urinary sodium excretion after VE and no pre-treatment (placebo) and VE and BNP pre-treatment (BNP) is shown in Figure 3. Among normal control subjects, there was significantly greater natriuresis with BNP pre-treatment compared to placebo. Further, and in contrast to VE alone, BNP pre-treatment significantly increased UNaV at 30 and 60 min among PSD subjects and at 30 min among PDD subjects (with a strong trend at 60 min, p = 0.07). Similar to UNaV, and whereas there was a trend toward reduction in UcGMPV among PSD and PDD subjects with placebo, there were significant increases in urinary cGMP at 30 and 60 min among PSD and PDD subjects with BNP pre-treatment (Fig. 4). Importantly, the response to BNP pre-treatment was similar among all 3 groups for UNaV and UcGMPV (baseline compared to 60 min; p > 0.05 for all between-group comparisons).
Figure 3. Urinary Sodium Excretion After Volume Expansion With BNP Pre-Treatment.
Urinary sodium excretion in (A) normal control subjects, (B) PSD subjects, and (C) PDD subjects at baseline, 30 min after volume expansion, and 60 min after volume expansion, after placebo (open bars) or subcutaneous B-type natriuretic peptide (SQ BNP) (solid bars) administration. *p < 0.05, ‡p = 0.07 versus baseline, and †p < 0.05 versus placebo as measured by t test using a Bonferroni correc- tion for multiple comparisons. Abbreviations as in Figure 1.
Figure 4. Urinary cGMP Excretion After Volume Expansion With BNP Pre-Treatment.
Urinary cyclic guanosine monophosphate (cGMP) excretion in (A) normal control subjects, (B) PSD subjects, and (C) PDD subjects at baseline, 30 min after volume expansion, and 60 min after volume expansion, after placebo (open bars) or subcutaneous B-type natriuretic peptide (SQ BNP) (solid bars) administration. *p < 0.05, ‡p = 0.06, §p = 0.08 versus baseline, and †p < 0.05 versus placebo as measured by t test using a Bonferroni correction for multiple comparisons. Abbreviations as in Figure 1.
Echocardiographic parameters after VE with placebo and BNP in all 3 groups are shown in Table 3. There was a trend for EF to increase and medial E/e= and right ventricular systolic pressure to decrease with BNP pre-treatment in all 3 groups, but this did not reach statistical significance. However, in both the PSD group and the PDD group, pre-treatment with BNP resulted in an improvement of diastolic function as assessed by Doppler criteria (see Methods). In the PSD group, 6 (30%) subjects had an improvement of diastolic function by 1 grade with BNP pre-treatment compared to 2 (10%) subjects with placebo. In the PDD group, 10 (56%) subjects had an improvement of diastolic function by 1 grade with BNP pre-treatment compared to no subjects with placebo.
Discussion
This study is the first to describe the cardiorenal response to VE in PDD subjects and compare it to PSD and normal subjects. Our results suggest that the renal response to VE is similarly impaired in both PSD and PDD subjects when compared to normal subjects. Specifically, renal cGMP activation paradoxically decreased among PSD and PDD subjects in response to acute VE, in contrast to an increase in normal subjects. The lack of activation of renal cGMP among PSD and PDD subjects was associated with no significant change in natriuresis compared to a significant increase among normal subjects. Importantly, administration of exogenous subcutaneous BNP before VE significantly increased urinary cGMP and natriuresis among PSD and PDD subjects in a manner similar to normal subjects. These results suggest an impaired natriuretic response to VE in both PSD and PDD that is rescued by exogenous BNP administration.
Underscoring the importance of understanding the pathophysiology of pre-clinical HF is that, despite advances in the treatment of HF, mortality and morbidity remain high (13). Importantly, previous studies demonstrate that although subjects with early HF may be asymptomatic, they have abnormalities in cardiorenal and humoral adaptations to sodium loading (3,21). Additional reports suggest that subjects with PSD have a reduced ability to increase plasma ANP in response to sodium loading, therefore exhibiting a tendency to retain sodium (11). The importance of the natriuretic peptide system in the response to VE in PSD is further underscored by evidence that antagonism of the natriuretic peptide receptor-A in an animal model was associated with significant urinary retention (10). In contrast to PSD, there is little information regarding the pathophysiology of PDD, and to date, there are no reports regarding the response to VE in PDD.
In this study, we report the cardiorenal response to acute VE in PDD subjects. Specifically, PDD subjects have a similarly impaired natriuretic response to VE as do PSD subjects when compared to normal control subjects. This blunted natriuretic response was associated with impaired renal activation of cGMP. Although we cannot exclude the possibility that PSD and PDD subjects may have a delayed natriuretic response to VE, even a delayed response to VE may have important negative sequelae on both renal and cardiac function.
The mechanism for the lack of increase in renal cGMP activation and natriuresis among PSD and PDD subjects is likely multifactorial. First, our results suggest a trend toward a divergent ANP response in PSD and PDD subjects compared to normal subjects, and decreased plasma ANP could account for the lack of activation of urinary cGMP among PSD and PDD subjects. A second mechanism may be related to up-regulation of phosphodiesterase type 5 in PSD and PDD, which would result in greater degradation of cGMP and impaired natriuresis (22). A third potential mechanism would be down-regulation of natriuretic peptide receptor-A in the kidney, leading to an impaired urinary cGMP response (23). It is also possible that the blunted natriuretic and urinary cGMP response in PSD and PDD subjects may be partially secondary to the lack of increased LV filling pressures after VE. However, despite the lack of increased LV filling pressures in normal control subjects, there was increased natriuresis and activation of urinary cGMP, suggesting that mechanisms beyond myocardial stretch (independent of the heart and cardiac filling pressures) may account for the blunted response in PSD and PDD. This finding is supported by the observation that in normal control subjects, there was no increase in plasma cGMP but significant increases in urinary cGMP, whereas among PSD and PDD subjects, there was no change in plasma cGMP but there were decreases in urinary cGMP after VE. A final potential mechanism is the up-regulation of neutral endopeptidase, which degrades the natriuretic peptides and is found most abundantly in the renal tubular cells (24).
In the second aim of the current study, we show that SQ BNP is able to rescue the impaired renal activation of cGMP and natriuresis after VE in PSD and PDD subjects. Specifically, SQ BNP is capable of significantly increasing urinary cGMP as well as natriuresis in PSD and PDD subjects to levels comparable to those of normal controls. These results suggest that, while there is impaired renal activation of cGMP in PSD and PDD, administration of SQ BNP is able to overcome this potential pathophysiologic alteration and increase natriuresis. It is important to note that whereas there were trends toward decreased right ventricular systolic pressure and medial E/e= after SQ BNP, they did not achieve statistical significance. It is possible that with longer duration of treatment, the sodium excretion properties of SQ BNP would result in significant changes in LV filling pressures. In combination with previous studies, which reported that ANP improved the renal response to VE in humans with symptomatic HF treated with an angiotensin-converting enzyme inhibitor (25), these findings suggest that potentiating the natriuretic peptide system may improve cardiorenal and humoral function in PSD and PDD.
Study limitations
The current study has several limitations. First are the different baseline characteristics of the normal, PSD, and PDD subjects. It is possible that the baseline differences in age and sex as well as comorbidities confound the study’s results. However, the baseline characteristics of our patient population are consistent with those of previous epidemiological studies demonstrating that there are significant differences in age and sex as well as in comorbidities between PSD subjects and PDD subjects (7). By study design, the normal control subjects lacked any cardiovascular comorbidities and took no cardiovascular medications to enable a comparison of PSD and PDD subjects to truly normal control subjects. Importantly, adjustment for age in our analysis did not significantly alter our results. A second limitation is that we did not define a mechanism for the impaired renal cGMP activation, and further studies will be required to clarify a mechanism. A third limitation is that, in the current study, we assessed a 1-time dose of SQ BNP, and future studies will be required to determine the effects of chronic SQ BNP therapy in pre-clinical HF.
Conclusions
In summary, we report that both PSD and PDD are characterized by a lack of activation of urinary cGMP and natriuresis in response to VE. The lack of appropriate natriuresis may contribute to volume overload and play a role in the progression and development of symptomatic HF. Importantly, administration of SQ BNP resulted in significant activation of urinary cGMP and natriuresis among PSD and PDD subjects comparable to that of normal control subjects. These findings warrant further studies to determine whether chronic SQ BNP administration can prevent the progression of PSD or PDD to symptomatic HF.
Acknowledgments
This research was supported by grants from the National Institutes of Health PO1 HL 76611, R01 HL 84155, and NIH/NCRR CTSA Grant Number UL1 RR024150. Dr. Simari has received research funding and royalties from Anexon, Inc. and is the inventor of related technology. Dr. Chen has received research grants from Scios Inc., Niles Therapeutics, and Anexon; and through Mayo Clinic has patented and licensed designer natriuretic peptides other than BNP.
Abbreviations and Acronyms
- ANP
atrial natriuretic peptide
- BNP
B-type natriuretic peptide
- cGMP
cyclic guanosine monophosphate
- DD
diastolic dysfunction
- EF
ejection fraction
- HF
heart failure
- LV
left ventricle
- PDD
pre-clinical diastolic dysfunction
- PSD
pre-clinical systolic dysfunction
- SQ
subcutaneous
- UcGMPV
urinary cyclic guanosine monophosphate excretion
- UNaV
urinary sodium excretion
- VE
volume expansion
Footnotes
Subcutaneous B-type natriuretic peptide was provided by Scios (Mountain View, California) at no cost to the authors; company representatives had no role in the design of the trial, access to the data, or input into the manuscript.
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;53:1343–1382. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 3.Volpe M, Tritto C, De Luca N, et al. Failure of atrial natriuretic factor to increase with saline load in patients with dilated cardiomyopathy and mild heart failure. J Clin Invest. 1991;88:1481–1489. doi: 10.1172/JCI115458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volpe M, Tritto C, DeLuca N, et al. Abnormalities of sodium handling and of cardiovascular adaptations during high salt diet in patients with mild heart failure. Circulation. 1993;88:1620–1627. doi: 10.1161/01.cir.88.4.1620. [DOI] [PubMed] [Google Scholar]
- 5.Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–2289. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 6.Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol. 1995;26:1565–1574. doi: 10.1016/0735-1097(95)00381-9. [DOI] [PubMed] [Google Scholar]
- 7.Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ., Jr Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 8.Chen HH, Lainchbury JG, Senni M, Bailey KR, Redfield MM. Diastolic heart failure in the community: clinical profile, natural history, therapy, and impact of proposed diagnostic criteria. J Card Fail. 2002;8:279–287. doi: 10.1054/jcaf.2002.128871. [DOI] [PubMed] [Google Scholar]
- 9.Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006;92:1259–1264. doi: 10.1136/hrt.2005.080150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens TL, Burnett JC, Kinoshita M, Matsuda Y, Redfield MM., Jr A functional role for endogenous atrial natriuretic peptide in a canine model of early left ventricular dysfunction. J Clin Invest. 1995;95:1101–1108. doi: 10.1172/JCI117757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez MJ, Wong SK, Kishimoto I, et al. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995;378:65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 12.Oliver PM, Fox JE, Kim R, et al. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci U S A. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 14.The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 15.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–e82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosetta Stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 17.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura RA, Appleton CP, Redfield MM, Ilstrup DM, Holmes DR, Jr, Tajik AJ. Noninvasive Doppler echocardiographic evaluation of left ventricular filling pressures in patients with cardiomyopathies: a simultaneous Doppler echocardiographic and cardiac catheterization study. J Am Coll Cardiol. 1996;28:1226–1233. doi: 10.1016/S0735-1097(96)00315-4. [DOI] [PubMed] [Google Scholar]
- 19.Burnett JC, Jr, Kao PC, Hu DC, et al. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–1147. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 20.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 21.Volpe M, Tritto C, Mele AF, et al. Impairment of atrial natriuretic factor response to acute saline load in hypertensives with family history of cardiovascular accidents. J Hypertens. 1991;9(Suppl):254–255. [PubMed] [Google Scholar]
- 22.Chen HH, Huntley BK, Schirger JA, Cataliotti A, Burnett JC., Jr Maximizing the renal cyclic 3’–5’-guanosine monophosphate system with type V phosphodiesterase inhibition and exogenous natriuretic peptide: a novel strategy to improve renal function in experimental overt heart failure. J Am Soc Nephrol. 2006;17:2742–2747. doi: 10.1681/ASN.2006020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickey DM, Flora DR, Bryan PM, Xu X, Chen Y, Potter LR. Differential regulation of membrane guanylyl cyclases in congestive heart failure: natriuretic peptide receptor (NPR)-B, not NPR-A, is the predominant natriuretic peptide receptor in the failing heart. Endocrinology. 2007;148:3518–3522. doi: 10.1210/en.2007-0081. [DOI] [PubMed] [Google Scholar]
- 24.Chen HH, Schirger JA, Chau WL, et al. Renal response to acute neutral endopeptidase inhibition in mild and severe experimental heart failure. Circulation. 1999;100:2443–2448. doi: 10.1161/01.cir.100.24.2443. [DOI] [PubMed] [Google Scholar]
- 25.Tomiyama H, Watanabe G, Abe M, Okazaki R, Yoshida H, Doba N. Systemic and renal effects of atrial natriuretic peptide in patients with heart failure treated with angiotensin-converting enzyme inhibitor or in acute saline solution loading. Am Heart J. 2001;141:422–427. doi: 10.1067/mhj.2001.112782. [DOI] [PubMed] [Google Scholar]