B-type natriuretic peptide (BNP) is a cardiac derived peptide hormone, which consists of a 17-AA ring structure created by a disulfide bond joining two cysteine residues with distinct N and C terminal extensions. BNP binds to its particulate guanylyl cyclase receptor (GC) -A to activate the second messenger cyclic guanylyl-monophosphate (cGMP). Studies have established widespread pleuripotent actions which result in natriuresis, vasorelaxation, inhibition of renin and aldosterone, enhanced myocardial relaxation, inhibition of fibrosis and hypertrophy, promotion of cell survival, angiogenesis, and inhibition of inflammation.1,2 The clinical therapeutic potential of BNP continues to emerge with studies demonstrating it protects against diabetic nephropathy when genetically overexpressed by gene transfer,3 lowers blood pressure by oral delivery in experimental hypertension4 and is cardioprotective when administered to humans undergoing cardiopulmonary bypass surgery.5
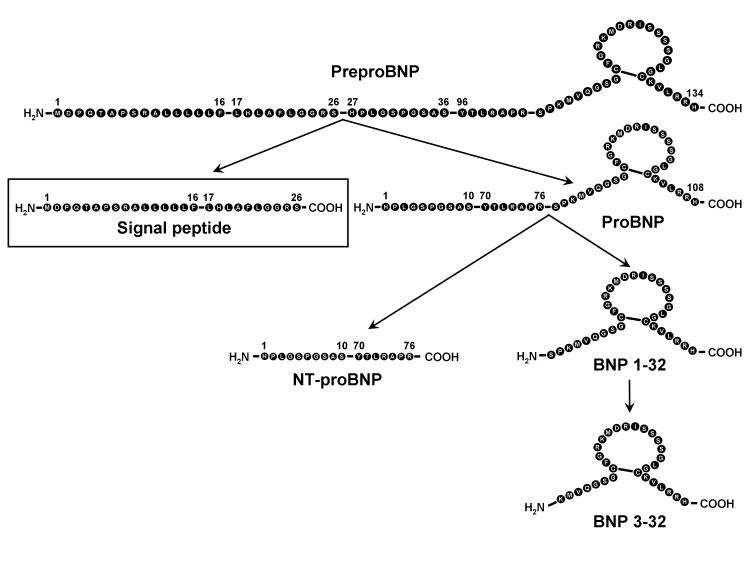
Most recently studies have focused on the processing of BNP from its production to its degradation. BNP is produced as a preprohormone, which subsequently is processed into a prohormone by cleavage of an N-terminal signal peptide (Figure 1). Human preproBNP is a 134-AA peptide which is cleaved to the 108-AA proBNP.6 Processing of proBNP to mature BNP is mediated by corin while a role for furin has been implicated.7,8 Human BNP, a 32-AA peptide, is released from the myocardium in response to various physiologic and pathophysiologic stimuli, such as myocardial wall stretch with evidence that myocardial ischemia releases BNP.9 Once released BNP is cleared by the natriuretic peptide clearance receptor (NPR-C) which is widely expressed.1 Importantly, BNP is degraded by neutral endopeptidase (NEP) 24.1110 but also by dipeptidyl peptidase IV (DPPIV),11 the latter resulting in a novel BNP 3-32 which possesses altered cardiorenal actions and may uniquely function at the tissue level as a local acting BNP.
Figure 1.
PreproBNP amino acid sequences and processing. This figure illustrates the cleavage of BNP signal peptide which reported by Siriwardena et al, proBNP processing into mature BNP1-32, and its degradation to BNP 3-32.
Today, BNP is a widely used worldwide as a biomarker for heart failure (HF). Using state of the art FT-ICR mass spectrometry, in human HF, much of plasma BNP immunoreactivity measured by commonly used assays is due to altered circulating molecular forms with reduced cGMP-activating properties.12 We now know that BNP circulates as in various forms – its precursor proBNP1-108, mature BNP 1-32, N-terminal peptide proBNP, and BNP 3-32. Importantly in vitro analysis reported that only BNP 1-32 and BNP 3-32 could stimulate cGMP production in human cardiac fibroblasts and cardiomyocytes.13 Thus, patients with HF have low circulating “functional” BNP 1-32 levels while other non-functional BNPs, including proBNP levels, are higher than normal subjects.12,14 This functional deficiency state of active BNP may affect the progression of HF and the remodeling process. The utility of BNP (especially proBNP 1-108, mature BNP 1-32 and NT-proBNP) continues to grow with its use now as a prognostic biomarker for future adverse cardiovascular outcomes15, to guide therapy in HF,16 and as a biomarker for myocardial injury.17,18
Of great interest is the report by Siriwardena et al in the current issue (19), which advances our knowledge of the biology of BNP as well as its role as a biomarker for cardiovascular disease. These investigators explored the novel idea that the signal peptide of BNP (BNPsp) is secreted by the human heart and circulates in normal human subjects. They further tested the hypothesis that BNPsp would be a cardiac biomarker for the diagnosis of myocardial ischemia and injury. As noted in Figure 1, BNPsp is the N-terminal 26 amino acid sequence of preproBNP1-134. As stated above, proBNP1-108 or other BNP molecular forms except BNPsp have been reported to circulate in humans. In the current report, the authors had the innovative thought that BNPsp is also cleaved into a C-terminal 10 amino acid BNPsp17-26 by proteolysis as signal peptides in general are degraded by a signal peptide peptidase in the endoplasmic reticulum (ER) membrane as reported previously for other preprohormones. They next generated a specific radioimmunoassay with their own specific antibody, which would recognize human BNPsp17-26. It is also possible that their antibody also recognizes the complete intact 26 amino acid BNPsp requiring further studies including peptide isolation and sequence analysis.
In a most comprehensive approach, Siriwardena and co-workers performed a series of studies in human myocardial tissue, in normal human subjects and in humans with cardiovascular and renal disease. They first demonstrated that they could detect BNPsp17-26 in the extracts from human heart tissue specifically in both atrial and ventricular myocardium and like mature BNP 1-32, BNPsp17-26 was higher in atrial myocardium. The authors then reported that BNPsp17-26 was present in the circulation in normal humans and is secreted from the normal human heart. But surprisingly, they also report BNPsp17-26 is secreted from the head, kidney and lower limbs and cleared by the liver. This observation is in stark contrast to their data in the paper in which there was no evidence for mature BNP as determined with their NT-proBNP assay for secretion outside the heart.
The meaning of these data is unclear at the moment and will require further studies. However, they change our thinking regarding BNP especially in its processing and possibly where it may be produced. First, to our surprise, BNP’s signal peptide (BNPsp17-26) is secreted into the circulation. Conventional wisdom is that a signal peptide functions only intracellularly to translocate a preprohormone from site of production into the ER for processing to its prohormone and then ultimately to its release from the cell. Importantly, with rare exception, the signal peptide remains in the cell and is degraded, and then resynthesized and used. The current paper changes our thinking and suggests that a signal peptide like BNPsp17-26 is actually released and circulates. At this point one can conclude that this secretion/release could be a nonspecific cellular release or overflow of this peptide with no specific biological meaning. In contrast one could ask the speculative question of where BNPsp or BNPsp17-26 has an intrinsic function. The second part of this biological BNP conundrum from the current human studies is now the possibility that BNP is synthesized outside of the heart based upon a step up of BNPsp17-26 levels across the head, kidney, and lower limb in addition to the heart. This raises the compelling concept recently advanced by Kuhn et al that BNP may be synthesized locally in ischemic tissue outside of the heart in satellite cells to induce local angiogenesis(20 ).Studies clearly are needed to explore possible production of BNP in these important regions of the body outside the heart and address this new BNP biology provided by the findings of this important paper.
Most clinically relevant is the report in the current paper of circulating BNPsp17-26 in human STEMI. Specifically, BNPsp17-26 was increased in human STEMI. Further, this elevation was highly specific in terms of when BNPsp17-26 was increased as it related to the phase of STEMI. The authors found its increase only during the acute phase of STEMI (4 to 6 hours) after which it normalized while NT-proBNP was not increased during this initial acute phase but increased only after 12 hours. Further, BNPsp17-26 was increased before either Myoglobin C or Troponin I. Indeed, ROC analysis of BNPsp17-26 at 5 hours gave high sensitivity and specificity equivalent to Troponin I. The authors concluded that BNPsp17-26 might be a new and robust biomarker for early stage-myocardial infarction.
Again, these most interesting observations of BNPsp17-26 in STEMI raise important questions. Why was there an increase in BNPsp17-26 so rapidly with the onset of myocardial injury that preceded other well-established biomarkers for injury such as troponin? One could speculate that BNPsp and/or BNPsp17-26 is abundant in the cardiomyocyte and with any leakiness in the cell membrane BNPsp17-26 is leaked and then normalizes as it is depleted. A more risky speculation is that ischemia induces a novel secretory pathway to explain the current findings. It should be noted that BNPsp17-26 was not elevated when first measured in patients with STEMI but increased at 4 hours after STEMI elevation so that one still cannot say that BNPsp17-26 is acutely released at the very onset of myocardial ischemia and/or injury.
From a clinical perspective of biomarkers for STEMI, the current findings lay the foundation for more extensive human trials. As the authors emphasize, the current studies are small with only 25 subjects. A larger trial of STEMI and also acute coronary syndrome (ACS) is required. Not only do we need reconfirmation of the early activation of BNPsp17-26 but we also need to define its relationship to reperfusion, infarct size, outcomes, and its relationship to mature BNP, NT-proBNP, proBNP and other cardiac biomarkers. If the current findings are confirmed then BNPsp17-26 may significantly increase our armamentarium of cardiac biomarkers for myocardial ischemia and injury.
It should be stated that surprisingly BNPsp17-26 was not increased in the circulation in HF in contrast to NT-proBNP, BNP or proBNP. Again such an observation has biological and clinical relevance and implications for BNPsp17-26 as a biomarker. The lack of increase suggests the absence of regulated release and/or depletion of BNPsp17-26 with high rates of BNP synthesis. This lack of increase therefore excludes BNPsp17-26 as a biomarker for HF but more studies are needed in terms of etiology of HF (ischemic versus nonischemic), severity of HF and systolic versus diastolic HF. The elevation of plasma BNPsp17-26 in chronic kidney disease requires one statement and that is the mechanism could be related to decrease renal clearance or more intriguingly increased renal production. Again further studies are needed to address this interesting issue.
The authors are to be congratulated for such a comprehensive study from biology to biomarkers, which significantly advances the fields of BNP, and cardiac biomarkers. Conventionally, prepro-proteins including signal peptides are firstly synthesized and the signal peptide is recognized and deciphered by cellular sorting and translocation machinery. Prepro-proteins are then transported to numerous destinations, such as the nucleus, the ER, the Golgi apparatus, and the plasma membrane. After recognizing the signal peptide, the signal peptide is removed by specialized signal peptidases, and then the mature part of protein is thereby released, and the divided signal peptide is then degraded by signal peptide peptidases(21). Siriwardena and co-workers provide us evidence that this may not be the entire case for preproBNP. Specifically in the human, BNPsp (or BNP17-26), the signal peptide for human preproBNP, is released from the cardiomyocyte (presumably both atrial and ventricular) and circulates. The full biological significance of this seminal observation (i.e., regulation and function) remains to be defined. Of most clinical relevance is the plasma elevation of BNPsp17-26 with the acute phase of STEMI preceding standard biomarkers of myocardial injury. This key observation offers a rare opportunity for a new and novel biomarker for myocardial injury, which has the potential to enhance the care of patients with STEMI and reduce the burden of human cardiovascular disease. Thus once again, the field of natriuretic peptides has just gotten more interesting.
Acknowledgments
Sources of Funding This work was supported by National Institutes of Health grants PO1 HL7611 and RO1 HL36634.
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 2.Garbers DL, Chrisman TD, Wiegn P, Katafuchi T, Albanesi JP, Bielinski V, Barylko B, Redfield MM, Burnett JC., Jr. Membrane guanylyl cyclase receptors: an update. Trends Endocrinol Metab. 2006;17:251–258. doi: 10.1016/j.tem.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makino H, Mukoyama M, Mori K, Suganami T, Kasahara M, Yahata K, Nagae T, Yokoi H, Sawai K, Ogawa Y, Suga S, Yoshimasa Y, Sugawara A, Tanaka I, Nakao K. Transgenic overexpression of brain natriuretic peptide prevents the progression of diabetic nephropathy in mice. Diabetologia. 2006;49:2514–24. doi: 10.1007/s00125-006-0352-y. [DOI] [PubMed] [Google Scholar]
- 4.Cataliotti A, Chen HH, Schirger JA, Martin FL, Boerrigter G, Costello-Boerrigter LC, James KD, Polowy K, Miller MA, Malkar NB, Bailey KR, Burnett JC., Jr. Chronic actions of a novel oral B-type natriuretic peptide conjugate in normal dogs and acute actions in angiotensin II-mediated hypertension. Circulation. 2008;118:1729–36. doi: 10.1161/CIRCULATIONAHA.107.759241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mentzer RM, Jr., Oz MC, Sladen RN, Graeve AH, Hebeler RF, Jr., Luber JM, Jr., Smedira NG. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery:the NAPA Trial. J Am Coll Cardiol. 2007;49:716–26. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 6.Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 7.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A. 2000;97:8525–9. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawada Y, Inoue M, Kanda T, Sakamaki T, Tanaka S, Minamino N, Nagai R, Takeuchi T. Co-elevation of brain natriuretic peptide and proprotein-processing endoprotease furin after myocardial infarction in rats. FEBS Lett. 1997;400:177–82. doi: 10.1016/s0014-5793(96)01385-3. [DOI] [PubMed] [Google Scholar]
- 9.Goetze JP, Christoffersen C, Perko M, Arendrup H, Rehfeld JF, Kastrup J, Nielsen LB. Increased cardiac BNP expression associated with myocardial ischemia. Faseb J. 2003;17:1105–7. doi: 10.1096/fj.02-0796fje. [DOI] [PubMed] [Google Scholar]
- 10.Kenny AJ, Bourne A, Ingram J. Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C-type natriuretic peptide and some C-receptor ligands by endopeptidase-24.11. Biochem J. 1993;291(Pt 1):83–8. doi: 10.1042/bj2910083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boerrigter G, Costello-Boerrigter LC, Harty GJ, Lapp H, Burnett JC., Jr. Des-serine-proline brain natriuretic peptide 3-32 in cardiorenal regulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R897–901. doi: 10.1152/ajpregu.00569.2006. [DOI] [PubMed] [Google Scholar]
- 12.Hawkridge AM, Heublein DM, Bergen HR, 3rd, Cataliotti A, Burnett JC, Jr., Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci U S A. 2005;102:17442–7. doi: 10.1073/pnas.0508782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heublein DM, Huntley BK, Boerrigter G, Cataliotti A, Sandberg SM, Redfield MM, Burnett JC., Jr. Immunoreactivity and guanosine 3′,5′-cyclic monophosphate activating actions of various molecular forms of human B-type natriuretic peptide. Hypertension. 2007;49:1114–9. doi: 10.1161/HYPERTENSIONAHA.106.081083. [DOI] [PubMed] [Google Scholar]
- 14.Liang F, O’Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, Apple FS, Maisel AS, Pollitt NS, Protter AA. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol. 2007;49:1071–8. doi: 10.1016/j.jacc.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 15.McKie PM, Cataliotti A, Lahr BD, Martin FL, Redfield MM, Bailey KR, Rodeheffer RJ, Burnett JC., Jr. The prognostic value of N-terminal pro-B-type natriuretic peptide for death and cardiovascular events in healthy normal and stage A/B heart failure subjects. J Am Coll Cardiol. 55:2140–7. doi: 10.1016/j.jacc.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lainchbury JG, Troughton RW, Strangman KM, Frampton CM, Pilbrow A, Yandle TG, Hamid AK, Nicholls MG, Richards AM. N-terminal pro-B-type natriuretic peptide-guided treatment for chronic heart failure: results from the BATTLESCARRED (NT-proBNP-Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol. 2009;55:53–60. doi: 10.1016/j.jacc.2009.02.095. [DOI] [PubMed] [Google Scholar]
- 17.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–59. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 18.Mukoyama M, Nakao K, Obata K, Jougasaki M, Yoshimura M, Morita E, Hosoda K, Suga S, Ogawa Y, Yasue H. Augmented secretion of brain natriuretic peptide in acute myocardial infarction. Biochem Biophys Res Commun. 1991;180:431–6. doi: 10.1016/s0006-291x(05)81311-7. [DOI] [PubMed] [Google Scholar]
- 19.Siriwardena M, Kleffmann T, Ruygrok P, Cameron V, Yandle T, Nicholls G, Richards M, Pemberton C. BNP Signal Peptide Circulates In Human Blood: Evaluation As A Potential Biomarker Of Cardiac Ischemia. Circulation. 2010 doi: 10.1161/CIRCULATIONAHA.109.909937. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn M, Volker K, Schwarz K, Carbajo-Lozoya J, Flogel U, Jacoby C, Stypmann J, van Eickels M, Gambaryan S, Hartmann M, Werner M, Wieland T, Schrader J, Baba HA. The natriuretic peptide/guanylyl cyclase--a system functions as a stress-responsive regulator of angiogenesis in mice. J Clin Invest. 2009;119:2019–30. doi: 10.1172/JCI37430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tjalsma H, Bolhuis A, Jongbloed JD, Bron S, van Dijl JM. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol Mol Biol Rev. 2000;64:515–47. doi: 10.1128/mmbr.64.3.515-547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]