Abstract
The type III TGF-β receptor (TβRIII) is a ubiquitous co-receptor for TGF-β superfamily ligands with roles in suppressing cancer progression, in part through suppressing cell motility. Here we demonstrate that TβRIII promotes epithelial cell adhesion to fibronectin in a β-arrestin2 dependent and TGF-β/BMP independent manner by complexing with active integrin α5β1, and mediating β-arrestin2-dependent α5β1 internalization and trafficking to nascent focal adhesions. TβRIII-mediated integrin α5β1 trafficking regulates cell adhesion and fibronectin fibrillogenesis in epithelial cells, as well as α5 localization in breast cancer patients. We further demonstrate that increased TβRIII expression correlates with increased α5 localization at sites of cell-cell adhesion in breast cancer patients, while higher TβRIII expression is a strong predictor of overall survival in breast cancer patients. These data support a novel, clinically relevant role for TβRIII in regulating integrin α5 localization, reveal a novel crosstalk mechanism between the integrin and TGF-β superfamily signaling pathways and identify β-arrestin2 as a regulator of α5β1 trafficking.
Keywords: integrin α5β1, β-arrestin2, TGF-β, betaglycan
Introduction
Cell-cell and cell-extracellular matrix (ECM) adhesion regulate multiple biological processes including embryonic development, immune recognition, cellular differentiation, migration, proliferation and survival (1). These adhesion events also play prominent roles in malignant transformation and cancer progression (1). Cell adhesion is mediated by transmembrane receptors including integrins (2), immunoglobulin supergene family members (3) and transmembrane proteoglycans (4).
Focal adhesions (FA) are dynamic organelles that mediate cell–ECM adhesion, regulate force transmission and signal in response to receptor engagement with the ECM (5). Integrin containing FA’s form rapidly during cell spreading, which is dependent on integrin engagement with the ECM (6). Integrins participate in endocytic/exocytic cycles (7, 8), which regulate focal adhesion formation, adhesion and motility (9). However, little is known regarding mechanisms regulating integrin trafficking. During epithelial cell transformation and cancer progression, changes in cell-ECM adhesion response result in changes in integrin conformation and trafficking, which can then directly regulate cell motility, invasion and cell survival (8).
The TGF-β superfamily co-receptor, the type III TGF-β receptor (TβRIII/betaglycan), is a transmembrane proteoglycan that serves as a co-receptor for multiple TGF-β superfamily members (10-12). TβRIII has essential, non-redundant roles in regulating signaling through TβRII and TβRI (10, 13, 14) as well as independently of TβRII and TβRI (reviewed in (15)). TβRIII mediates TGF-β signaling independent of its ligand presentation role (16). In addition, the cell-surface levels of TβRIII and its interacting receptors, TβRII, TβRI, ALK3 and ALK6 are regulated, through the interaction of TβRIII with G-interacting protein-interacting protein, C terminus (GIPC) (17) and β-arrestin2 (18, 19). We, and others have demonstrated that TβRIII functions as a suppressor of cancer progression in a broad spectrum of human cancers (20-25). Mechanistically, TβRIII suppresses cancer progression, at least in part, through regulating random cell migration via β-arrestin2-mediated Cdc42 activation (26). The ability of TβRIII to suppress cancer progression cannot be explained by its ligand presentation role, as many of these roles, including its ability to inhibit cell migration, occur independently of TGF-β superfamily ligands and TGF-β superfamily signaling (26). As specific integrins, including integrin α5β1, regulate cell adhesion and random cell migration (27), here we investigate a role for TβRIII in regulating integrin function.
Results
TβRIII promotes epithelial cell adhesion, focal adhesion formation and integrin signaling during epithelial cell spreading on fibronectin
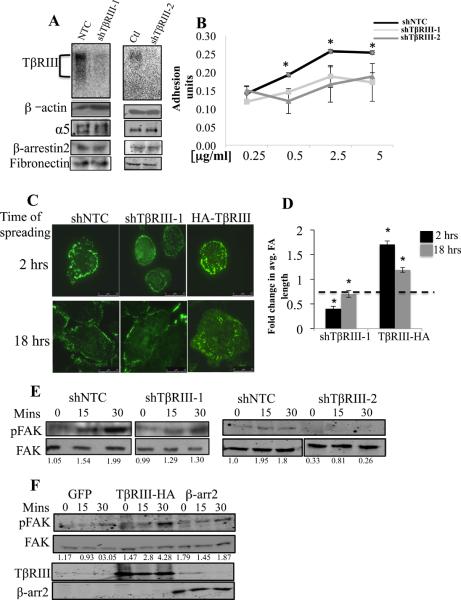
To determine whether TβRIII regulates epithelial cell adhesion to ECM components, we silenced TβRIII with short hairpin interfering RNA’s targeting TβRIII (shTβRIII-1 and shTβRIII-2) in the normal mammary epithelial cell line MCF10A, with either scrambled shRNA (shCtl) or non-targeting shRNA (shNTC) as controls (Figure 1A, sFigure 1A). Silencing TβRIII expression via shTβRIII-1 or shTβRIII-2 to TβRIII did not alter the expression of α5 integrin, its substrate, fibronectin (FN), the TβRIII interacting protein β-arrestin2 (Figure 1A), or expression of the other TGF-β receptors, TβRII and TβRI (sFigure 1C, D). MCF10A cells adhered to FN, laminin and collagen in a dose and time dependent manner (sFigure 2). However shRNA to TβRIII significantly reduced adhesion to FN (Figure 1B, sFigure 2A, B) but had no significant effect on adhesion to laminin or collagen (sFigure 2C-F). Further, silencing TβRIII expression effectively reduced adhesion of MDA-MB231 breast cancer and Ovca420 ovarian cancer cells to FN (sFigure 3), supporting a general role for TβRIII in regulating cell adhesion to FN.
Figure 1. TβRIII mediates epithelial cell adhesion to fibronectin, regulates focal adhesion formation and FAK activation in response to spreading on FN.
MCF10A mammary epithelial cells adenovirally infected with either shTβRIII-1 or shTβRIII-2 to human TβRIII in parallel with controls (shNTC or shCtl) were (A) assessed for cell surface expression of TβRIII by 125I-TGF-β binding and crosslinking, and for total cellular β-arrestin2, fibronectin and integrin α5 expression, with β-actin as a loading control, and (B) assessed for adhesion to the indicated concentrations of FN for 30 minutes and the adhesion level plotted (mean+/−SD ,*p<0.001). Adhesion assays were performed as described in Methods with BSA as a negative control. (C) MCF10A cells were infected with adenovirus expressing shTβRIII-1, control (shNTC), or TβRIII (HA-TβRIII), plated on FN coated cover slips for the indicated times, FAs were detected by anti-vinculin antibody against endogenous vinculin using TIRFM. (D) FA length and the mean +/− SEM were plotted relative to the respective controls (shNTC for shTβRIII; endogenous TβRIII for HA-TβRIII), * p<0.01. Length and intensity of 10 visibly distinct focal adhesions/cell was measured working clockwise from the top of the cell for 10 cells (100 FA measurements per condition). (E) MCF10A-shTβRIII-1 (left panel) or MCF10A-shTβRIII-2 (right panel) with MCF10A-NTC cells as controls were spread on 5 μg/ml FN coated wells for the indicated times and FAK activation (pFAK at Tyr 397), determined by western blot with total FAK as a loading control. Quantitation of the amount of pFAK relative to total FAK is presented below. (F) MCF10A cells transiently overexpressing either TβRIII or β-arrestin2 were spread on 5 μg/ml FN coated wells for the indicated times and FAK activation (pFAK at Tyr 397), determined by western blot with total FAK as a loading control. Quantitation of the amount of pFAK relative to total FAK is presented below.
Cell spreading, including formation of focal adhesions (FA), are early events required for efficient cell-ECM adhesion (28). Using Total Internal Reflection Fluorescence microscopy (TIRFM), we established that shRNA-mediated silencing of TβRIII significantly reduced mean FA length (Figure 1C, D) and decreased FA intensity (sTable 1, p<0.01) 2 hours post spreading in MCF10A cells relative to shNTC cells, with the decreases in FA intensity persisting up to 18 hours (Figure 1C, D, sTable 1). Conversely, transient overexpression of TβRIII increased FA length and intensity 2 hours post plating which persisted for 18 hours after plating (Figure 1C, D, sTable 1).
Integrin clustering during cell spreading results in the rapid recruitment of Focal Adhesion Kinase (FAK) to FA’s, FAK phosphorylation (Tyr397) and activation (29-32). Consistent with the effects of loss of TβRIII expression on FA formation, reducing TβRIII expression by either shTβRIII-1 or shTβRIII-2 significantly delayed and reduced FAK activation in response to spreading relative to control cells (Figure 1E). Conversely, increasing TβRIII expression increased the extent of FAK activation upon cell spreading relative to control cells (Figure 1F) consistent with increased focal adhesion length and intensity observed upon overexpressing TβRIII (Figure 1C, D). Taken together, these data support a role for TβRIII in mediating adhesion of epithelial and epithelial-derived cancer cells to FN via regulating nascent FA formation and downstream integrin signaling during cell spreading.
TβRIII controls epithelial cell adhesion to FN via its cytoplasmic domain and independent of TGF-β and BMP
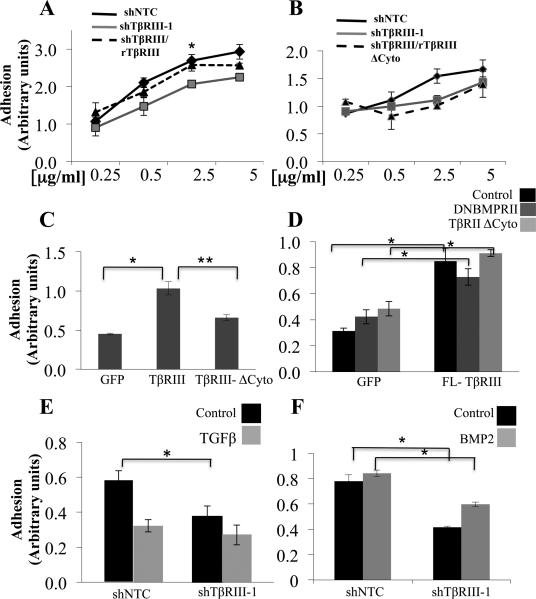
To investigate the mechanism by which TβRIII regulates epithelial cell adhesion to FN, we assessed the relative ability of TβRIII or TβRIIIΔCyto (lacking the cytoplasmic domain) to rescue shTβRIII-mediated decreases in adhesion to FN. While rat TβRIII rescued shTβRIII-mediated decreased in adhesion in MCF10A cells, rat TβRIIIΔCyto was unable to do so (Figure 2A, B, sFigure 1B). Similarly, increasing expression of TβRIII increased adhesion to FN, while increasing expression of TβRIIIΔCyto was only modestly effective in this regard (Figure 2C).
Figure 2. TβRIII mediate adhesion to FN via its cytoplasmic domain but independent of its co-receptor function.
(A-B) MCF10A-shTβRIII-1 (grey line), MCF10A-NTC (dark line) or MCF10A-shTβRIII-1 with rTβRIII rescue (dashed line (B)), or MCF10A-shTβRIII-1 with rTβRIIIΔCyto rescue (dashed line (C)), were plated on the indicated concentrations of FN for 30 minutes and the adhesion level plotted (mean+/−SD,*p<0.05). (C) MCF10A cells infected with adenovirus expressing either control (GFP), TβRIII or TβRIIIΔCyto, were plated on 5μg/ml FN coated dishes for 30 minutes and the adhesion level plotted (mean+/−SD,* **p<0.005). (D) MCF10A cells expressing either control (GFP) or TβRIII, followed by transfection with vector control, TβRIIΔCyto or DNBMPRII as indicated were plated on 5μg /ml FN for 30 minutes and the adhesion level plotted (mean +/− SD, *p<0.005). (E-F) Adhesion of either MCF10A-NTC or MCF10A-shTβRIII1 cells on 5μg/ml FN for 30 minutes in the presence of 200pM TGF-β (E) or 10nM BMP-2 (F), *p<0.05.
To directly assess the contribution of the two major classes of ligands for TβRIII, TGF-β and BMP, we assessed adhesion to FN in the presence of exogenous TGF-β1 or BMP-2. TGF-β1 reduced cell adhesion to FN (Figure 2E), while BMP-2 had a modest effect on increasing adhesion to FN in MCF10A cells, (Figure 2F). However, shTβRIII decreased adhesion in the presence of either TGF-β1 or BMP-2 (Figure 2E, F), albeit to a lesser extent in the presence of TGF-β1. As TβRIII could function to regulate adhesion via sequestration of TGF-β superfamily ligands through production of soluble TβRIII (sTβRIII), we assessed the ability of sTβRIII to regulate the adhesion of MCF10A cells to FN. Treatment with sTβRIII at concentrations which inhibit TGF-β superfamily signaling (26) had no effect on adhesion (sFigure 4A). Further, effectively blocking TGF-β signaling with dominant negative TβRII (TβRIIΔCyto) (sFigure 4B) or the ALK-5/TβRI inhibitor, SB431542 or BMP signaling with dominant negative BMPRII (BMPRIIΔT, sFigure 4D) (33, 34) had no effect on TβRIII-mediated adhesion to FN (Figure 2D, sFigure 4B), suggesting that TβRIII mediates the adhesion of epithelial cells to FN largely independent of its role in regulating TGF-β superfamily signaling.
TβRIII requires active α5β1 to regulate cell adhesion to FN
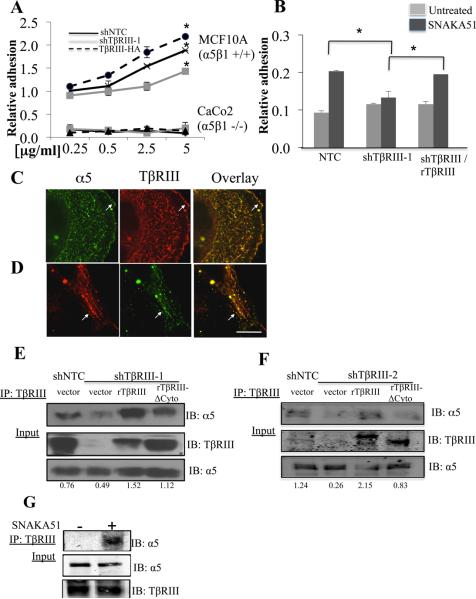
Integrin α5β1 is the main receptor for FN (35). While shTβRIII decreased adhesion and overexpressing TβRIII increased adhesion of integrin α5β1 positive MCF10A cells to FN (Figure 3A), increasing or decreasing TβRIII expression did not alter the adhesion of integrin α5β1 null Caco-2 cells to FN ((36), Figure 3A). To investigate whether integrin α5β1 activation regulated TβRIII-mediated adhesion, we utilized K562 leukemia cells, which express predominantly inactive α5β1 (37). Activation of integrin α5β1 with either stimulatory anti-β1 and/or SNAKA51 antibody promoted K562 cell adhesion to FN ((37), Figure 3B). While shTβRIII had no effect on K562 cell adhesion to FN in the absence of α5β1 activation (Figure 3B, sFigure 4E), in the context of SNAKA51-induced α5β1 activation and K562 cell adhesion to FN, shTβRIII potently decreased K562 cell adhesion to FN and this effect could be rescued by rat TβRIII (Figure 3B, sFigure 4E).
Figure 3. TβRIII-mediated adhesion to FN is dependent on integrin α5β1.
(A) TβRIII was either silenced (shTβRIII-1) or over expressed in MCF10A (α5β1+/+) and Caco2 cells (α5β1−/−) cells and FN dose response adhesion assays performed, *p<0.05. (B) K562 cells infected with shTβRIII-1 or control (shNTC), followed by transfection with rat TβRIII as indicated and adhesion assays performed on FN (5μg /ml) either in the presence or absence of SNAKA 51 (10μg/ml) (mean+/−SE,*p<0.05). (C-D) Fluorescent confocal microscopy of endogenous α5 and endogenous TβRIII in MCF10A cells plated on 5μg/ml FN coated coverslips. Representative example of colocalization at the cell surface (C, arrows) and intracellular vesicles (D, arrows), Bar=11μm. (E) MCF10A-shTβRIII-1 or shTβRIII-2 (F) cells and MCF10A NTC (control) cells transfected with vector, ratTβRIII or ratTβRIIIΔCyto as indicated, were lysed, immunoprecipitated with antibody to TβRIII and immunoblotted with antibody to endogenous α5, with assessment of < 5% of the cell lysate for TβRIII and α5. Quantitation of the amount of α5 precipitated relative to the total amounts of α5 is presented. (G) K562 cells pre-incubated in the presence and absence of SNAKA51 antibody were lysed, immunoprecipitated and immunoblotted as in E, F.
Based on the role of both TβRIII and integrin α5β1 for adhesion to FN, we examined whether TβRIII formed complexes with α5β1. In MCF10A cells endogenous TβRIII and integrin α5 co-localized both at the plasma membrane and in intracellular vesicles (Figure 3C, 3D), while no colocalization was observed in cells lacking TβRIII (sFigure 5A). In addition, endogenous integrin α5 and TβRIII co-immunoprecipitated (Figure 3E, 3F), shTβRIII (shTβRIII-1 and shTβRIII-2), decreased the association of α5 with TβRIII (Figures 3E, F), and this could be rescued with expression of full-length rat TβRIII, but to lesser extent by rat TβRIIIΔCyto (Figure 3E, 3F). Consistent with a role for active integrin α5β1, while α5 did not interact with TβRIII in the absence of α5β1 activation, SNAKA51 strongly induced the interaction of endogenous integrin α5 and TβRIII (Figure 3G). These data strongly support the preferential association of TβRIII with activated α5β1 via TβRIII’s cytoplasmic domain, and a role for α5β1 activation in mediating the effects of TβRIII on adhesion to FN.
TβRIII mediates the trafficking of active integrin α5β1
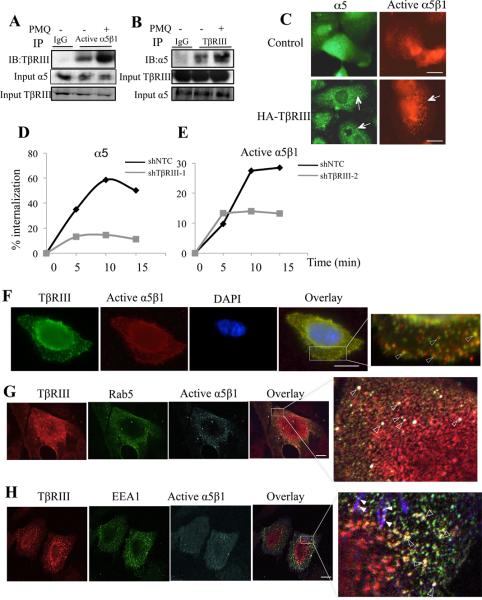
The ability of epithelial cells to spread on the ECM is dependent on the amount of active and/or total integrin at the cell surface (38). While TβRIII interacted and co-localized with α5β1 both on the cell surface and internal vesicles, TβRIII did not alter total integrin α5 levels (Figure 1A, Figure 3E, F), suggesting that they associated at the cell surface and cointernalized (39, 40). Consistent with this hypothesis, primaquine (PMQ), which preserves endosomal integrin complexes, significantly increased the TβRIII and α5β1 interaction (Figure 4A, B). Further, increasing expression of TβRIII modestly increased vesicular staining of endogenous α5 and significantly increased vesicular staining of endogenous activated α5β1 (Figure 4C), while shRNA-mediated silencing of TβRIII significantly decreased the rate and extent of α5β1 internalization (Figure 4D, E). To directly establish whether TβRIII and α5β1 co-internalized together we cell surface labeled active α5β1 and TβRIII and examined their co-internalization. We observed rapid co-internalization of the two proteins into intracellular vesicles (Figure 4F, sFigure 5B), supporting a model in which TβRIII interacts with activated α5β1 at the cell surface and promotes the internalization and trafficking of α5β1 into endocytic vesicles.
Figure 4. TβRIII regulates the internalization and trafficking of integrin α5β1.
(A) MCF10A cells pre-incubated with or without primaquine (PMQ, 0.6μM) for 10 minutes lysed, were immunoprecipitated with anti-active α5β1 or IgG control, and immunoblotted with anti-TβRIII, with assessment of 5% of the cell lysate for α5 and TβRIII levels as indicated, or (B) immunoprecipitated with anti-TβRIII and immunoblotted with antibody to endogenous α5 with assessment of 5% of the cell lysate for TβRIII and α5 levels as indicated. (C) Fluorescent microscopy was used to assess the localization of endogenous α5 (green) or active α5β1 (red) in MCF10A cells expressing endogenous TβRIII (control) or exogenous TβRIII (HA-TβRIII). Arrows indicate the intracellular vesicle formation following over expression of HA-TβRIII. Bar=3.75μm. (D-E) Serum starved MCF10A-NTC (dark line) or MCF10A-shTβRIII-1 (grey line) were surface labeled at 4°C with either anti-α5 antibody (D) or anti-active α5β1 (SNAKA51, E), and the receptors internalized at 37°C in the presence of serum containing media for the indicated times. Mean Fluorescent Intensity (MFI) of internalized antibody relative to T0 was determined. Data representative of 5 individual experiments is presented. (F) Fluorescent microscopy analysis of cell surface, active α5β1 labeled using SNAKA 51 and TβRIII antibody, after allowing internalization at 37°C for 5 minutes (T5). Fixed cells were simultaneously stained and imaged for SNAKA 51 (red), TβRIII (green) and DAPI (blue, nucleus). Overlay is presented, with yellow (arrow) indicating colocalization as shown using arrowheads in magnified areas of the boxed regions. Bar= 3.6 μm. (G-H) Fluorescent confocal microscopy analysis of MCF10A cells, surface labeled with anti-active- α5β1 (SNAKA51) and TβRIII and fixed 5 mins post internalization. Cells were simultaneously labeled for either Rab5 (G), or EEA1 (H) and representative images presented. TβRIII-α5β1 complexes colocalize into Rab5 or EEA1 positive early endosomes as shown in arrowheads in magnified areas of the boxed regions. Endogenous Rab5 colocalizes with TβRIII/α5β1 complexes into early endosomes (solid and empty arrowheads), many of which are located near adhesion sites (empty arrowheads). Bar= 10 μm.
During early endocytosis, the GTPase Rab5 regulates the passage of cargos from the cell surface/plasma membrane into the early endosome (41). To establish the early endocytic fate of TβRIII and active α5β1, we stimulated MCF10A cells with SNAKA51 and stimulated internalization for 5 mins at 37°C. Fluorescent confocal analysis indicated that after 3-5 mins of internalization at 37°C, TβRIII and α5β1 integrin colocalized into early Rab5 positive vesicles (Figure 4G). Similar results were obtained by using EEA1 as a surrogate for early endosomes (Figure 4H). Many of the colocalized vesicles localized near adhesion sites (Figure 4H, empty arrow heads), supporting a role for TβRIII in regulating α5β1-mediated FA formation and cell adhesion by promoting internalization/recycling of active α5β1.
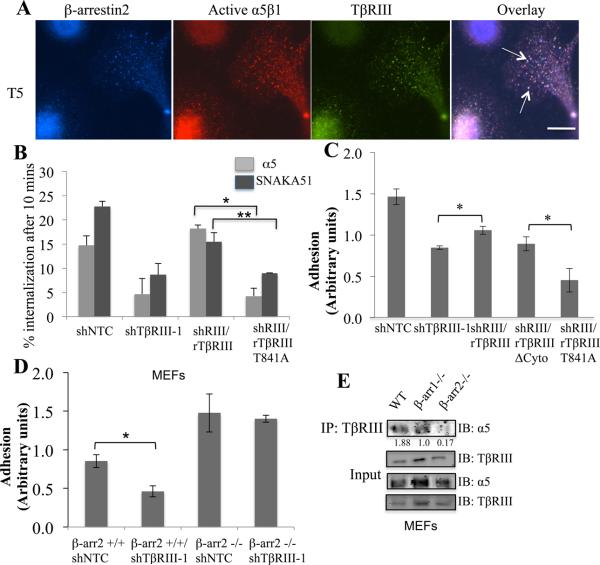
β-arrestin2 mediates TβRIII’s interaction with α5β1, internalization and TβRIII-dependent adhesion to FN and fibrillogenesis
TβRIII internalizes and mediates the internalization of other interacting TGF-β superfamily receptors via the scaffolding protein β-arrestin2, which binds directly to the cytoplasmic domain of TβRIII (18, 19). Consistent with a role for β-arrestin2 in TβRIII-mediated α5β1 internalization, cell surface labeled active α5β1 co-localized with β-arrestin2 within 5 minutes of internalization (sFigure 5C), and cell surface labeled active α5β1 co-localized with TβRIII and β-arrestin2 in endocytic vesicles 5 minutes post internalization (Figure 5A). Further, while over-expression of TβRIII increased α5 and active α5β1 internalization (sFigure 5D), over-expression of TβRIII-T841A, which is unable to bind β-arrestin2 (18), was unable to do so. Similarly, while wild type rat TβRIII rescued shTβRIII-mediated decreases in α5 and activated α5β1 internalization, rat TβRIIIT841A was unable to do so (Figure 5B).
Figure 5. β-arrestin2 is essential for TβRIII–α5β1 interaction, TβRIII-mediated cell adhesion and integrin internalization.
(A) MCF10A cells transiently expressing β-arrestin2 were cell surface labeled with anti- active α5β1 (SNAKA51) and TβRIII for 30 min at 4°C, and allowed to internalize at 37°C for 5 minutes (T5) followed by rapid cooling to 4°C. Cells labeled with SNAKA51 (red) and similarly for TβRIII (green) and β-arrestin2 (blue) were visualized simultaneously. Images are pseudo colored and triple overlay is presented with white vesicles indicating vesicles of triple overlap. Bar= 3.8μm. (B) Percent internalization of α5 or active α5β1 in MCF10A-shTβRIII or shNTC cells, followed by transfection with ratTβRIII or ratTβRIII-T841A as indicated relative to T0. Data represents the mean of 2 experiments, (mean+/−SEM, *p<0.005,**p< 0.05). (C) Indicated cell lines were used for adhesion assays on 5 μg/ml fibronectin for 30 minutes and the adhesion level plotted (mean+/−SD, *p<0.05). (D) β-arrestin2 −/− or β-arrestin2 +/+ MEF’s, were infected with shTβRIII or shNTC and incubated on 5 μg/ml fibronectin surfaces for 30 minutes and adhesion level plotted (mean+/−SD,*p<0.01), (E) TβRIII was immunoprecipitated from either wild type, β-arrestin1 −/− and β-arrestin2 −/− MEF’s and immunoblotted with antibody to endogenous α5 with assessment of <5% of the cell lysate for TβRIII and α5 levels as indicated. Quantitation of the ration of α5 immunoprecipitated to the levels of total α5 is presented.
As the TβRIII/β-arrestin2 interaction had an essential role in TβRIII-mediated regulation of α5β1 internalization, we determined whether the interaction of TβRIII with β-arrestin2 mediates cell adhesion to FN. While wild type rat TβRIII rescued the adhesion defect induced by shTβRIII, rat TβRIII-T841A was unable to do so (Figure 5C). In a reciprocal manner, while increasing TβRIII expression increased adhesion to FN, increasing TβRIII-T841A expression had no effect on adhesion to FN (sFigure 5E). Interestingly, while increasing TβRIII expression increased FAK activation (Figure 1F), increasing expression of β-arrestin2 had a modest effect (Figure 1F), suggesting that the expression of β-arrestin2 is not rate limiting. Further, silencing TβRIII expression in mouse embryonic fibroblast cells (MEF) from β-arrestin2 −/− mice (42) had no significant effect on adhesion to FN, while silencing TβRIII expression in β-arrestin2 +/+ MEFs significantly reduced adhesion to FN (Figure 5D). β-arrestin2 was also required for the interaction of TβRIII with α5, as endogenous α5 and TβRIII co-immunoprecipitated in β-arrestin2 +/+ MEFs, but not in β-arrestin2 −/− MEFs (Figure 5E). The contribution of β-arrestin2 to TβRIII’s role in adhesion and interaction with α5β1 appears to be specific as silencing TβRIII expression in mouse embryonic fibroblast cells (MEF) from β-arrestin1 −/− mice resulted in a significant reduction in cell adhesion to FN (sFigure 6A), demonstrating that β-arrestin1 was not required to mediate TβRIII’s role in cell adhesion. In addition, TβRIII was capable of interacting with α5 integrin in β-arrestin1−/− MEF’s (Figure 5E), indicating that β-arrestin1 was not required to mediate this interaction.
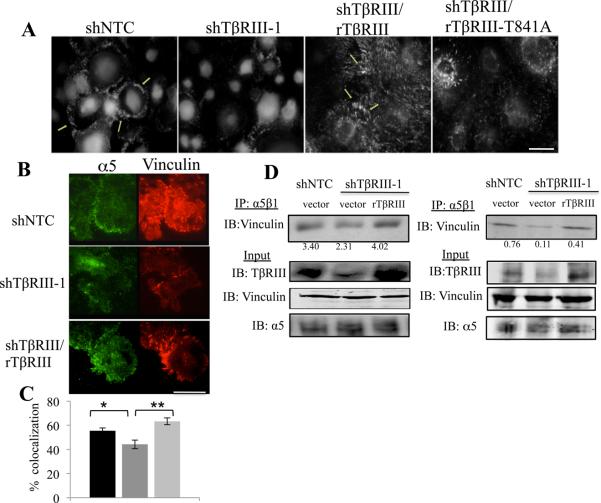
As TβRIII interacts with α5β1 to facilitate FA formation and cell-matrix adhesion to FN, we examined the role of TβRIII in regulating an important aspect of fibronectin function, fibronectin fibrillogenesis. In comparison with shNTC cells, shTβRIII significantly impaired fibronectin fibrillogenesis (Figure 6A), without altering FN levels (Figure 1A). Additionally, while rat wild type TβRIII rescued fibrillogenesis, rat TβRIII-T841A was unable to do so (Figure 6A). Conversely, while overexpressing TβRIII was able to significantly increase fibronectin fibrillogenesis, TβRIII-T841A expression was only partially able to stimulate fibrillogenesis (sFigure 7A). These data support a model in which TβRIII-β-arrestin2 mediate interactions with activated α5β1 to regulate its internalization and trafficking, cell adhesion to FN and FN fibrillogenesis.
Figure 6. TβRIII/β-arrestin2 regulates FN fibrillogenesis and incorporation of integrin α5β1 into sites of focal adhesions.
(A) Fluorescent microscopy analysis of endogenous FN fibrils in indicated cell lines using anti-fibronectin antibody. Bar= 3.5μm. (B) TIRF microscopy of MCF10A shTβRIII, control shNTC cells or shTβRIII cells transfected with rat TβRIII cells plated on FN coated coverslips, labeled with anti-vinculin and anti-α5 antibody, Bar=25μm. (C) Quantitation of colocalization of endogenous integrin α5 and vinculin was performed as described in Methods and is presented below (*p<0.05,**p<0.0005). (D) α5β1 (SNAKA51) – vinculin co-precipitation in indicted cell lines. Cells were lysed, immunoprecipitated with antibody activated integrin α5β1 (SNAKA 51) and immunoblotted with anti-vinculin, with assessment of <5% of cell lysate for TβRIII, α5 and vinculin levels. Quantitation of the relative amounts of immunoprecipitated vinculin relative to the total amounts of vinculin is presented.
TβRIII controls the trafficking of active integrin α5β1 to focal adhesions
As TβRIII has effects on adhesion, fibrillogenesis and FA formation, and colocalizes in early endocytic vesicles proximal to sites of adhesion, we investigated whether TβRIII regulates the localization of α5β1 to FA’s during cell spreading. Using TIRFM, we established that integrin α5 co-localized with the FA protein, vinculin, with shTβRIII significantly decreasing α5/vinculin co-localization, which was rescued with rat TβRIII (Figure 6B,C). Consistent with the TIRFM results, α5 or activated α5β1 co-immunoprecipitated vinculin (Figure 6D, sFigure 7B), shTβRIII decreased the ability of α5 and vinculin (sFigure 7B) as well as the ability of active α5β1 and vinculin to interact (Figure 6D), and these shTβRIII-mediated decreases were rescued by rat TβRIII (Figure 6D, sFigure 7B). The ability to shRNA-mediated silencing of TβRIII expression to have a greater effect on disrupting activated α5β1-vinculin interactions (Figure 6D) is consistent with a specific role for TβRIII in mediating trafficking of active α5β1 to FA’s, supporting a functional link between TβRIII interacting with activated α5β1 and trafficking α5β1 to sites of adhesion.
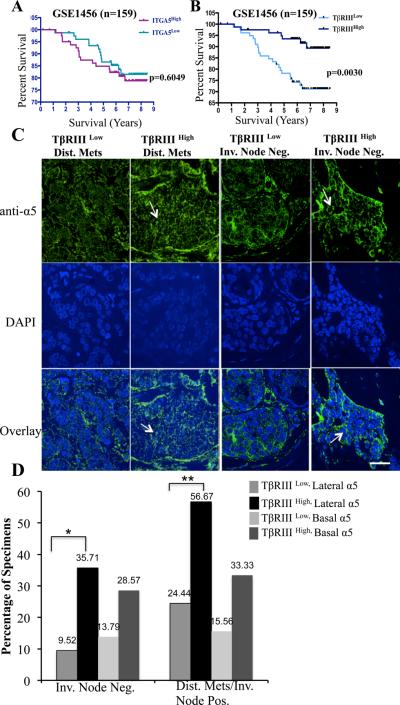
TβRIII alters integrin α5 localization in human breast cancer specimen.
TβRIII regulates integrin trafficking and integrin α5 localization and incorporation to sites of adhesion (Figures 5, 6) in vitro. To investigate whether TβRIII regulated integrin α5 expression or localization in the context of human breast cancer we examined α5 expression and localization in a breast cancer tissue array containing 252 breast cancers, where we have demonstrated decreased TβRIII protein expression from normal, to DCIS to lymph node negative invasive breast cancer (20). Consistent with our in vitro studies, no significant correlation between α5 integrin expression and TβRIII expression at the gene expression level was observed (data not shown). There was also no significant correlation with α5 gene expression and survival in 2 independent gene expression data sets (Figure 7A, sFigure 8A), suggesting that integrin α5 localization may be an important determinant of its function. Consistent with this hypothesis, α5 exhibited distinct localization patterns in normal mammary epithelial cells and cancer cells, either at the lateral surface of cells in ductal regions or cell clusters as described previously (43), at the basal surface, or diffusely (sFigure 8E, Figure 7C). Correlating these data with TβRIII expression in these tissues (20), we noted marked increases in lateral localization of α5 in invasive breast cancer specimens expressing high TβRIII (Figure 7C, D). While the proportion of samples with high basal localization also increased with high TβRIII expression, these differences were not statistically significant (Figure 7D). The data support a model in which TβRIII regulates integrin α5 localization through trafficking of α5β1 to increase lateral α5 localization at sites of cell-cell adhesion in breast cancer clinical specimens.
Figure 7. TβRIII regulates integrin α5 protein localization in tumor tissues and is a strong predictor of overall survival in breast cancer patients.
(A-B) Kaplan-Meier survival analysis of a gene expression data set stratified by α5 (A) or TβRIII (B) expression is presented. No association between α5 expression and survival was observed. Decreased TβRIII expression is associated with significantly decreased long-term survival in patients with breast cancer. p values (log-rank) are as indicated (p<0.005). C) Representative confocal fluorescence images of α5 localization in breast tumor tissue as indicated in either invasive node negative or distant metastatic and node positive tissue with low or high TβRIII expression. Scale bar = 22.5 μm. Levels of TβRIII were determined previously (20). α5 localization was scored independent of overall levels, as either 0-4 lateral or basal and categorized as low (0-1) and high (2-4). Note; diffuse α5 staining in specimen with low TβRIII versus increased lateral localization (arrows) in specimen with high TβRIII. (D) Summary of the quantification of the localization, percentages are presented in either Invasive node negative or distant metastasis (Dis. Met)/Invasive Node positive specimen that were combined, *, ** p < 0.05, 2-tailed Fisher’s exact probability.
Loss of TβRIII expression correlates with reduced overall survival in breast cancer patients independent of integrin α5 expression
To investigate the functional relationship and overall survival outcome due to TβRIII and α5 expression, we queried the same two data sets, GSE3494 and GSE1456 using median expression value for TβRIII and integrin α5 (IGTA5) to delineate high versus low gene expression. In both datasets, reduced TβRIII gene expression was significantly associated with decreased overall survival (Figure 7B, sFigure 8B) supporting our previous findings that low TβRIII expression was significantly associated with a decrease in recurrence-free survival (20). While there was a trend towards decreased survival between patients with high TβRIII /high α5 and patients with high TβRIII/low α5 this was not statistically significant (sFigure 8C, D). These data demonstrate that while integrin α5 expression levels are not a major determinant of overall survival, TβRIII expression levels are a major driver of overall survival in breast cancer patients. Taken together, these data support a model in which TβRIII suppresses breast cancer progression at least in part through regulating the localization of integrin α5.
Discussion
Here we demonstrate that TβRIII, via its cytoplasmic domain, stimulates β-arrestin2 dependent endocytosis and trafficking of activated integrin α5β1 to focal adhesions, promoting focal adhesion formation, cell adhesion to FN and FN fibrillogenesis in epithelial cells. TβRIII also regulates α5 integrin localization to sites of adhesion in breast cancer tissues, with TβRIII expression being a major driver of α5 integrin localization and breast cancer survival. Further, we demonstrate that integrin α5 expression levels are not predictors of overall survival, suggesting that TβRIII-mediated localization of integrin α5 may be an important regulator of disease progression.
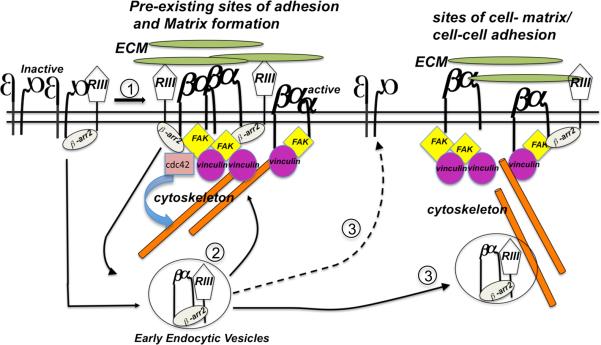
TβRIII has been best characterized as a TGF-β superfamily co-receptor. However, neither stimulating TGF-β superfamily signaling with TGF-β1 or BMP-2, nor inhibiting signaling with dominant negative TβRII or BMPRII had an effect on TβRIII-mediated cellular adhesion to fibronectin (Figure 2), supporting a co-receptor function independent role for TβRIII in regulating cell adhesion. While we have previously demonstrated that the TβRIII-β-arrestin2 interaction can regulate the trafficking of interacting TGF-β receptors, TβRII and TβRI/ALK5 (18), as well BMP receptors, ALK3 and ALK6 (19), here we demonstrate a novel interacting partner for TβRIII outside of the TGF-β superfamily-signaling pathway, namely integrin α5β1. The data herein support a model (Figure 8) in which TβRIII interacts with activated α5β1 during epithelial cell spreading and/or adhesion via β-arrestin2, enhancing early integrin α5β1 endocytosis, ensuring the recycling of integrin α5β1 to sites of newly forming sites of adhesion and fibrillogenesis (Figure 8).
Figure 8. Model for the TβRIII/ β-arrestin2 dependent integrin α5β1 trafficking and function in epithelial and epithelial-derived cancer cells.
At sites of epithelial cell spreading/adhering to FN, TβRIII, via its cytoplasmic domain interaction with the scaffolding protein, β-arrestin2, interacts with Cdc42 (26) and active integrin α5β1 that is engaged with fibronectin fibrils (1). This interaction promotes the Rab5 dependent internalization of the active pool of α5β1 (2). Upon endocytosis, α5β1 is recycled back from TβRIII/β-arrestin2/Rab5 positive vesicles (2) to the cell surface (3) to newly forming focal adhesions at the cell surface or sites of cell-cell adhesion as observed in human tissues (3). In adherent cells, rapid internalization and recycling of active integrins could play a role in regulating random cell migration of cancer cells as observed previously for TβRIII dependent migration of cancer cells via Cdc42 (26) and integrin α5β1 dependent migration (27). Alternatively, rapid internalization and recycling of active integrins could regulate integrin α5β1 and TβRIII dependent cell-cell adhesion of normal epithelial cells, loss of which could result in disruption of cell polarity and cancer initiation/progression.
Interestingly, while β-arrestin2 was required for TβRIII to stimulate adhesion in mouse embryonic fibroblasts (MEFs), β-arrestin2 (−/−) MEFs exhibited a significant increase in adhesion to FN. As β-arrestin2−/− MEF’s induced FAK activation upon cell spreading to the same extent as wild type MEF’s (sFigure 6B), β-arrestin2 appears to have a complex role in regulating cell adhesion to FN. While β-arrestin2 has been shown to be required for the induction and strengthening of integrin-mediated leukocyte adhesion during CXCR2-driven extravasation (44) and has been hypothesized to play roles as a scaffolding protein for cytoskeletal proteins (45), data presented here are the first direct demonstration of β-arrestin2 as a regulator of epithelial cell adhesion, focal adhesion formation and integrin α5β1 trafficking. The contribution of β-arrestin2 to cell adhesion, both TβRIII dependent and independent appear to specific to β-arrestin2, as β-arrestin1 cannot substitute for β-arrestin2 in the context of TβRIII and loss of β-arrestin1 expression had no effect on adhesion to FN (sFigure 6A). The TβRIII- independent contributions of β-arrestin2 to cell adhesion remain to be explored.
Consistent with the observed effects of TβRIII on focal adhesion formation, TβRIII is required for integrin-mediated FAK activation (46), suggesting that TβRIII regulates integrin-mediated inside-out signaling, and facilitating integrin engagement with its ligand resulting in activation of FAK. Interestingly, FAK activation has been demonstrated to activate Cdc42 through binding and phosphorylation of the Cdc42 effector N-WASP (47), suggesting that TβRIII might activate Cdc42 (26), at least in part, through regulating integrin-mediated FAK activation (Figure 8). In addition, as loss of TβRIII expression decreases fibrillogenesis in the absence of any significant change in either total integrin α5β1 expression or FN expression, TβRIII may regulate integrin-mediated outside in signaling as well. Whether the TβRIII-mediated regulation of integrin α5β1 internalization, trafficking, and biology extends to other members of the integrin family, and under which contexts, remains to be explored.
Our data on integrin function and trafficking and previously published observations on TβRIII’s effects on persistent migration and Cdc42 activation suggest that spatial-temporal localization of active integrin α5β1, as impacted by the TβRIII–β-arrestin2 interaction (Figure 8), function to determine the extent and ability of cancer cells to move and invade. The reduced accumulation of α5 at FA’s and reduced fibrillogenesis mediated by loss of TβRIII expression would promote rapid FA turnover and faster migration and invasion, consistent with our previously published finding that shTβRIII expression increases cancer cell migration and invasion (48) (22). Overall, our data suggest a model in which the ability of TβRIII to suppress cancer progression is through the regulation and maintenance of matrix stability and proper accumulation of activated integrin α5β1. Loss of TβRIII expression, as observed in many cancers, would contribute to the destabilization of the matrix and changes in activated integrin α5 localization, increased motility and invasion and progression of cancer. Consistent with this model, we observed specific effects of TβRIII on α5 localization in breast tumor tissues to sites of cell-cell contact (lateral localization). Recently, increased expression of integrin α5 was found to be associated with ErbB2-positive breast cancers that also resulted in changes in α5 localization (43) possibly due to disruption of cell polarity. Interestingly, while TβRIII expression was strongly associated with increased survival, we found no association of α5 levels with survival in two distinct gene expression data sets, in contrast to a previous study implicating integrin α5 levels as a predictor of overall survival in breast cancer patients (49). Our data on the role of TβRIII in regulating integrin trafficking and the ECM during cancer progression and TβRIII’s role in regulating integrin α5 localization in patient samples suggest that it is not the expression level, but the localization of activated integrin α5 that maybe an important factor in disease progression and outcome. Whether assessment of the localization of integrin α5, or of other integrins and signaling molecules will provide useful prognostic or predictive information in the clinical management of patients is currently under investigation.
Materials and Methods
Cell Culture, Transfection and Reagents
Human epithelial cell lines MCF10A, SKOV3, Ovca420 were cultured as described previously (26, 50). K562 and Caco2 cells were purchased from ATCC. β-arrestin2 mouse embryonic cell lines were a gift from Dr. Bob Lefkowitz (42). All cell lines were grown at 37°C in a humidified incubator with 5% CO2. Antibodies and adenoviral constructs are described in supplementary methods. Antibodies to FL-TβRIII were obtained from R&D systems, cell signaling (catalog# 2519) or described previously (20). Integrin α5 (Catalog # 4705), integrin β1 (Catalog # 4706), β-arrestin2 (Catalog # 3857), FAK (Catalog # 3283), total FAK (Catalog # 3285), Rab5 (catalog# 2143), and EEA1 (catalog# 2411) antibodies were obtained from Cell Signaling. The fibronectin antibody (Catalog # SC71114) was from Santa Cruz Technologies and vinculin antibody (Catalog # CP74) was from Calbiochem. BMPRII short (BMPRIIΔT) was a kind gift from Liliana Attisano (33), SNAKA 51 antibody was a kind gift from Martin J Humphries. Human FN, rat-tail collagen and laminin were obtained from Sigma and BD Biosciences.
DNA/Adenoviral constructs, shRNA/siRNA
All TβRIII deletion constructs and shRNA constructs have been described previously (26). All transient transfections and shRNA adenoviral infections were performed as described previously (22, 26, 51).
Adhesion assays
96 well microtiter plates were pre-coated with ECM proteins and saturated with 1% bovine serum albumin. Adherent cells were detached using 5mM EDTA and resuspended at a cell density of 10-30,000 cells/ml (depending on the cell line) in serum free media with or without the indicated stimuli and plated on the ECM coated microtiter plates. Following adhesion for indicated times, cells were washed in PBS and fixed in 4% paraformaldehyde and then stained with crystal violet for 20 minutes. The stain was washed, dried and color solubilized using 2% SDS for 20 minutes. Absorbance was read at 595nm on the Wallac Victor Counter (PerkinElmer Life Sciences) and values normalized to BSA only controls.
Immunoprecipitation and Western blotting
Cells were lysed in buffer containing 20mM Hepes pH7.4, 150mM NaCl, 2mM EDTA, 10% glycerol, 10mM NaF and 0.5% NP40 in the presence of protease inhibitors. Cells were lysed in buffer, incubated for 20 minutes on ice, and then centrifuged at 15,000g, 20 minutes at 4°C. Equivalent amounts of protein were immunoprecipitated overnight with the antibody of interest and immune complexes were recovered on either protein G-Sepharose or protein A-Sepharose (GE Healthcare). Immunoprecipitates were washed four times with lysis buffer, twice with the same buffer without Tween-20, and then separated by SDS-PAGE. Proteins were then transferred to a Hybond-C extra nitrocellulose membrane (Amersham), probed with antibodies of interest, and detected by an enhanced chemiluminescence technique (PerkinElmer).
Fibrillogenesis
Following infection and/or transfection of the cells, cells were seeded onto glass coverslips in a 6 well dish at a cell density of 2×105cells/well and left to adhere for an additional 24-48 hours in medium depleted of serum. Cells were then washed with PBS and fixed with 4% paraformaldehyde on ice, permeabilized in 0.1% Triton X-100/PBS on ice for 1 min and incubated with anti-fibronectin antibody for 1 h. Cells were labeled with secondary antibody conjugated to Alexa Fluor 488 for 30 minutes at room temperature.
Immunofluorescence staining of Formalin fixed paraffin embedded (FFPE) tissue array
was performed using Sodium Citrate buffer pH 6.0 with 0.05% Tween 20 in a pressure cooker for antigen retrieval and performed essentially as described previously (52). Array was stained with integrin α5 (Cell Signaling) antibody overnight at 4°C followed by Alexa-Fluor conjugated secondary anti- rabbit 488. Array was counterstained for nuclei with DAPI. Images were collected using a Zeiss LSM 510 inverted confocal microscope using either a 40x/1.3 NA oil, 63x/1.4 oil Plan-Apochromat, objective. For scoring purposes, array was scored ‘blinded’ for TβRIII levels and scored on an epi-flourescent microscope.
Internalization assays
Were performed as described previously (53) and MFI (Mean fluorescence intensity) of the cells, subsequent to stripping un-internalized antibody determined using flow cytometry.
Conventional confocal scanning for colocalization studies and fluorescence microscopy
For immunflourescence analysis of colocalized proteins upon internalization, endogenous integrin α5β1 was cell surface labeled for 30 minutes at 4°C using either integrin α5 antibody or 10 μg/ml SNAKA51 antibody, washed and transferred to pre-warmed media and allowed to recover at 37°C for 5 minutes to induce endocytosis. Cells were processed for IF and were imaged either by using a Leica SP5 confocal laser-scanning microscope (Leica) or a TE2000 inverted fluorescence microscope. Percent colocalization was determined by determining % pixel overlap using Metamorph software.
TIRF-based microscopy and focal adhesion analysis
Thirty-five mm glass bottom culture dishes (MatTek) coated with 5μg/mL fibronectin (Calbiochem) were used to image fixed cells on a Leica AM TIRF microscope. Length and intensity of 10 visibly distinct focal adhesions/cell was measured working clockwise from the top of the cell for 10 cells (100 FA measurements per condition). Intensity of a line drawn through the dark background was subtracted to account for differences in background intensity.
Gene expression analysis
Clinical data, including overall survival, and raw expression data (.CEL) files were acquired from NCBI Gene Expression Omnibus (GEO) for GSE3494 (n=251) and GSE1456 (n=159). Data was MAS5 normalized using Affymetrix Expression Console (Version 1.0). The Affymetrix HG-U133A plus 2 probe set annotation file (release 29) was acquired from the Affymetrix website and probe sets identified for TβRIII (204731_at) and IGTA5 (201389_at). The median expression value for TGFβR3 and IGTA5 was used to delineate high versus low gene expression. A Kaplan-Meier survival curve was generated using GraphPad Prism 4.0.
Statistical analysis
Significance of results was assessed using two-tailed Student’s t test unless otherwise indicated. *p values are as indicated in the Figures and p values <0.05 were considered significant. Error bars represent either mean +/− SE or mean +/−SD as indicated. Data are representative of a minimum of three independent experiments, unless otherwise indicated.
Supplementary Material
Acknowledgements
We sincerely thank Sue Craig and Martin Humphries for generously providing us with SNAKA51, L. Attisano for BMPRII construct, Tam How and Alisha Holtzhausen for technical help in generation of the ratTβRIII-T841A plasmid and plasmid purifications respectively, Duke University Light Microscopy facility for help in training individuals with the use of the TIRFM and confocal microscopes.
Financial Support: This work was supported in part by NIH Grants R01-CA135006 and R01-CA136786 (GCB), Komen for the Cure Grants KG090154 and SAC100002 (GCB) and Department of Defense grant W81 XWH-09-1-0265 (KM).
Footnotes
Conflict of Interest: Authors report no conflict of interest.
References
- 1.Edelman GM, Crossin KL. Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem. 1991;60:155–90. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- 2.Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loftus JC, Smith JW, Ginsberg MH. Integrin-mediated cell adhesion: the extracellular face. J Biol Chem. 1994 Oct 14;269(41):25235–8. [PubMed] [Google Scholar]
- 4.Ruoslahti E, Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–9. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- 5.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 6.Morgan MR, Byron A, Humphries MJ, Bass MD. Giving off mixed signals--distinct functions of alpha5beta1 and alphavbeta3 integrins in regulating cell behaviour. IUBMB Life. 2009 Jul;61(7):731–8. doi: 10.1002/iub.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bretscher MS. Cells can use their transferrin receptors for locomotion. Embo J. 1992 Feb;11(2):383–9. doi: 10.1002/j.1460-2075.1992.tb05066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellinen T, Ivaska J. Integrin traffic. J Cell Sci. 2006 Sep 15;119(Pt 18):3723–31. doi: 10.1242/jcs.03216. [DOI] [PubMed] [Google Scholar]
- 9.Bretscher MS. Moving membrane up to the front of migrating cells. Cell. 1996 May 17;85(4):465–7. doi: 10.1016/s0092-8674(00)81246-5. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Casillas F, Wrana JL, Massague J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993 Jul 2;73(7):1435–44. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- 11.Kirkbride KC, Townsend TA, Bruinsma MW, Barnett JV, Blobe GC. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J Biol Chem. 2008 Mar 21;283(12):7628–37. doi: 10.1074/jbc.M704883200. [DOI] [PubMed] [Google Scholar]
- 12.Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, et al. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000 Mar 23;404(6776):411–4. doi: 10.1038/35006129. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Casillas F, Payne HM, Andres JL, Massague J. Betaglycan can act as a dual modulator of TGF-beta access to signaling receptors: mapping of ligand binding and GAG attachment sites. J Cell Biol. 1994 Feb;124(4):557–68. doi: 10.1083/jcb.124.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stenvers KL, Tursky ML, Harder KW, Kountouri N, Amatayakul-Chantler S, Grail D, et al. Heart and liver defects and reduced transforming growth factor beta2 sensitivity in transforming growth factor beta type III receptor-deficient embryos. Mol Cell Biol. 2003 Jun;23(12):4371–85. doi: 10.1128/MCB.23.12.4371-4385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilandzic M, Stenvers KL. Betaglycan: A multifunctional accessory. Mol Cell Endocrinol. 2011 Apr 28; doi: 10.1016/j.mce.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Blobe GC, Schiemann WP, Pepin MC, Beauchemin M, Moustakas A, Lodish HF, et al. Functional roles for the cytoplasmic domain of the type III transforming growth factor beta receptor in regulating transforming growth factor beta signaling. J Biol Chem. 2001 Jul 6;276(27):24627–37. doi: 10.1074/jbc.M100188200. [DOI] [PubMed] [Google Scholar]
- 17.Blobe GC, Liu X, Fang SJ, How T, Lodish HF. A novel mechanism for regulating transforming growth factor beta (TGF-beta) signaling. Functional modulation of type III TGF-beta receptor expression through interaction with the PDZ domain protein, GIPC. J Biol Chem. 2001 Oct 26;276(43):39608–17. doi: 10.1074/jbc.M106831200. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Kirkbride KC, How T, Nelson CD, Mo J, Frederick JP, et al. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003 Sep 5;301(5638):1394–7. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- 19.Lee NY, Kirkbride KC, Sheu RD, Blobe GC. The transforming growth factor-beta type III receptor mediates distinct subcellular trafficking and downstream signaling of activin-like kinase (ALK)3 and ALK6 receptors. Mol Biol Cell. 2009 Oct;20(20):4362–70. doi: 10.1091/mbc.E09-07-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong M, How T, Kirkbride KC, Gordon KJ, Lee JD, Hempel N, et al. The type III TGF-beta receptor suppresses breast cancer progression. J Clin Invest. 2007 Jan;117(1):206–17. doi: 10.1172/JCI29293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finger EC, Turley RS, Dong M, How T, Fields TA, Blobe GC. TbetaRIII suppresses non-small cell lung cancer invasiveness and tumorigenicity. Carcinogenesis. 2008 Mar;29(3):528–35. doi: 10.1093/carcin/bgm289. [DOI] [PubMed] [Google Scholar]
- 22.Gordon KJ, Dong M, Chislock EM, Fields TA, Blobe GC. Loss of type III transforming growth factor beta receptor expression increases motility and invasiveness associated with epithelial to mesenchymal transition during pancreatic cancer progression. Carcinogenesis. 2008 Feb;29(2):252–62. doi: 10.1093/carcin/bgm249. [DOI] [PubMed] [Google Scholar]
- 23.Hempel N, How T, Dong M, Murphy SK, Fields TA, Blobe GC. Loss of betaglycan expression in ovarian cancer: role in motility and invasion. Cancer Res. 2007 Jun 1;67(11):5231–8. doi: 10.1158/0008-5472.CAN-07-0035. [DOI] [PubMed] [Google Scholar]
- 24.Turley RS, Finger EC, Hempel N, How T, Fields TA, Blobe GC. The type III transforming growth factor-beta receptor as a novel tumor suppressor gene in prostate cancer. Cancer Research. 2007 Feb 1;67(3):1090–8. doi: 10.1158/0008-5472.CAN-06-3117. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 25.Bilandzic M, Chu S, Farnworth PG, Harrison C, Nicholls P, Wang Y, et al. Loss of betaglycan contributes to the malignant properties of human granulosa tumor cells. Mol Endocrinol. 2009 Apr;23(4):539–48. doi: 10.1210/me.2008-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mythreye K, Blobe GC. The type III TGF-{beta} receptor regulates epithelial and cancer cell migration through {beta}-arrestin2-mediated activation of Cdc42. Proc Natl Acad Sci U S A. 2009 May 1; doi: 10.1073/pnas.0812879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White DP, Caswell PT, Norman JC. alpha v beta3 and alpha5beta1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J Cell Biol. 2007 May 7;177(3):515–25. doi: 10.1083/jcb.200609004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009 Jan;10(1):21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 29.Parsons JT, Schaller MD, Hildebrand J, Leu TH, Richardson A, Otey C. Focal adhesion kinase: structure and signalling. J Cell Sci Suppl. 1994;18:109–13. doi: 10.1242/jcs.1994.supplement_18.16. [DOI] [PubMed] [Google Scholar]
- 30.Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8487–91. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994 Mar;14(3):1680–8. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–6. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee-Hoeflich ST, Causing CG, Podkowa M, Zhao X, Wrana JL, Attisano L. Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis. Embo J. 2004 Dec 8;23(24):4792–801. doi: 10.1038/sj.emboj.7600418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu F, Ventura F, Doody J, Massague J. Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol Cell Biol. 1995 Jul;15(7):3479–86. doi: 10.1128/mcb.15.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akiyama SK, Yamada SS, Chen WT, Yamada KM. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989 Aug;109(2):863–75. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuwada SK, Li X. Integrin alpha5/beta1 mediates fibronectin-dependent epithelial cell proliferation through epidermal growth factor receptor activation. Mol Biol Cell. 2000 Jul;11(7):2485–96. doi: 10.1091/mbc.11.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark K, Pankov R, Travis MA, Askari JA, Mould AP, Craig SE, et al. A specific alpha5beta1-integrin conformation promotes directional integrin translocation and fibronectin matrix formation. J Cell Sci. 2005 Jan 15;118(Pt 2):291–300. doi: 10.1242/jcs.01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005 Oct;17(5):509–16. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Woods AJ, White DP, Caswell PT, Norman JC. PKD1/PKCmu promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. Embo J. 2004 Jul 7;23(13):2531–43. doi: 10.1038/sj.emboj.7600267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts MS, Woods AJ, Dale TC, Van Der Sluijs P, Norman JC. Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of alpha v beta 3 and alpha 5 beta 1 integrins. Mol Cell Biol. 2004 Feb;24(4):1505–15. doi: 10.1128/MCB.24.4.1505-1515.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zerial M, McBride H. Rab proteins as membrane organizers. Nature reviews Molecular cell biology. [Review] 2001 Feb;2(2):107–17. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 42.Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci U S A. 2001 Feb 13;98(4):1601–6. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haenssen KK, Caldwell SA, Shahriari KS, Jackson SR, Whelan KA, Klein-Szanto AJ, et al. ErbB2 requires integrin alpha5 for anoikis resistance via Src regulation of receptor activity in human mammary epithelial cells. Journal of cell science. 2010 Apr 15;123(Pt 8):1373–82. doi: 10.1242/jcs.050906. [Research Support, U.S. Gov’t, Non-P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molteni R, Crespo CL, Feigelson S, Moser C, Fabbri M, Grabovsky V, et al. Beta-arrestin 2 is required for the induction and strengthening of integrin-mediated leukocyte adhesion during CXCR2-driven extravasation. Blood. 2009 Jul 30;114(5):1073–82. doi: 10.1182/blood-2008-10-183699. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 45.DeFea KA. Stop that cell! Beta-arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu Rev Physiol. 2007;69:535–60. doi: 10.1146/annurev.physiol.69.022405.154804. [DOI] [PubMed] [Google Scholar]
- 46.Toutant M, Costa A, Studler JM, Kadare G, Carnaud M, Girault JA. Alternative splicing controls the mechanisms of FAK autophosphorylation. Mol Cell Biol. 2002 Nov;22(22):7731–43. doi: 10.1128/MCB.22.22.7731-7743.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu X, Suetsugu S, Cooper LA, Takenawa T, Guan JL. Focal adhesion kinase regulation of N-WASP subcellular localization and function. J Biol Chem. 2004 Mar 5;279(10):9565–76. doi: 10.1074/jbc.M310739200. [DOI] [PubMed] [Google Scholar]
- 48.Mythreye K, Blobe GC. Proteoglycan signaling co-receptors: Roles in cell adhesion, migration and invasion. Cell Signal. 2009 May 8; doi: 10.1016/j.cellsig.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nam JM, Onodera Y, Bissell MJ, Park CC. Breast cancer cells in three-dimensional culture display an enhanced radioresponse after coordinate targeting of integrin alpha5beta1 and fibronectin. Cancer Research. 2010 Jul 1;70(13):5238–48. doi: 10.1158/0008-5472.CAN-09-2319. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003 Jul;30(3):256–68. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 51.You HJ, Bruinsma MW, How T, Ostrander JH, Blobe GC. The type III TGF-beta receptor signals through both Smad3 and the p38 MAP kinase pathways to contribute to inhibition of cell proliferation. Carcinogenesis. 2007 Dec;28(12):2491–500. doi: 10.1093/carcin/bgm195. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 52.Robertson D, Savage K, Reis-Filho JS, Isacke CM. Multiple immunofluorescence labelling of formalin-fixed paraffin-embedded (FFPE) tissue. BMC Cell Biol. 2008;9:13. doi: 10.1186/1471-2121-9-13. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts M, Barry S, Woods A, van der Sluijs P, Norman J. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr Biol. 2001 Sep 18;11(18):1392–402. doi: 10.1016/s0960-9822(01)00442-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.