Abstract
The major cardiac syndromes, myocardial infarction and heart failure, are responsible for a large portion of deaths worldwide. Genetic and pharmacological manipulations indicate that cell death is an important component in the pathogenesis of both diseases. Cells die primarily by apoptosis or necrosis, and autophagy has been associated with cell death. Apoptosis has long been recognized as a highly regulated process. Recent data indicate that a significant subset of necrotic deaths is also highly programmed. In this review, we discuss the molecular mechanisms that underlie these forms of cell death and their interconnections. Because of their regulated nature, the possibility is raised that small molecules aimed at inhibiting cell death may provide novel therapies for these common and lethal heart syndromes.
Keywords: cell death, apoptosis, necrosis, myocardial infarction, heart failure
INTRODUCTION
Cells die primarily by apoptosis or necrosis. Apoptosis is a highly regulated mode of cell suicide.1 While necrosis has traditionally been regarded as passive and unregulated, data accumulated over the past decade indicates that a substantial proportion of necrotic deaths are actively executed by the cell in a highly regulated manner. This form of necrosis is sometimes referred to as regulated or programmed. Both apoptosis and necrosis play critical roles in normal biology including prenatal development and postnatal homeostasis.2 Accordingly, when increased, decreased, or mislocalized, cell death plays major roles in human diseases, including cardiovascular disease, cancer,3, 4 diabetes,5, 6 sepsis,7 and some neurological disorders.8, 9
Apoptosis is characterized by cell shrinkage,10 fragmentation into membrane-enclosed “apoptotic bodies”, and phagocytosis of these corpses by macrophages, or occasionally, neighboring cells.11 When this clean-up operation is efficient, inflammation is avoided. ATP levels in apoptotic cells are reasonably well maintained both because of continued production and decreased expenditures.12, 13 The net result of apoptosis is the stealth deletion of individual cells within a tissue. In contrast, necrosis is characterized by loss of plasma membrane integrity, cellular and organellar swelling, and marked inflammation. ATP levels are dramatically reduced in necrotic cells, both because of severe mitochondrial damage that cripples ATP generation as well as unrestrained energy expenditures.14 The chicken and egg relationships between ATP deficits and loss of plasma membrane integrity remain unclear. Similarly, while it is tempting to speculate that the decision of a doomed cell to undergo apoptosis versus necrosis is determined by energetics, this possibility has not yet been definitively established.
MECHANISMS OF CELL DEATH
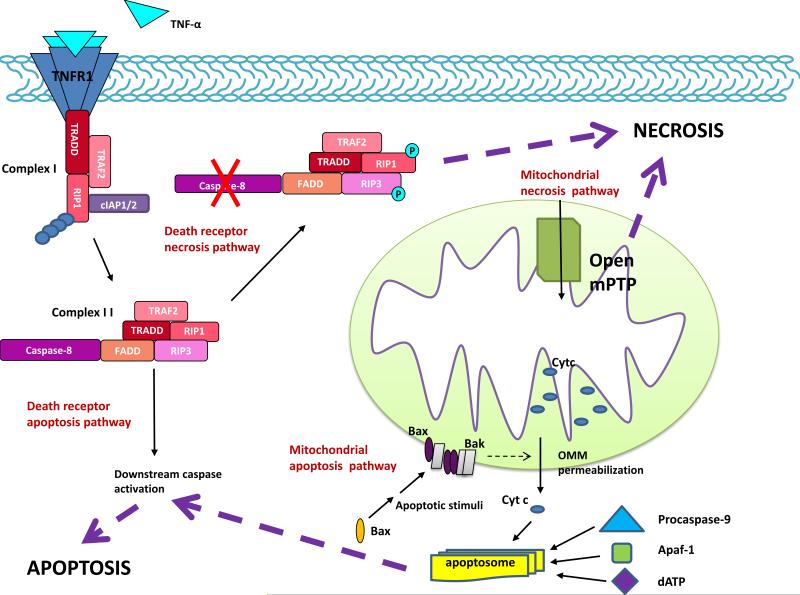
Apoptosis and necrosis are mediated by distinct, but highly overlapping central pathways (Figure). The extrinsic pathway involves cell surface death receptors and the intrinsic pathway utilizes the mitochondria and endoplasmic reticulum (ER). These pathways, which mediate both apoptosis and necrosis, are linked by multiple biochemical and functional connections.. Extrapolating this degree of connectivity, the possibility is raised that these cell death mechanisms comprise a single unified death machinery. However, given the morphological differences among types of cell death and the presumption that each arose at a specific time in evolution for a specific purpose, the notion of a unified model remains to be established.
Figure. Cell death pathways.
Apoptosis and necrosis are mediated by death receptor (extrinsic) and mitochondrial (intrinsic) pathways. In the death receptor pathway, a death ligand (e.g. TNF-α) binds its cognate death receptor to trigger assembly of either the DISC (not shown) or complex I. When RIP1 is K63-polyubiquinated by cIAP1/2, complex I signals survival through NF-kB activation (not shown). If (a) death receptor dissociates from complex I, (b) the complex is endocytosed, (c) RIP1 undergoes deubiquitiniation, and (d) a FADD-RIP3 complex is recruited, complex II is formed. This complex signals apoptosis or necrosis depending on procaspase-8 activity. Activation of procaspases-8 leads to cleavage and activation of downstream procaspases that proteolyze cellular proteins to bring about apoptosis. Procaspase-8 also cleaves RIP1 and RIP3, to preclude necrosis. In contrast, with caspase-8 inhibition, RIP1 and RIP3 undergo a series of cross-phosphorylation events that trigger necrosis by a variety of mechanisms (see text). In the mitochondrial pathway, the critical event in apoptosis is permeabilization of the outer mitochondrial membrane (OMM), which results in release of mitochondrial apoptogens (e.g. cytochrome c) to the cytoplasm. Complex interactions among Bcl-2 family members (e.g. Bax and Bak) mediate OMM permeabilization (see text). Once in the cytoplasm, cytochrome c stimulates assembly of the apoptosome, a multiprotein complex in which procaspase-9 is activated. Procaspase-9 goes on to activate downstream procaspases. In contrast, the defining event in necrosis is opening of the mitochondrial permeability transition pore (mPTP) in the inner membrane, which (a) collapses the electrical gradient across the IMM leading to cessation of ATP synthesis and (b) promotes the influx of water into the mitochondrial matrix resulting in severe mitochondrial swelling. Multiple connections exist between these pathways. TNF-α, tumor necrosis factor α, TNFR1, tumor necrosis factor receptor 1, RIP1, receptor interacting protein 1, cIAP1/2, cellular inhibitor of apoptosis 1 and 2, FADD, Fas-associated via death domain, RIP3, receptor interacting protein 3, TRADD, TNF receptor-associated death domain, TRAF2, TNFR-associated factor 2, Cyt c, cytochrome c, Bax, Bcl-2 associated X protein, Bak, Bcl-2 homologous antagonist/killer, Apaf-1, apoptotic protease activating factor-1.
Extrinsic (death receptor) pathway: apoptosis and necrosis
In the death receptor pathway, a variety of death ligands bind their cognate receptors to trigger cell death. Some of these ligands are soluble (e.g. tumor necrosis factor (TNF)-α), and some are bound to the surface of other cells (e.g. Fas ligand). The efficiency with these ligands to induce death varies with cell type. Recent work has shown that the same death ligands may induce apoptosis or necrosis, the choice mediated by downstream events.
Binding of ligand to receptor induces the formation of either of two multiprotein complexes: the Death Inducing Signaling Complex (DISC) and complex I.15, 16 The DISC signals apoptosis, while Complex I can signal either apoptosis, necrosis, or cell survival. The DISC has been studied most intensively in the context of Fas ligand/Fas signaling, and complex I in the setting of TNF/TNF receptor 1 signaling. However, which ligand/receptor combinations employ the DISC versus complex I, or both, is incompletely understood.
In DISC formation, the binding of death ligand induces a conformational change in the cytosolic domain of the death receptor, which recruits an adaptor protein (e.g. Fas-associated via death domain (FADD), TNF receptor-associated death domain (TRADD)).17 This adaptor protein, in turn, binds upstream procaspases-8 or -10 to form the DISC.15, 17 Procaspases are the zymogen form of caspases, cystenyl proteases that cut following aspartic acid residues.18 Within the DISC, procaspases-8 and -10 are activated through a forced proximity mechanism.19, 20 Once activated, these caspases cleave and activate downstream procaspases-3 and -7. Caspases-3 and -7 then cut hundreds of cellular proteins to bring about apoptotic death through mechanisms that are incompletely understood. In most cells, activation of the extrinsic pathway alone is insufficient to kill, and requires amplification through the intrinsic pathway. One means by which amplification is achieved is through the cleavage of the Bcl-2 family protein BH3-interacting domain death agonist (Bid) by caspase-8, following which truncated Bid translocates to the mitochondria and contributes to outer mitochondrial membrane (OMM) apoptotic events described below.21
In the assembly of complex I, the binding of death ligand to receptor recruits TRADD, which recruits receptor interacting protein 1 (RIP1, a serine/threonine kinase), cellular Inhibitor of Apoptosis Proteins (IAP) 1 and 2, and TNF receptor-associated factor 2 and 5 (TRAF 2 and 5).16 RIP1 undergoes K63-polyubiquitination by cIAP1 and -2.22, 23 This provides a platform for the recruitment of additional kinases that activate NF-kB, resulting in the transcription of survival proteins.24 However, following dissociation of death receptor, endocytosis, deubiquitination of RIP1, and recruitment of a FADD-RIP3 complex, complex I morphs into complex II.25, 26 Complex II signals apoptosis when FADD recruits procaspase-8 leading to its activation by forced proximity.16, 19 Caspase-8 not only activates downstream caspases to bring about apoptosis, it also cleaves RIP1 and RIP3 abrogating their ability to signal necrosis (see below).27 If caspase-8 activity is inhibited experimentally or by certain viral or cancer proteins, apoptosis is blocked, obligating the cell to undergo necrosis in this pathway.28, 29 Necrosis is triggered by the interaction of RIP1 with RIP3, a second serine/threonine kinase, resulting in a complex series of cross-phosphorylation events. Necrostatin-1, a small molecule inhibitor of the kinase activity of RIP1, ablates necrosis in the death receptor pathway.30
Events in this pathway downstream of RIP1 and RIP3 are incompletely understood, but include phosphorylation by RIP3 of mixed lineage kinase domain-like protein (MLKL),31 phosphoglycerate mutase (PGAM5, a mitochondrial phosphatase),32 and certain catabolic enzymes (glutamate dehydrogenase 1 (GLUD1), glutamate ammonia ligase (GLUL), and glycogen phosphorylase (PYGL)), the latter potentially eliciting necrosis through the generation of ROS.33 The effects of ROS at the mitochondria will be discussed below. In addition, ROS-mediated DNA damage leads to overactivation of poly(ADP-ribose) polymerase-1(PARP-1), a nuclear enzyme that consumes NAD+ leading to significant ATP consumption, a key feature of necrosis.34 Other downstream events that have been implicated in death receptor necrosis signaling include activation of calpains, phospholipases, lipoxygenases, and sphingomyelinases and permeabilization of lysosomes. For further details, the reader is referred to a recent review.35
Intrinsic (mitochondrial/ER) pathway: apoptosis and necrosis
Mitochondria and ER are central to both apoptotic and necrotic signaling, and the intrinsic pathway mediates a more diverse array of death stimuli than does the death receptor pathway. These include deprivation of nutrients, oxygen, and survival factors, oxidative stress, DNA damage, proteotoxic stress, and chemical and physical toxins. Current understanding suggests that the pathways and events that mediate apoptosis and necrosis at the mitochondria are spatially and mechanistically distinct. The primary event in apoptosis is permeabilization of the OMM resulting in the release of apoptogens.1 In contrast, the defining event in primary necrosis is the early opening of a channel in the inner mitochondrial membrane (IMM) termed the mitochondrial permeability transition pore (mPTP).36
Mitochondrial signaling: apoptosis
The main regulators of the mitochondrial apoptosis pathway are the Bcl-2 family proteins.37 In addition, as will be discussed below, recent data also implicates these proteins in the regulation of necrosis. The Bcl-2 family is comprised of both antiapoptotic (e.g. Bcl-2, Bcl-xL, Mcl-1) and proapoptotic members, and the proapoptotics are further divided into multidomain (e.g. Bax, Bak) and BH3-only proteins (multiple members).37 In healthy cells, Bax resides primarily in the cytosol. In response to death stimuli, Bax undergoes conformational activation and translocates to the mitochondria, where it inserts into the OMM.38 Apoptotic signals also stimulate the conformational activation of Bak, which is constitutively localized to the OMM.39 Within the OMM, Bax and Bak homo- and hetero-oligomerize to bring about OMM permeabilization through poorly understood mechanisms.40 The noxious stimuli that activate Bax and Bak are transduced from various locations in the cell via specific BH3-only proteins. For example, loss of the survival signals insulin and IGF-1 leads to activation of the BH3-only protein Bad by decreasing Bad phosphorylation and permitting its release from the 14-3-3 protein.41 The means by BH3-only proteins activate Bax and Bak is complex. Certain BH3-only proteins called “activators” (e.g. Bim, Bid) bind directly to Bax (and possibly Bak) to conformationally activate these proteins. Other BH3-only proteins called “sensitizors” displace the activator BH3-only proteins from anti-apoptotics such as Bcl-2 and Bcl-xL. Conversely, anti-apoptotic Bcl-2 proteins inhibit Bax and Bak by sequestering the BH3-only activators, and possibly also through direct interactions with Bax and Bak.38
Permeabilization of the OMM leads to the release of apoptogens, including cytochrome c, Smac/Diablo, Omi/HtrA2, Apoptosis Inducing Factor (AIF), and Endonucleas e G (Endo G) from the mitochondria to the cytosol. Cytosolic cytochrome c and dATP bind to the adaptor protein Apaf-1 resulting in a presumed conformational change that stimulates Apaf-1 oligomerization and its recruitment of upstream procaspase-9 into a complex termed the apoptosome.42, 43 Procaspase-9 is activated by forced proximity within this complex and goes on to cleave and activate procaspases-3 and -7. Apoptosis is opposed by IAP family members, the same proteins that act in the death receptor necrosis pathway to signal survival through their K63-polyubiquination of RIP1. In the mitochondrial apoptosis pathway, these IAPs inhibit already activated downstream caspases by occluding access of substrates to the active sites of these caspases.44-46 The apoptogens Smac/Diablo and Omi/HtrA2 reverse caspase inhibition by IAPs through binding to IAPs and displacing the caspases.47-50 In addition, Omi/HtrA2 possesss serine protease activity that cleaves XIAP, an IAP.51 AIF, which in combination with perhaps EndoG, causes fragmentation of DNA from ~200 to 50 kb fragments, has been hypothesized to mediate a form of caspase-indendent cell death.52, 53 However, it is possible that the primary role of AIF-induced DNA damage is to further augment activation of PARP-1 leading to ATP depletion during necrosis.
A host of inhibitors oppose these apoptosis pathways. These include FADD-like IL-1β-converting enzyme inhibitory protein (FLIP) which inhibits DISC assembly and/or function,54 anti-apoptotic Bcl-2 proteins that block release of mitochondrial apoptogens, and IAP family members that inhibit already activated downstream caspases as described. While these inhibitors act on either the death receptor or mitochondrial apoptosis pathways, Apoptosis Repressor with Caspase recruitment domain (ARC) inhibits both pathways by disrupting DISC assembly and inhibiting Bax activation.55 ARC expression was initially believed to be limited to cardiac and skeletal myocytes and neurons, but recent data shows that is also induced at high levels in cancer cells56-58 and hypoxic pulmonary artery smooth muscle cells in vivo.59
Mitochondrial signaling: necrosis
In contrast to OMM permeabilization in apoptosis, the defining event of necrosis at the mitochondria is opening of the mPTP, a pore in the IMM. In healthy mitochondria, the IMM is impermeable to water, ions, and even single protons. As substrates are metabolized in the mitochondrial matrix resulting in the transport of electrons along the respiratory chain, protons are pumped from the matrix to the intermembrane space. This creates an electrochemical gradient (ΔΨm) between the intermembrane space and matrix, which provides the potential energy necessary to drive ATP synthesis. Necrotic stimuli, such as Ca2+, trigger opening of the mPTP.60 Ca2+-induced mPTP opening can be potentiated by ROS, alkalosis and depletion of ATP or ADP.61, 62 Opening of the mPTP causes abrupt loss of ΔΨm leading to cessation of mitochondrial ATP synthesis. In addition, mPTP opening allows water to rush down its osmotic gradient into the matrix, leading to mitochondrial swelling, and sometimes frank rupture of the OMM. Although rupture of the OMM can cause release of cytochrome c and activate caspases,63 it is unclear how much engagement of downstream apoptosis signaling contributes to cell death in the mitochondrial necrosis pathway given the other cataclysmic events precipitated by mPTP opening. However, as will be discussed below, potential caspase activation during necrosis complicates interpretation of assays such as TUNEL, which are traditionally assumed to be specific to apoptosis.
Despite extensive research in the field, the components of the mPTP remain unknown. The adenine nucleotide translocase (ANT) and phosphate carrier in the IMM, voltage-dependent anion channel (VDAC) and peripheral benzodiazepine receptor in the OMM, hexokinase which is loosely attached to the cytosolic face of the OMM, and cyclophilin D (a peptidyl prolyl cis-trans isomerase) in the matrix have been proposed to be components of the pore.36 However, genetic studies have excluded ANT,64 VDAC,65 and cyclophilin D63, 66 as core pore components, although ANT and cyclophilin D are important positive regulators of pore opening.63, 64, 66
Necrosis can occur as a primary event or secondary to apoptosis, the latter when the disposal of apoptotic bodies is delayed. Delayed clean-up occasionally occurs in vivo, and is almost always observed at late time points in cell culture.11 In primary necrosis, mPTP opening occurs early, before cytochrome c release. If mPTP opening takes place during apoptosis, it occurs coincident with or following cytochrome c release. In this case, mPTP opening may result from caspase-dependent events.67 Although the kinetics differ markedly, these observations explain why loss of ΔΨm may provide a marker for both necrosis and apoptosis.
How cell death stimuli connect with the mitochondrial necrosis machinery is incompletely understood. Some classic activators of this pathway, such as ischemia and ischemia-reperfusion, induce mPTP opening through Ca2+ and ROS. In addition, activators of the death receptor necrosis pathway may ultimately engage the mitochondrial necrosis pathway through links that were previously discussed. It is likely, however, that additional connections/pathways exist.
ER-mediated apoptosis and necrosis
The ER mediates the synthesis and proper folding of multiple proteins, some post-translational modifications, trafficking of newly synthesized proteins to the Golgi apparatus, lipid biosynthesis, and Ca2+ homeostasis. These effects are critical for normal cellular functioning. Under certain conditions, however, the ER can also mediate cell death – both apoptosis and necrosis. Considerable controversy exists as to the precise mechanisms by which the ER contributes to cell death and the mechanisms that mediate the switch from adaptation to death. While adaptive and death responses could be mediated by parallel pathways, the involvement of shared signaling components implicates the same pathways in both outcomes. For example, misfolded proteins in the ER lumen elicit a response mediated by ER transmembrane sensors PKR-like ER kinase (PERK), inositol-requiring protein 1α (IRE1α), and activating transcription factor 6 (ATF6). These proteins activate complex transcriptional and post-transcrptional cascades to re-establish ER homeostasis. However, it is thought that, when various ER stresses (e.g. misfolded proteins, oxidative stress, certain lipids) fail to be resolved in a timely manner, death may result.68
While the precise ER-specific machinery by which cell death is promoted remains incompletely understood, the transcription factor C/EBP homologous protein (CHOP) has been clearly implicated. CHOP, which is activated downstream of the ER transmembrane sensors, induces the expression of pro-apoptotic proteins Bim,69 TREB3,70 and DR571 and represses that of Bcl-2.72 Another important death mediator is Ca2+, which transits from the ER lumen to the mitochondria, to trigger apoptosis or necrosis through mechanisms that are discussed in the section on cross-talk between mitochondrial apoptosis and necrosis pathways. Less clear are potential roles for various caspases ,73, 74 JNKs,75 other ER membrane proteins ,76 and cleavage of multiple mRNAs by IRE1α,77 which also possesses endonuclease activity.
Autophagy-associated cell death
Autophagy is a process in which the cell breaks down its own proteins and lipds. This provides energy during periods of starvation and stress, a means for the disposal of long-lived proteins, and a mechanism for protein quality control.78 Accordingly, in organisms ranging from yeast to mammals, autophagy is a survival mechanism. That said, too much autophagy has been hypothesized to cause cell death, a process referred to as autophagic cell death or, more accurately, as autophagy-associated cell death. It is plausible that self-cannibalization could result in cell death. However, at this point in time, a direct causal link between autophagy and cell death has not been definitively demonstrated. One impediment in establishing this connection is the absence of markers for autophagy-associated death - in distinction to the existence of abundant markers for autophagy itself. In most experiments, an intervention is used to alter rates of autophagy, the success of which is confimed with autophagy markers, and this manipulation is then correlated with histological markers of cell death (e.g. TUNEL). While it is possible that autophagy kills cells indirectly through another form of cell death (see below), an autophagy-specific mode of killing has not been identified. Questions remain even regarding the interpretation of electron micrographs showing presumably dead or dying cells that contain autophagic vacuoles because it is unclear whether autophagy in this situation represents a pathogenic mechanism, a compensatory process, or is unrelated to the presumed cell death.79 There are, however, some convincing data supporting a role for autophagy in cell death, e.g. during regression of the salivary gland in Drosophila development.80 In addition, we will highlight studies linking autophagy to cell death during myocardial infarction and heart failure in the section on heart disease below.
Although a dedicated machinery for autophagy-associated cell death has not been identified, physical and functional connections between key autophagy and cell death proteins have been recognized and might provide insights into interrelationships between these processes.81 In the discussion to follow, the reader is referred to a number of comprehensive reviews dealing with autophagy.78, 82, 83 Beclin-1, a protein involved in autophagosome formation, contains a BH3 domain analogous to those in BH3-only proteins, which as discussed above promote apoptosis. The Bcl-2-Beclin-1 interaction inhibits the pro-autophagic function of Beclin-1 in response to starvation without interfering with anti-apoptotic function of Bcl-2. Moreover, multiple BH3-only proteins can displace Beclin-1 from Bcl-2 to promote autophagy.81
Connections between cell death pathways
We have previously discussed connections that link (a) death receptor apoptosis with mitochondrial apoptosis pathways (e.g. Bid); and (b) death receptor apoptosis with death receptor necrosis pathways (caspase-8 activity as a decision point in apoptosis versus necrosis in this pathway). In this section, we consider molecules/pathways connecting (a) necrosis signaling at death receptors with that at the mitochondria; and (b) mitochondrial apoptosis and necrosis pathways.
Cross-talk between death receptor and mitochondrial necrosis pathways
As previously discussed, activation of the death receptor pathway signals necrosis when caspase-8 is inhibited.28, 29 Induction of necrosis in this paradigm is abrogated by the absence of Bax/Bak or cyclophilin D, genetically linking death receptor and mitochondrial necrosis events.84, 85 Second, RIP1 translocates to the mitochondria when activated in the death receptor necrosis pathway, although its mitochondrial actions are not yet understood.86 Third, activation of RIP1 and RIP3 in the death receptor pathway stimulates ROS production through NOX1 and GLUD1/GLUL/PYGL1 activation respectively,33, 87 and as discussed, ROS is a strong potentiator of Ca2+-induced mPTP opening. Fourth, as discussed previously, RIP3 activation in the death receptor pathway also triggers cell death through phosphorylation of the mitochondrial phosphatase PGAM5.32 Other connections are likely to become evident as these pathways are understood in more detail.
Cross-talk between mitochondrial apoptosis and necrosis pathways
We have previously discussed some connections between these pathways including how OMM rupture (not permeabilization) in necrosis may result in cytochrome c release, and how caspase activation in apoptosis may trigger late mPTP opening. Another important connection involves Bcl-2 proteins, which unite apoptosis and necrosis signaling at the mitochondria through their effects on Ca2+ handling at the ER.88 Bax, which induces OMM permeabilization during apoptosis, also increases the concentration of Ca2+ in the ER lumen, such that a larger Ca2+ bolus is released when the ER is presented with a death stimulus. ER Ca2+ transits to the mitochondria either through the cytoplasm or via direct connections between mitochondria and ER .89, 90 Increases in mitochondrial Ca2+ can trigger mPTP opening and necrosis or apoptosis through mechanisms that have not yet been defined. Bcl-2 opposes these Bax-induced effects at both the mitochondria and ER.
CELL DEATH IN HEART DISEASE
Myocardial infarction
Surgical occlusion of the left coronary artery is used as a surrogate for acute thrombosis in animal models of ST-segment elevation (STEMI) myocardial infarction. This process is usually studied in the context of reperfusion (“ischemia-reperfusion”, I/R) because of the clear benefit of restoring blood flow in human myocardial infarction. It should be noted, however, that despite the net effect of reperfusion to reduce infarct size, the introduction of blood into an ischemic zone generates ROS, Ca2+, and alkalosis, all inducers of mPTP opening.36 For this reason, significant research is directed toward reducing “reperfusion injury”.91 Another point relevant to interpreting data from rodent models of ischemia-reperfusion is that, despite rare reports to the contrary, it is unusual for genetic or pharmacological manipulations to reduce infarct size in the setting of prolonged ischemia without reperfusion (“permanent occlusion”), another reason why most studies employ I/R.
Cell Death in myocardial infarction
In both permanent occlusion and I/R models of myocardial infarction, a large burst of cell death takes place within the area rendered ischemia over the first 6-24 hours.92 Lesser amounts of cell death takes place in the peri-infarct zone, initially the result of residual ischemia, but persisting due to cardiac remodeling driven by the loss of contractile units in the infarct. A yet lower magnitude of cell death continues for months in the remote myocardium as remodeling progresses.93 In this section, we focus on cardiac myocyte death in the ischemic zone.
During myocardial infarction, cardiac myocytes in the ischemic zone die by both apoptosis and necrosis. Surprising, the magnitudes of each form of cell death remain unclear. The impediment has been limitations of current assays to definitively distinguish between apoptosis and necrosis in tissue from animals subjected to myocardial infarction. For example, although the primary consequence of mPTP opening during necrosis is cessation of ATP synthesis, the accompanying mitochondrial swelling can result in OMM rupture and cytochrome c release. It is unclear how often OMM rupture occurs in this situation, but the potential release of cytochrome c confounds the interpretation of assays based on caspase activation and DNA fragmentation (e.g. TUNEL). Solutions include the direct evaluation of plasma membrane integrity in vivo using a variety of approaches and electron microscopy, although the latter is limited by differential sensitivities for the detection of necrotic versus apoptotic cells. While these techniques have been employed to some extent, a rigorous quantification of apoptosis and necrosis during myocardial infarction is needed.
Apoptosis in myocardial infarction
Multiple studies have demonstrated a causal connection between cardiac myocyte apoptosis and myocardial infarction. Both the death receptor and mitochondrial pathways have been shown to be critical. Hearts of mice lacking Fas (Ipr mice) exhibit smaller infarcts in response to I/R, when studied as isolated preparations or in vivo.94, 95 Given that death signals related to I/R potently activate the mitochondrial pathway, the reasons underlying the importance of the death receptor pathway in this process are not obvious. One explanation may be that death ligands themselves are important mediators of I/R, and in support of this, Fas ligand appears in the coronary effluent of isolated hearts during the reperfusion phase. Another possibility may be that activation of the death receptor pathway provides another input into activation of the mitochondrial apoptosis pathway through truncated Bid.
Cardiac-specific overexpression of Bcl-2 decreases infarct size and cardiac dysfunction following I/R in vivo.96, 97 In addition, deletion of Bax reduces infarct size in isolated hearts subjected to I/R.98 Bax deletion has also been reported to cause mild reductions in infarct size following permanent occlusion in vivo.99 Absence of PUMA, a p53 responsive BH3-only protein, reduces infarct in isolated, perfused hearts subjected to I/R.100 Thus, Bcl-2 family members modulate infarct size.
Cardiac overexpression of cIAP2 results also in smaller infarcts in isolated perfused hearts subjected to I/R.101 This effect may result from the inhibition of already activated downstream caspases by IAPs by cIAP2 and/or its K63-polyubiquitination of RIP1 which activates the death receptor survival pathway. UCF-101, a small molecule inhibitor of the serine protease activity of Omi/HtrA2, decreases infarct size following I/R.102, 103 Pancaspase inhibitors provide varying degrees of reduction in the size of infarcts elicited by I/R.104-107 Overexpression of ARC, which inhibits both death receptor and mitochondrial apoptosis pathways, also decreases infarct size after I/R.108 The fact that multiple manipulations of apoptosis pathways affect infarct size provides confidence that this form of cell death is involved in myocardial infarction.
Necrosis in myocardial infarction
Regulated necrosis has also been demonstrated to play a role in the development of myocardial infarction. Necrostatin, the inhibitor of the kinase activity of RIP1, reduces infarct size in response to I/R in vivo. Interestingly, its cardioprotective effect is dependent on the presence of cyclophilin D, suggesting connections between RIP1 and mitochondrial necrosis events.109
Bax and Bak have recently been shown to regulate necrosis. In addition to reducing infarct size, deletion of Bax and Bak markedly reduces the degree of necrotic injury in the hearts of mice subjected to I/R. These effects occurs through a pathway distinct from the regulation of apoptosis by Bax and Bak, as evidenced by the ability of oligomerization-deficient Bax mutants, which cannot support apoptosis, but retain the ability to mediate necrosis.110
Mice lacking cyclophilin D, a positive regulator of mPTP opening, demonstrate decreased infarct size following I/R.63, 66 Pharmacologic inhibition of cyclophilin D, using cyclosporine A or sangliferin A, also reduces infarct size.111-114 A pilot study has translated this work to a small number of patients with ST-segment elevation myocardial infarction. When superimposed on angioplasty and stenting, cyclosporine A resulted in statistically significant reduction in infarct size as measured by serum levels of creatine kinase, but not troponin I, and by magnetic resonance imaging.115 While significant reductions in infarct size persisted at 6 months post-myocardial infarction, only a non-statistically significant trend toward preserved cardiac function was observed.116 Thus, further work is needed to assess the efficacy of this cardioprotective strategy in humans.
Taken together, these studies demonstrate that both apoptosis and necrosis contribute to the pathogenesis of myocardial infarction.
Autophagy-associated death in myocardial infarction
Autophagy is induced during both I/R and permanent occlusion. However, the mechanisms and the consequences of this induction appear to be different.117 During permanent occlusion, AMPK is activated and inhibits mTOR, a potent inhibitor of autophagy. Consequently, autophagy is induced. Inhibition of autophagy by transgenic overexpression of dominant negative AMPK resulted in worsening of infarct size in response to permanent occlusion.117 Similar results were obtained when autophagy was inhibited by overexpression of Rheb ,118 overexpression of a dominant negative form of GSK3β, or deletion of one allele of GSK3β .119 Thus, consistent with the survival role of autophagy during starvation, these data suggest that autophagy protects the myocardium during prolonged ischemia. During I/R, however, Beclin-1 levels increase to activate autophagy. Mice in which one Beclin-1 allele has been inactivated exhibit smaller infarcts in this situation.117 Similar results were found when autophagy was decreased by loss of fuction manipulations of GSK3β as described above .119 These and other studies120, 121 suggest that autophagy is associated with a protective role during ischemia and a pathogenic role during I/R. Further investigation is needed, however, to determine the extent to which alterations in autophagy explain these changes in infarct size.
Cell death and heart failure
Apoptosis in heart failure
In contrast to myocardial infarction in which there is an explosive and short-lived burst of cell death, the absolute percentage of apoptotic cardiac myocytes in failing human hearts is quite low (0.08-0.25% as assessed by TUNEL). However, this percentage of cardiac myocyte apoptosis is ~10-100-fold higher that that observed in control hearts (0.001-0.01%).122-124 These data suggest the hypothesis that low, but elevated levels of cardiac myocyte apoptosis, result over time in cumulative loss of cardiac myocytes and heart failure. This possibility was first tested in transgenic mice with a conditionally-activatable procaspase-8 allele, which showed that rates of cardiac myocyte apoptosis as low as 0.023% elicit a lethal dilated cardiomyopathy. Control mice overexpressing an enzymatically-dead procaspase-8 remained normal.125 These data establish the sufficiency of clinically-relevant levels of apoptosis to induce heart failure.
Conversely, the necessity of cardiac myocyte apoptosis for heart failure was tested using pancaspase inhibition in a model of peripartum cardiomyopathy.126 This was induced by cardiac-specific overexpression of Gαq, a surrogate for humoral stimuli relevant to heart failure. Pregnancy precipitated lethal heart failure in 30% of Gαq transgenic mice. Pre-treatment with a pancaspase inhibitor reduced cardiac myocyte apoptosis, preserved heart function, and completely rescued mortality. These data demonstrate the necessity of cardiac myocyte apoptosis for heart failure in this model. These concepts have also been extended to other models. For example, following myocardial infarction, deletion of Bcl-2/adenovirus E1b 19kD interacting protein 3 (Bnip3), a BH3-like protein, reduced pathological remodeling in the perinfarct zone and resultant heart failure.127
Necrosis in heart failure
Cardiac myocyte necrosis may also play a role in heart failure. Cardiac myocyte-specific transgenic overexpression of the β2-α subunit of the L-type Ca2+ channel resulted in Ca2+ overload, mPTP opening, necrosis, and cardiac dysfunction.128 This phenotype was rescued by deletion of ppif encoding cyclophilin D, but not overexpression of Bcl-2, suggesting that heart failure in this model is attributable to cardiac myocyte necrosis. Similarly, doxorubicin-induced cardiomyopathy was ameliorated by knockout ppif. In contrast to myocardial infarction, involvement of necrosis in heart failure is somewhat unexpected. While this interpretation may be correct, it is important to also consider recently discovered effects of cyclophilin D on cardiac metabolism.129 Future work will be needed to determine the magnitude of cardiac myocyte necrosis in failing hearts and the general applicability to pathogenesis of this syndrome.
Autophagy-associated death in heart failure
A previous study of failing human hearts has suggested that autophagy-associated cell death is the most common form of cellular demise during heart failure.130 However, the markers used to diagnose various forms of cell death in this study were not specific. Stronger data concerning the relationship of autophagy and heart failure have been provided by genetic loss- and gain-of-function studies. Atg5 deletion in the heart precipitates ventricular enlargement and cardiac dysfunction after hemodynamic overload implying that autophagy is a compensatory mechanism during heart failure .131 In contrast, Beclin-1+/- mice subjected to pressure overload exhibited decreased pathological remodeling and cardiac dysfunction, while Beclin-1 overexpression resulted in the opposite .132 The explanation for the conflicting results in the Atg5 and Beclin-1 studies is not known, but may be related to differences in the genetic manipulations or apparent severity of pressure overload. Therefore, the role of autophagy in the pathogenesis of pressure overload-induced heart failure is not clear. On the other hand, deletion of one allele of Beclin-1 worsens cardiac remodeling and function and mortality in response to proteotoxic stress induced by transgenic overexpression of the R120G mutant of αβ crystallin, a model of desmin-related cardiomyopathy.133 Thus, in keeping with its role in disposing of defective proteins, autophagy plays a protective role in heart failure initiated by proteotoxicity. Taken together, these data highlight that autophagy may be protective in response to some cardiomyopathic stimuli and pathogenic in response to others.
CONCLUDING REMARKS
This review discusses the role of cell death in the major syndromes that affect the heart: myocardial infarction and heart failure. Although myocardial infarction and heart failure are complex and involve multiple cellular processes, the data indicate that cell death plays a critical role in the pathogenesis of both syndromes. The regulated nature of much of the cell death in these diseases opens up the possibility of manipulating death pathways to therapeutic advantage. Given its acute nature, myocardial infarction is currently the most attractive target. An important issue in this setting is how the drug will access tissue in which the blood supply is compromised. One possibility is drug delivery at the time of reperfusion. However, administration even prior to reperfusion may have beneficial effects on the peri-infarct region as well as potentially extending the window for effective reperfusion. Heart failure may also be a viable target, but potential oncogenic effects of chronic cell death inhibition are a concern. To circumvent this obstacle will require the development of approaches to target drug to the myocardium. The hope is that, in combination with therapies directed at atherosclerosis and plaque rupture, small molecule approaches to decrease the susceptibility of the myocardium to cell death will limit tissue damage and ultimately reduce mortality.
ACKNOWLEDGMENTS
We thank Gloria Kung and Wendy M. McKimpson for their critical comments on the manuscript.
SOURCES OF FUNDING
This work was supported by grants from National Institutes of Health 5R01HL060665-14 (RNK), 5P60DK020541-35 (RNK), 5P30CA013330-39 (RNK), and 5T32AG023475-08 (RSW), and the A. G. Leventis Foundation (KK). R.N.K. is supported by the Dr. Gerald and Myra Dorros Chair in Cardiovascular Disease. We are most grateful to the Wilf Family for their ongoing generosity and support.
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Cotter TG. Apoptosis and cancer: The genesis of a research field. Nat Rev Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 5.Gurzov EN, Eizirik DL. Bcl-2 proteins in diabetes: Mitochondrial pathways of beta-cell death and dysfunction. Trends Cell Biol. 2011;21:424–431. doi: 10.1016/j.tcb.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 8.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin d deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in alzheimer's disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan J. Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis. 2009;14:469–477. doi: 10.1007/s10495-008-0304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr JF. Shrinkage necrosis: A distinct mode of cellular death. J Pathol. 1971;105:13–20. doi: 10.1002/path.1711050103. [DOI] [PubMed] [Google Scholar]
- 11.Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140:798–804. doi: 10.1016/j.cell.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Sun XM, Butterworth M, MacFarlane M, Dubiel W, Ciechanover A, Cohen GM. Caspase activation inhibits proteasome function during apoptosis. Mol Cell. 2004;14:81–93. doi: 10.1016/s1097-2765(04)00156-x. [DOI] [PubMed] [Google Scholar]
- 13.Soldani C, Scovassi AI. Poly(adp-ribose) polymerase-1 cleavage during apoptosis: An update. Apoptosis. 2002;7:321–328. doi: 10.1023/a:1016119328968. [DOI] [PubMed] [Google Scholar]
- 14.Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (atp) concentration: A switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent apo-1 (fas/cd95)-associated proteins form a death-inducing signaling complex (disc) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Micheau O, Tschopp J. Induction of tnf receptor i-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 17.Peter ME, Krammer PH. The cd95(apo-1/fas) disc and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 18.Pop C, Salvesen GS. Human caspases: Activation, specificity, and regulation. J Biol Chem. 2009;284:21777–21781. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 20.Bao Q, Shi Y. Apoptosome: A platform for the activation of initiator caspases. Cell Death Differ. 2007;14:56–65. doi: 10.1038/sj.cdd.4402028. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of bid by caspase 8 mediates the mitochondrial damage in the fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 22.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. Ciap1 and ciap2 facilitate cancer cell survival by functioning as e3 ligases that promote rip1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of ikk by tnfalpha requires site-specific ubiquitination of rip1 and polyubiquitin binding by nemo. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E, Arora V, Mak TW, Lacasse EC, Waring J, Korneluk RG. Both ciap1 and ciap2 regulate tnfalpha-mediated nfkappab activation. Proc Natl Acad Sci U S A. 2008;105:11778–11783. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Du F, Wang X. Tnf-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase rip by caspase-8 prompts tnf-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase rip as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 29.Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P. Inhibition of caspases increases the sensitivity of l929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of rip1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of rip3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase pgam5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 33.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. Rip3, an energy metabolism regulator that switches tnf-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 34.Los M, Mozoluk M, Ferrari D, Stepczynska A, Stroh C, Renz A, Herceg Z, Wang ZQ, Schulze-Osthoff K. Activation and caspase-mediated inhibition of parp: A molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol Biol Cell. 2002;13:978–988. doi: 10.1091/mbc.01-05-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kung G, Konstantinidis K, Kitsis RN. Programmed necrosis, not apoptosis, in the heart. Circ Res. 2011;108:1017–1036. doi: 10.1161/CIRCRESAHA.110.225730. [DOI] [PubMed] [Google Scholar]
- 36.Halestrap AP. A pore way to die: The role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans. 2010;38:841–860. doi: 10.1042/BST0380841. [DOI] [PubMed] [Google Scholar]
- 37.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The bcl-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walensky LD, Gavathiotis E. Bax unleashed: The biochemical transformation of an inactive cytosolic monomer into a toxic mitochondrial pore. Trends Biochem Sci. 2011;36:642–652. doi: 10.1016/j.tibs.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. Vdac2 inhibits bak activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 40.Antignani A, Youle RJ. How do bax and bak lead to permeabilization of the outer mitochondrial membrane? Curr Opin Cell Biol. 2006;18:685–689. doi: 10.1016/j.ceb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Danial NN. Bad: Undertaker by night, candyman by day. Oncogene. 2008;27(Suppl 1):S53–70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- 42.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and datp-dependent formation of apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 43.Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X, Akey CW. Three-dimensional structure of the apoptosome: Implications for assembly, procaspase-9 binding, and activation. Mol Cell. 2002;9:423–432. doi: 10.1016/s1097-2765(02)00442-2. [DOI] [PubMed] [Google Scholar]
- 44.Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun C, Fesik SW, Liddington RC, Salvesen GS. Structural basis for the inhibition of caspase-3 by xiap. Cell. 2001;104:791–800. doi: 10.1016/s0092-8674(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 45.Chai J, Shiozaki E, Srinivasula SM, Wu Q, Datta P, Alnemri ES, Shi Y. Structural basis of caspase-7 inhibition by xiap. Cell. 2001;104:769–780. doi: 10.1016/s0092-8674(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Park YC, Rich RL, Segal D, Myszka DG, Wu H. Structural basis of caspase inhibition by xiap: Differential roles of the linker versus the bir domain. Cell. 2001;104:781–790. [PubMed] [Google Scholar]
- 47.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating iap inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 48.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of diablo, a mammalian protein that promotes apoptosis by binding to and antagonizing iap proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 49.Faccio L, Fusco C, Chen A, Martinotti S, Bonventre JV, Zervos AS. Characterization of a novel human serine protease that has extensive homology to bacterial heat shock endoprotease htra and is regulated by kidney ischemia. J Biol Chem. 2000;275:2581–2588. doi: 10.1074/jbc.275.4.2581. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R. A serine protease, htra2, is released from the mitochondria and interacts with xiap, inducing cell death. Mol Cell. 2001;8:613–621. doi: 10.1016/s1097-2765(01)00341-0. [DOI] [PubMed] [Google Scholar]
- 51.Yang QH, Church-Hajduk R, Ren J, Newton ML, Du C. Omi/htra2 catalytic cleavage of inhibitor of apoptosis (iap) irreversibly inactivates iaps and facilitates caspase activity in apoptosis. Genes Dev. 2003;17:1487–1496. doi: 10.1101/gad.1097903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 53.Li LY, Luo X, Wang X. Endonuclease g is an apoptotic dnase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 54.Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, Peter ME, Yang X. C-flip(l) is a dual function regulator for caspase-8 activation and cd95-mediated apoptosis. EMBO J. 2002;21:3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nam YJ, Mani K, Ashton AW, Peng CF, Krishnamurthy B, Hayakawa Y, Lee P, Korsmeyer SJ, Kitsis RN. Inhibition of both the extrinsic and intrinsic death pathways through nonhomotypic death-fold interactions. Mol Cell. 2004;15:901–912. doi: 10.1016/j.molcel.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 56.Mercier I, Vuolo M, Madan R, Xue X, Levalley AJ, Ashton AW, Jasmin JF, Czaja MT, Lin EY, Armstrong RC, Pollard JW, Kitsis RN. Arc, an apoptosis suppressor limited to terminally differentiated cells, is induced in human breast cancer and confers chemo- and radiation-resistance. Cell Death Differ. 2005;12:682–686. doi: 10.1038/sj.cdd.4401631. [DOI] [PubMed] [Google Scholar]
- 57.Mercier I, Vuolo M, Jasmin JF, Medina CM, Williams M, Mariadason JM, Qian H, Xue X, Pestell RG, Lisanti MP, Kitsis RN. Arc (apoptosis repressor with caspase recruitment domain) is a novel marker of human colon cancer. Cell Cycle. 2008;7:1640–1647. doi: 10.4161/cc.7.11.5979. [DOI] [PubMed] [Google Scholar]
- 58.Carter BZ, Qiu YH, Zhang N, Coombes KR, Mak DH, Thomas DA, Ravandi F, Kantarjian HM, Koller E, Andreeff M, Kornblau SM. Expression of arc (apoptosis repressor with caspase recruitment domain), an antiapoptotic protein, is strongly prognostic in aml. Blood. 2011;117:780–787. doi: 10.1182/blood-2010-04-280503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaiman AL, Damico R, Thoms-Chesley A, Files DC, Kesari P, Johnston L, Swaim M, Mozammel S, Myers AC, Halushka M, El-Haddad H, Shimoda LA, Peng CF, Hassoun PM, Champion HC, Kitsis RN, Crow MT. A critical role for the protein apoptosis repressor with caspase recruitment domain in hypoxia-induced pulmonary hypertension. Circulation. 2011;124:2533–2542. doi: 10.1161/CIRCULATIONAHA.111.034512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crompton M, Costi A, Hayat L. Evidence for the presence of a reversible ca2+-dependent pore activated by oxidative stress in heart mitochondria. Biochem J. 1987;245:915–918. doi: 10.1042/bj2450915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin a of a ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- 62.Halestrap AP. Calcium-dependent opening of a non-specific pore in the mitochondrial inner membrane is inhibited at ph values below 7. Implications for the protective effect of low ph against chemical and hypoxic cell damage. Biochem J. 1991;278(Pt 3):715–719. doi: 10.1042/bj2780715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin d reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 64.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The adp/atp translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin d-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 67.Ricci JE, Munoz-Pinedo C, Fitzgerald P, Bailly-Maitre B, Perkins GA, Yadava N, Scheffler IE, Ellisman MH, Green DR. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex i of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 68.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. Er stress triggers apoptosis by activating bh3-only protein bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 70.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. Trb3, a novel er stress-inducible gene, is induced via atf4-chop pathway and is involved in cell death. EMBO J. 2005;24:1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamaguchi H, Wang HG. Chop is involved in endoplasmic reticulum stress-induced apoptosis by enhancing dr5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 72.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 74.Saleh M, Vaillancourt JP, Graham RK, Huyck M, Srinivasula SM, Alnemri ES, Steinberg MH, Nolan V, Baldwin CT, Hotchkiss RS, Buchman TG, Zehnbauer BA, Hayden MR, Farrer LA, Roy S, Nicholson DW. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature. 2004;429:75–79. doi: 10.1038/nature02451. [DOI] [PubMed] [Google Scholar]
- 75.Lei K, Davis RJ. Jnk phosphorylation of bim-related members of the bcl2 family induces bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mathai JP, Germain M, Shore GC. Bh3-only bik regulates bax,bak-dependent release of ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J Biol Chem. 2005;280:23829–23836. doi: 10.1074/jbc.M500800200. [DOI] [PubMed] [Google Scholar]
- 77.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. Ire1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sridhar S, Botbol Y, Macian F, Cuervo AM. Autophagy and disease: Always two sides to a problem. J Pathol. 2012;226:255–273. doi: 10.1002/path.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in drosophila. Cell. 2007;131:1137–1148. doi: 10.1016/j.cell.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levine B, Sinha S, Kroemer G. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y, Klionsky DJ. The regulation of autophagy - unanswered questions. J Cell Sci. 2011;124:161–170. doi: 10.1242/jcs.064576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Irrinki KM, Mallilankaraman K, Thapa RJ, Chandramoorthy HC, Smith FJ, Jog NR, Gandhirajan RK, Kelsen SG, Houser SR, May MJ, Balachandran S, Madesh M. Requirement of fadd, nemo, and bax/bak for aberrant mitochondrial function in tumor necrosis factor alpha-induced necrosis. Mol Cell Biol. 2011;31:3745–3758. doi: 10.1128/MCB.05303-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to tnf-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 86.Temkin V, Huang Q, Liu H, Osada H, Pope RM. Inhibition of adp/atp exchange in receptor-interacting protein-mediated necrosis. Mol Cell Biol. 2006;26:2215–2225. doi: 10.1128/MCB.26.6.2215-2225.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim YS, Morgan MJ, Choksi S, Liu ZG. Tnf-induced activation of the nox1 nadph oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 88.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. Bax and bak regulation of endoplasmic reticulum ca2+: A control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 89.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 90.De Stefani D, Bononi A, Romagnoli A, Messina A, De Pinto V, Pinton P, Rizzuto R. Vdac1 selectively transfers apoptotic ca2+ signals to mitochondria. Cell Death Differ. 2012;19:267–273. doi: 10.1038/cdd.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 92.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996;74:86–107. [PubMed] [Google Scholar]
- 93.Sam F, Sawyer DB, Chang DL, Eberli FR, Ngoy S, Jain M, Amin J, Apstein CS, Colucci WS. Progressive left ventricular remodeling and apoptosis late after myocardial infarction in mouse heart. Am J Physiol Heart Circ Physiol. 2000;279:H422–428. doi: 10.1152/ajpheart.2000.279.1.H422. [DOI] [PubMed] [Google Scholar]
- 94.Jeremias I, Kupatt C, Martin-Villalba A, Habazettl H, Schenkel J, Boekstegers P, Debatin KM. Involvement of cd95/apo1/fas in cell death after myocardial ischemia. Circulation. 2000;102:915–920. doi: 10.1161/01.cir.102.8.915. [DOI] [PubMed] [Google Scholar]
- 95.Lee P, Sata M, Lefer DJ, Factor SM, Walsh K, Kitsis RN. Fas pathway is a critical mediator of cardiac myocyte death and mi during ischemia-reperfusion in vivo. Am J Physiol Heart Circ Physiol. 2003;284:H456–463. doi: 10.1152/ajpheart.00777.2002. [DOI] [PubMed] [Google Scholar]
- 96.Brocheriou V, Hagege AA, Oubenaissa A, Lambert M, Mallet VO, Duriez M, Wassef M, Kahn A, Menasche P, Gilgenkrantz H. Cardiac functional improvement by a human bcl-2 transgene in a mouse model of ischemia/reperfusion injury. J Gene Med. 2000;2:326–333. doi: 10.1002/1521-2254(200009/10)2:5<326::AID-JGM133>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 97.Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of bcl-2 attenuates apoptosis and protects against myocardial i/r injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 98.Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, Pannet H, Shneyvays V, Shainberg A, Goldshtaub V, Tobar A, Vidne BA. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284:H2351–2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 99.Hochhauser E, Cheporko Y, Yasovich N, Pinchas L, Offen D, Barhum Y, Pannet H, Tobar A, Vidne BA, Birk E. Bax deficiency reduces infarct size and improves long-term function after myocardial infarction. Cell Biochem Biophys. 2007;47:11–20. doi: 10.1385/cbb:47:1:11. [DOI] [PubMed] [Google Scholar]
- 100.Toth A, Jeffers JR, Nickson P, Min JY, Morgan JP, Zambetti GP, Erhardt P. Targeted deletion of puma attenuates cardiomyocyte death and improves cardiac function during ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;291:H52–60. doi: 10.1152/ajpheart.01046.2005. [DOI] [PubMed] [Google Scholar]
- 101.Chua CC, Gao J, Ho YS, Xiong Y, Xu X, Chen Z, Hamdy RC, Chua BH. Overexpression of iap-2 attenuates apoptosis and protects against myocardial ischemia/reperfusion injury in transgenic mice. Biochim Biophys Acta. 2007;1773:577–583. doi: 10.1016/j.bbamcr.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu HR, Gao E, Hu A, Tao L, Qu Y, Most P, Koch WJ, Christopher TA, Lopez BL, Alnemri ES, Zervos AS, Ma XL. Role of omi/htra2 in apoptotic cell death after myocardial ischemia and reperfusion. Circulation. 2005;111:90–96. doi: 10.1161/01.CIR.0000151613.90994.17. [DOI] [PubMed] [Google Scholar]
- 103.Bhuiyan MS, Fukunaga K. Inhibition of htra2/omi ameliorates heart dysfunction following ischemia/reperfusion injury in rat heart in vivo. Eur J Pharmacol. 2007;557:168–177. doi: 10.1016/j.ejphar.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 104.Yaoita H, Ogawa K, Maehara K, Maruyama Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation. 1998;97:276–281. doi: 10.1161/01.cir.97.3.276. [DOI] [PubMed] [Google Scholar]
- 105.Holly TA, Drincic A, Byun Y, Nakamura S, Harris K, Klocke FJ, Cryns VL. Caspase inhibition reduces myocyte cell death induced by myocardial ischemia and reperfusion in vivo. J Mol Cell Cardiol. 1999;31:1709–1715. doi: 10.1006/jmcc.1999.1006. [DOI] [PubMed] [Google Scholar]
- 106.Huang JQ, Radinovic S, Rezaiefar P, Black SC. In vivo myocardial infarct size reduction by a caspase inhibitor administered after the onset of ischemia. Eur J Pharmacol. 2000;402:139–142. doi: 10.1016/s0014-2999(00)00477-5. [DOI] [PubMed] [Google Scholar]
- 107.Yang W, Guastella J, Huang JC, Wang Y, Zhang L, Xue D, Tran M, Woodward R, Kasibhatla S, Tseng B, Drewe J, Cai SX. Mx1013, a dipeptide caspase inhibitor with potent in vivo antiapoptotic activity. Br J Pharmacol. 2003;140:402–412. doi: 10.1038/sj.bjp.0705450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pyo JO, Nah J, Kim HJ, Chang JW, Song YW, Yang DK, Jo DG, Kim HR, Chae HJ, Chae SW, Hwang SY, Kim SJ, Cho C, Oh CG, Park WJ, Jung YK. Protection of cardiomyocytes from ischemic/hypoxic cell death via drbp1 and pme2glydh in cardio-specific arc transgenic mice. J Biol Chem. 2008;283:30707–30714. doi: 10.1074/jbc.M804209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lim SY, Davidson SM, Mocanu MM, Yellon DM, Smith CC. The cardioprotective effect of necrostatin requires the cyclophilin-d component of the mitochondrial permeability transition pore. Cardiovasc Drugs Ther. 2007;21:467–469. doi: 10.1007/s10557-007-6067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, Yang Y, Calvert JW, Lindsten T, Thompson CB, Crow MT, Gavathiotis E, Dorn GW, 2nd, O'Rourke B, Kitsis RN. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci U S A. 2012;109:6566–6571. doi: 10.1073/pnas.1201608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin a acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-d at a different site from cyclosporin a. J Biol Chem. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- 112.Griffiths EJ, Halestrap AP. Protection by cyclosporin a of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 113.Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 114.Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, Ovize M. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol. 2005;38:367–374. doi: 10.1016/j.yjmcc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 115.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 116.Mewton N, Croisille P, Gahide G, Rioufol G, Bonnefoy E, Sanchez I, Cung TT, Sportouch C, Angoulvant D, Finet G, Andre-Fouet X, Derumeaux G, Piot C, Vernhet H, Revel D, Ovize M. Effect of cyclosporine on left ventricular remodeling after reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:1200–1205. doi: 10.1016/j.jacc.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 117.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of amp-activated protein kinase and beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 118.Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, Condorelli G, Sadoshima J. Rheb is a critical regulator of autophagy during myocardial ischemia: Pathophysiological implications in obesity and metabolic syndrome. Circulation. 2012;125:1134–1146. doi: 10.1161/CIRCULATIONAHA.111.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhai P, Sciarretta S, Galeotti J, Volpe M, Sadoshima J. Differential roles of gsk-3beta during myocardial ischemia and ischemia/reperfusion. Circ Res. 2011;109:502–511. doi: 10.1161/CIRCRESAHA.111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 121.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA, Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 122.Guerra S, Leri A, Wang X, Finato N, Di Loreto C, Beltrami CA, Kajstura J, Anversa P. Myocyte death in the failing human heart is gender dependent. Circ Res. 1999;85:856–866. doi: 10.1161/01.res.85.9.856. [DOI] [PubMed] [Google Scholar]
- 123.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 124.Saraste A, Pulkki K, Kallajoki M, Heikkila P, Laine P, Mattila S, Nieminen MS, Parvinen M, Voipio-Pulkki LM. Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur J Clin Invest. 1999;29:380–386. doi: 10.1046/j.1365-2362.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 125.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hayakawa Y, Chandra M, Miao W, Shirani J, Brown JH, Dorn GW, 2nd, Armstrong RC, Kitsis RN. Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of galpha(q) transgenic mice. Circulation. 2003;108:3036–3041. doi: 10.1161/01.CIR.0000101920.72665.58. [DOI] [PubMed] [Google Scholar]
- 127.Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, Li H, Kirshenbaum LA, Hahn HS, Robbins J, Jones WK, Dorn GW. Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of bnip3 restrains postinfarction remodeling in mice. J Clin Invest. 2007;117:2825–2833. doi: 10.1172/JCI32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, Karch J, Gabel S, Farber J, Force T, Brown JH, Murphy E, Molkentin JD. Cyclophilin d controls mitochondrial pore-dependent ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest. 2010;120:3680–3687. doi: 10.1172/JCI43171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kostin S, Pool L, Elsasser A, Hein S, Drexler HC, Arnon E, Hayakawa Y, Zimmermann R, Bauer E, Klovekorn WP, Schaper J. Myocytes die by multiple mechanisms in failing human hearts. Circ Res. 2003;92:715–724. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- 131.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 132.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tannous P, Zhu H, Johnstone JL, Shelton JM, Rajasekaran NS, Benjamin IJ, Nguyen L, Gerard RD, Levine B, Rothermel BA, Hill JA. Autophagy is an adaptive response in desmin-related cardiomyopathy. Proc Natl Acad Sci U S A. 2008;105:9745–9750. doi: 10.1073/pnas.0706802105. [DOI] [PMC free article] [PubMed] [Google Scholar]