Abstract
Endothelial cells (ECs) play important roles in cutaneous inflammation, in part, by release of inflammatory chemokines/cytokines. Because dermal blood vessels are innervated by sympathetic nerves, the sympathetic neurotransmitter norepinephrine (NE) and the co-transmitter adenosine-5’-triphosphate (ATP) may regulate expression of EC inflammatory factors. We focused on IL-6 regulation because it has many inflammatory and immune functions, including participation in Th17 cell differentiation. Strikingly, NE and ATP synergistically induced release of IL-6 by a human dermal microvascular endothelial cell line (HMEC-1). Adrenergic antagonist and agonist studies indicated that the effect of NE on induced IL-6 release is primarily mediated by β2-adrenergic receptors (ARs). By real-time PCR IL-6 mRNA was also synergistically induced in HMEC-1 cells. This synergistic effect of NE and ATP was reproduced in primary human dermal endothelial cells (pHDMECs) and is also primarily mediated by β2-ARs. Under conditions of stress, activation of the symphathetic nervous system may lead to release of ATP and NE by sympathetic nerves surrounding dermal blood vessels with induction of IL-6 production by ECs. IL-6 may then participate in immune and inflammatory processes including generation of Th17 cells. Production of IL-6 in this manner might explain stress-induced exacerbation of psoriasis, and perhaps, other skin disorders involving Th17-type immunity.
Keywords: endothelial cells, norepinephrine, adenosine-5’-triphosphate, IL-6
1. Introduction
Endothelial cells (ECs) are strategically located between the blood and tissue compartments and, therefore, are in a position to play important roles in the initiation and regulation of inflammation [1]. In part, this is through the release of inflammatory chemokines/chemokines which allow them to communicate with other cells and organs and thus modulate immune activities [2–4]. They also express adhesion molecules that mediate rolling, adhesion and transmigration of leukocytes out of the vasculature and into tissues such as the skin [5, 6]. Endothelial cells produce a number of chemokines that bind to and signal through specific receptors on leukocytes, ultimately attracting them to areas of inflammation [3, 7], as well as cytokines including IL-6.
The last several decades have provided strong evidence that the nervous system and immune system are involved in functional cross talk. Interactions between the nervous, immune and endocrine systems are mediated by numerous molecules including cytokines, neurotransmitters, neuropeptides, hormones and their respective receptors. These interactions play an important role in many immune responses including inflammatory diseases and host susceptibility [8–11].
Stress has complex effects on the immune system and can affect both innate and acquired immunity. Stressors may be physical or psychological and can be acute or chronic. The stress response is controlled by elements of the central and peripheral nervous systems. Stress has been shown to have stimulative or inhibitory effects on the immune system depending on the type, duration and intensity of the stressor applied [12–14].
Under conditions of stress, two main neurological pathways are activated, the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system (SNS). Activation of these two pathways results in the release of several types of stress hormones including glucocorticoids, and catecholamines from the adrenal medulla and, especially, norepinephrine by sympathetic nerve termini. These two pathways play major roles in integrating and regulating different immune responses [15, 16]. A third axis, the neurotrophin neuropeptide axis also plays a role [17]. Recent evidence suggests a link between stress and disease susceptibility, especially chronic inflammatory diseases including rheumatoid arthritis, asthma, atherosclerosis and irritable bowel disease as well as psoriasis and certain other skin diseases [16, 18–20].
The SNS innervates both primary (bone marrow and thymus) and secondary (spleen and lymph nodes) immune organs, as well as the skin and other organs and tissues. [15, 21–25]. The SNS also innervates the vasculature allowing it to regulate vasomotor functions and release of blood cells from the blood marrow. Recent evidence indicates the SNS is important in regulation of proinflammatory conditions [11, 26] and that sympathetic neurotransmitters have an important role in regulating immune and inflammatory responses [10, 15, 26].
It has long been hypothesized that stress can influence certain skin conditions such as rosacea, psoriasis and atopic dermatitis [18, 27–31]. Accumulating experimental evidence indicates that the neuroendocrine system plays a key role in cutaneous inflammation [20, 32–34]. The SNS within skin is supplied by postganglionic fibers of the paravertebral chain ganglia [35, 36]. NE released from sympathetic varicose axon terminals diffuses from the release site; thus, NE transmits its signals nonsynaptically to immune cells and the endothelium. Circulating NE, as well as that released from SNS peripheral nerves locally, may modulate immune function by binding to ARs expressed on immune cells, often resulting in changes in cytokine/chemokine production. ARs are heteromeric 7-transmembrane spanning G-coupled-proteins and are subdivided into 3 classes, each of which contains three members; α1 (α1A, α1B, α1D), a2 (α2A, α2B, α2C); and β (β1, β2, and β3). ARs are also present on endothelial cells [37, 38]. In human and mouse skin the β2-AR appears to be the most abundant AR. It is the sub-type of β-ARs expressed by the major cell types found in skin including keratinocytes [39, 40], fibroblasts [41] and melanocytes [42]. However, α-ARs are also present in the skin [43, 44]. It has previously been shown that NE enhances lipopolysaccharide-induced IL-6 release from cells of the human dermal microvascular endothelial cell line HMEC-1 [45].
Adenosine-5’-triphosphate (ATP) participates in many intra- and extracellular functions [46, 47]. Extracellular ATP may act in an autocrine or paracrine manner and exerts important effects on many cell types. ATP can also be released from many cell types and is a sympathetic co-transmitter along with NE and neuropeptide Y [48–50]. ATP binds to purinergic P2 receptors, which belong to either the ionotropic P2X receptor family (ligand gated channels) or the metabotropic P2Y receptor family (G protein-coupled receptors), with activation of downstream signaling pathways [46, 51, 52]. Puringenic receptors are also expressed by macro- and micro-vascular endothelial cells [53]. We have demonstrated that HMEC-1 cells express mRNA for several P2 receptors [54]. We have also previously shown that ATP (as well as ATPγS, a hydrolysis-resistant long-lived analog of ATP) increases the secretion of IL-6 and the chemokines CXCL8 (interleukin-8), CCL2 (monocyte chemoattractant protein-1) and CXCL1 (growth related oncogene-) by HMEC-1 cells as well as by primary human dermal microvascular endothelial cells (pHDMECs) [54, 55]. ATPγS also upregulates expression of intercellular adhesion molecule 1 (ICAM-1) by HMEC-1 cells [54]. We hypothesized that under conditions of stress, activation of symphathetic nerves may lead to release of ATP by nerves associated with dermal vessels followed by release of cytokines/chemokines by endothelial cells and upregulation of ICAM-1 leading to enhanced recruitment of inflammatory cells into skin interstitium.
Under conditions of stress, activation of symphathetic nerves may lead to release of both ATP and NE in the vicinity of dermal blood vessels. Complex interactions between the effect of ATP and that of NE may regulate the ability of endothelial cells to release certain types of cytokines/chemokines. In this regard, it has been reported that exposure of rat thymic epithelial cells to both NE and ATP resulted in an additive effect on IL-6 synthesis [56]. However, the influence of co-transmitters on immune responses and cutaneous inflammation, particularly at the endothelial level is poorly understood. In this study we have examined the effect of the sympathetic co-transmitters NE and ATP on IL-6 release by the dermal microvascular endothelial cell line HMEC-1 and pHDMECs. We focused on the cytokine IL-6 because it is involved in differentiation of Th17 cells [57–65], which are now believed to be key in the pathogenesis of psoriasis [63–72].
2. Materials and Methods
2.1 Reagents
Norepinephrine was purchased from EMD Biosciences, Inc. (La Jolla, CA). ATP (cell culture grade) and phentolamine (Phent) were from Sigma-Aldrich (St. Louis, MO). Propranolol (Prop), ICI 118,151 (ICI), isoproterenol (Iso) and salbutamol (Sal) were from Tocris (Ellisville, MO). The high capacity cDNA kit and Power SYBER Green Master Mix were obtained from Applied Biosystems (Foster City, CA).
2.2 Cell culture and media
HMEC-1 cells were a gift from T.J. Lawley (Emory University, Atlanta, GA). This cell line was created by immortalizing HDMECs via simian virus 40 transformation and retains many properties of native dermal microvascular endothelial cells including cell adhesion molecule expression and cytokine/chemokine production [2, 73]. HMEC-1 cells were maintained in endothelial cell basal media (EBM; Lonza, Walkersville, MD), supplemented with 10% heat inactivated fetal bovine serum (FBS; Gemini, Bio-Products, Sacramento, CA), 100 U/ml penicillin, 100 µg/ml streptomycin, (Mediatech, Manassas, VA), 10 ng/ml epidermal growth factor (BD Biosciences, Bedford, MA) and 1 µg/ml hydrocortisone (Sigma-Aldrich, St. Louis, MO). Cells were maintained at 37°C in a humidified atmosphere with 5% CO2. In experiments that examined the effect of drugs on cytokine production or RNA transcription, cells were incubated in EMB supplemented with 2% FBS and penicillin/streptomycin only and referred to as depleted media (DM). Primary neonatal foreskin human dermal microvascular endothelial cells (neonatal pHDMECs) were from pooled donors and were obtained commericially (Lonza, Walkersville, MD). Primary endothelial cells were grown in endothelial cell basal 2 media (EBM 2) supplemented with the EGM –2 MV single Quotes (Lonza), containing supplements and growth factors (hydrocortisone, hEGF, FBS, VEGF, hFGF-B, R3-IGF-1, ascorbic acid and gentamicin/amphotericin-B).
2.3 Cytokine ELISAs
HMEC-1 cells were plated and adhered in 12-well plates at 2×105 cells/well in complete media. After approximately 4 hrs cells were switched to depleted media and incubated overnight. Sixteen hours later the media was replaced with fresh depleted media and cells were treated with various concentrations of NE and/or ATP for the times indicated and supernatants were harvested. For neonatal pHDMECs, 0.15×105 cells/well were plated in 12-well plates in CM media and incubated overnight. Medium was replaced with fresh CM and cells were treated with NE and/or ATP as indicated and supernatants were harvested 8 hrs later. IL-6 quantitation was performed by sandwich enzyme-linked immunosorbent assay (ELISA) with matched antibody pairs and standards from BD Biosciences (San Jose, CA). Optical density was determined using a Versamax microplate reader (Molecular Devices, Sunnyvale, CA) and analyzed with Softmax software.
2.4 RNA isolation and real-time PCR
For RNA isolation, 0.5×106 cells were plated in 35 mm dishes in 2 ml of complete medium or 0.25×106 cells per well in 1 ml complete media in 12-well plates, allowed to adhere for approximately 4 hours and then were cultured in depleted medium overnight. After the appropriate treatment and time, total RNA was extracted using the RNeasy Plus Mini Kit from Qiagen (Valencia, CA), which includes a genomic DNA eliminator column. cDNA was synthesized from 1 µg of RNA using a high capacity cDNA synthisis kit from Applied Biosystem (Foster City, CA). IL-6 expression levels were analyzed by real-time PCR using the Power SYBER Green Master Mix (Applied Biosystem) and primers obtained from Invitrogen with the ABI 7900HT instrument (Applied Biosystems). Gyceraldehyde–3-phosphate-dehydrgenase (GAPDH) was used as an internal control and data were analyzed by the relative comparative Ct method [74, 75]. RNA primers used were as follows. GAPDH: forward 5’-TGGTATCGTGGAAGGACTCA-3’, reverse 5’- CCAGTAGAGGCGGGATGAT-3’, IL-6: forward 5’-GACAGCCACTCACCTCTTCA-3’, reverse 5’-CCTCTTTGCTGCTTTCACAC-3’. Melting curve analysis was performed to verify the specificity of primer amplification products. RNA was normalized to GAPDH and given as fold expression level relative to media only control.
2.5 Viable cell count after various treatments
Two hundred-fifty thousand HMEC-1 cells per well were cultured in 12-well plates in medium containing the appropriate amount of norepinephrine with or without the addition of 100 µM or 50 µM ATP. After 24 hours, supernatants were collected and the cells were removed by trypsinazation and collected by centrifugation. Viable and dead cells were counted from triplicate wells for each condition by the Trypan blue exclusion method.
2.6 Statistical analysis
In each study, biomarker levels under multiple experimental conditions were measured. The experiments may be carried out in multiple plates and repeated in multiple experiments. Linear regression was used to estimate the contribution from each and/or combination of treatments while controlling for potential systematic differences in different experiments and/or different plates when possible. Hypotheses related to biomarker levels under different experimental conditions were then examined using simultaneous tests for General Linear Hypotheses [76]. P-values were adjusted for multiple comparisons by controlling the false discovery rate.
3. Results
3.1 Norepinephrine and ATP synergize in inducing IL-6 protein production by HMEC-1 cells
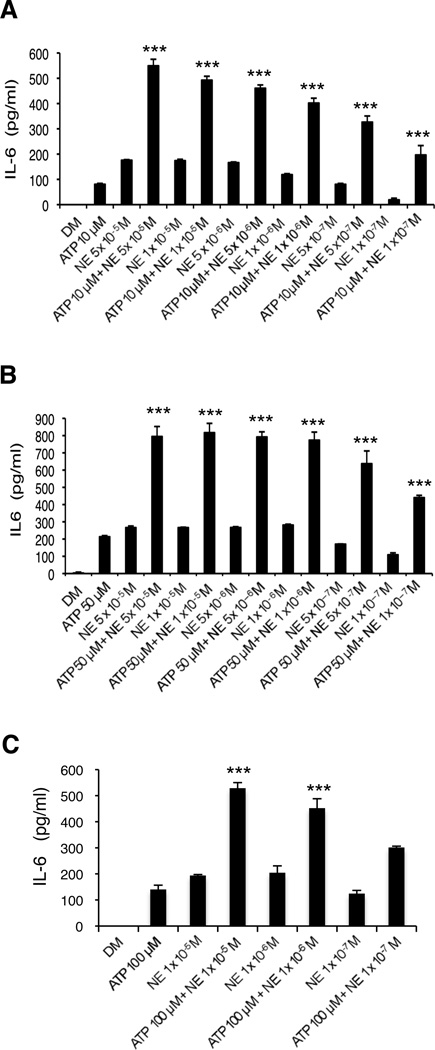
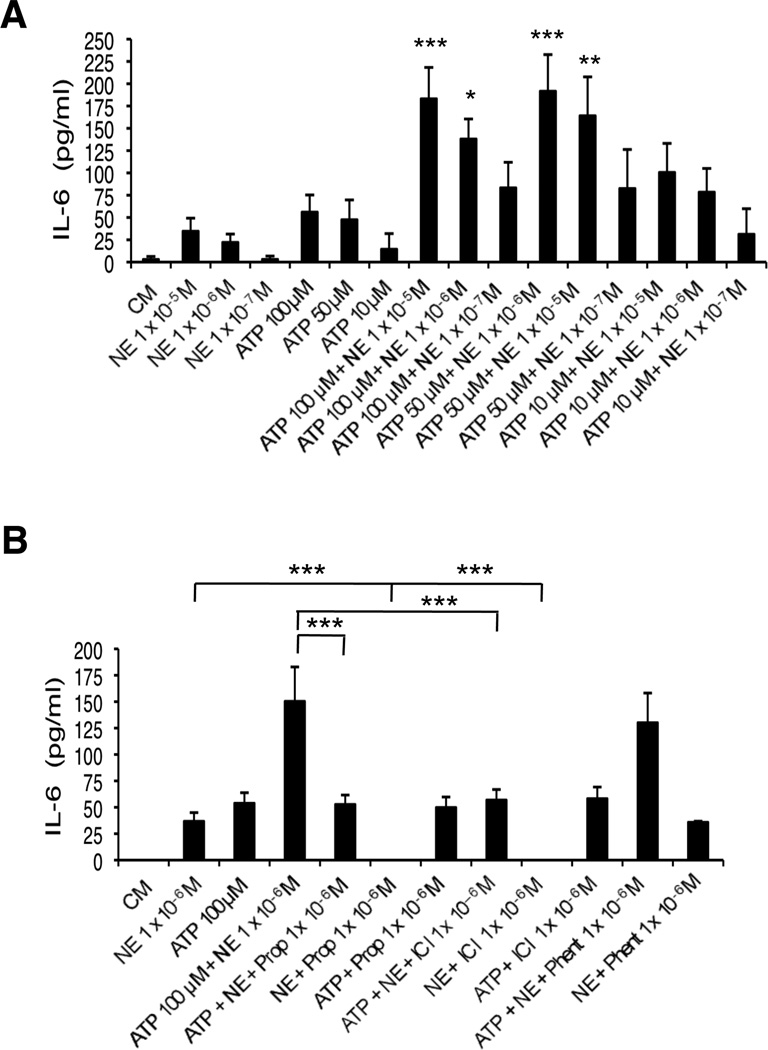
To determine the effect of ATP and NE on the production of the cytokine IL-6, HMEC-1 cells were treated with 10 M ATP, 50 M ATP or 100 M ATP in the presence or absence of various concentrations of NE (5×10−5 M to 1×10−7 M) (Figure 1A, 1B and 1C, respectively). After 24 hrs, supernatants were collected and analyzed for IL-6 content by ELISA. As shown in Figure 1, ATP and NE alone are each able to induce secretion of IL-6 at the concentrations tested. When added together, ATP and NE show remarkable synergy in the production of IL-6 at all combinations of concentrations examined. ATP (50 µM or 10 µM) and NE (1×10−5 M, 1×10−6 M or 1×10−7 M) also show striking synergy after only 8 hrs of incubation (data not shown).
Figure 1.
NE and ATP induce IL-6 secretion by HMEC-1 cells synergistically. Supernatants were collected after 24 hrs and analyzed by ELISA. (A) Cells treated with 10 µM ATP or each indicated NE concentration alone significantly increased IL-6 (p<0.001) versus DM. Cells treated with 10 uM ATP and each NE concentration induced IL-6 release synergistically. (B) Cells treated with 50 µM ATP or each indicated NE concentration alone significantly increased IL-6 (p<0.001) versus DM. Cells treated with 50 µM ATP and each NE concentration resulted in a synergistic release of IL-6. (C) Cells treated with 100 µM ATP or each NE concentration alone significantly increased IL-6 (p<0.001) compared to DM. Treatment of cells with 100 µM ATP and 10−5 M NE or 10−6 M NE resulted in synergistic induction of IL-6. A representative experiment of 3 (A), 3 (B) and 2 (C) is shown in each graph; results are the mean +/− SD of assays performed in triplicate. ***p<0.001 vs. additive effect of each agent alone.
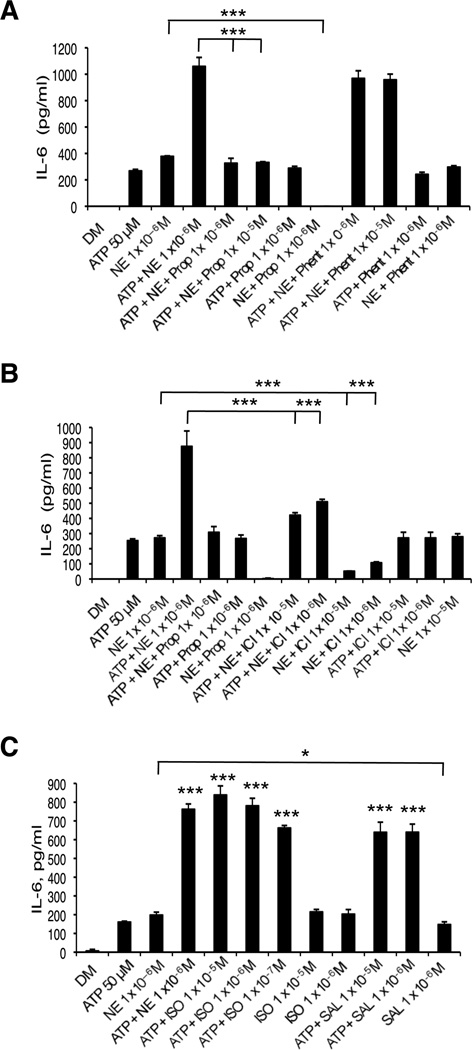
3.2-ARs mediate norepinephrine augmentation of ATP-induced IL-6 expression by HMEC-1 cells
To determine which class of ARs is responsible for the augmentation of ATP-induced activation of IL-6 by NE, experiments using receptor antagonists and agonists were performed. Initially, HMEC-1 cells were treated with the non-specific -AR antagonist propranolol or the -AR antagonist phentolamine. As shown in Figure 2A, when cells were treated in the presence of propranolol, the synergistic effect of ATP (50 µM) and NE (1 × 10−6 M) on IL-6 was completely abolished, while phentolamine had only a very small effect. Similarly, the presence of the 2-AR inhibitor ICI 118,551 resulted in a significant reduction in observed synergy (Figure 2B). Controls showed that NE induction of IL-6 could be blocked by propranolol and ICI 118,551 but not phentolamine. In addition, the adrenergic blockers had no effect on ATP-induced IL-6 release. These results indicated that NE augmented the ATP-induced release of IL-6 primarily through β2-ARs. Further experiments were done with the β-agonists isoproterenol (non-specific β) and salbutamol (β2-specific). Both agonists induced IL-6 release by HMEC-1 cells, similar to NE’s effect. ATP and isoproterenol together synergized in producing IL-6. The levels achieved were the same seen with ATP and NE. ATP and salbutamol also synergized in producing IL-6, although the levels were slightly less than those obtained with the same concentration of NE and isoproterenol (Figure 2C). These results provide further support for the role of β2-ARs in this response.
Figure 2.
Effect of β-adrenergic antagonists and agonists on IL-6 release by HMEC-1 cells. Supernatants were collected after 24 hrs and analyzed by ELISA. Cells treated with 50 µM ATP and 10−6 M NE resulted in synergistic induction of IL-6 release (p<0.001) as shown in each graph. (A) Pretreatment of cells with 10−5 M or 10−6 M Prop abolished this effect (***p<0.001) while pretreatment with phentolamine had little effect. 10−6 M Prop + 10−6 M NE is statistically different from 10−6 M NE alone (***p<0.001), but 10−6 M Prop + 50 µM ATP is not different from 50 µM ATP. (B) Pretreatment with 10−5 M or 10−6 M ICI reduced this effect (***p<0.001). 10−5 M ICI + 10−6 M NE and 10−6 M ICI + 10−6 M NE is statistically different from 10−6 M NE alone (***p<0.001), but 10−5 M ICI + 50 µM ATP and 10−6 M ICI + 50 µM ATP is not statistically different from 50 µM ATP alone. (C) Treatment with 50 µM ATP and ISO (10−5 M, 10−6 M or 10−7 M) resulted in synergistic induction of IL-6 release (***p< 0.001). There is no significant difference between 10−6 M NE and 10−5 M ISO or 10−6 M ISO. Treatment with 50 uM ATP and SAL (10−5 M or 10−6 M) resulted in synergistic IL-6 release (***p<0.001). One representative experiment of 2 is shown for each graph; results are the mean +/− SD of triplicate assays.
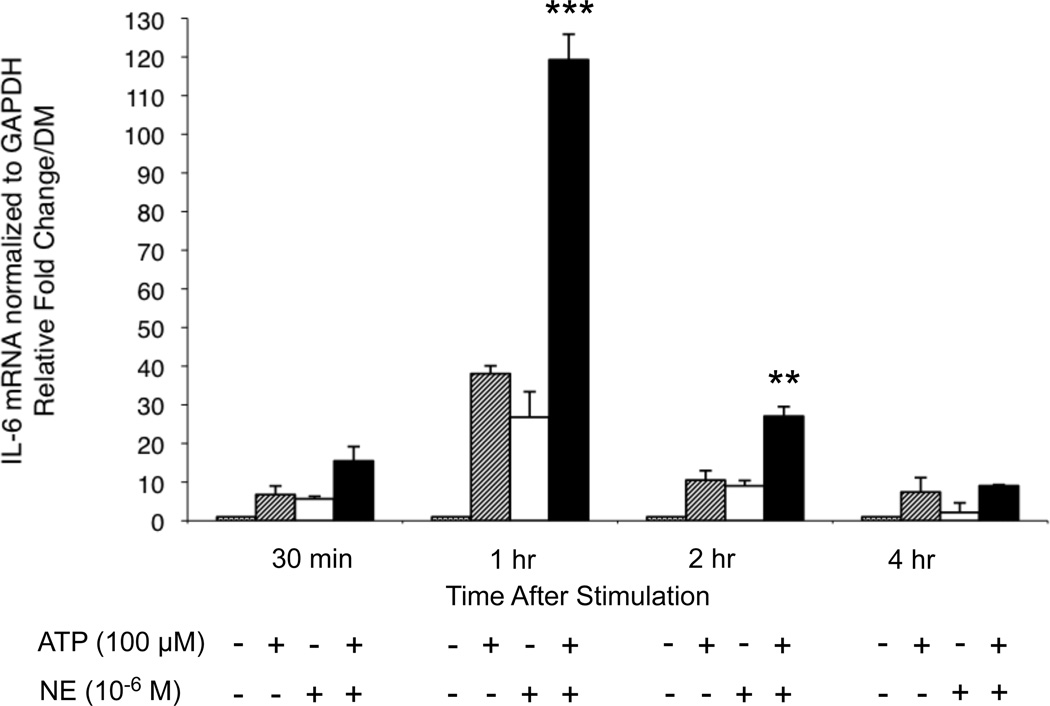
3.3 Real-time PCR analyses show increased levels of IL-6 mRNA in HMEC-1 cells treated with ATP and NE
To investigate the effect of ATP and NE on IL-6 mRNA expression, a real-time RT-PCR time course experiment was performed. ATP (100 M) and NE (1×10−6 M) each induced the synthesis of IL-6 mRNA at 30 min. 1 hr, 2 hr and 4 hr, with peak induction occurring after 1 hr after treatment (Figure 3). Treatment with both ATP and NE resulted in synergistic induction of IL-6 mRNA levels at 1 hr and 2 hr, with the largest effect observed at 1 hr. These results indicate that the increased levels of IL-6 protein observed in the ELISA assays are due, at least in part, to an increase in mRNA levels.
Figure 3.
Real-time PCR analysis of IL-6 mRNA levels. HMEC-1 cells were treated with 10−6 M NE and 100 µM ATP for the times indicated. Cells were lysed and total RNA was prepared. cDNA was synthesized from 1 µg RNA and qPCR was carried out using the Power SYBR green master mix and GAPDH as the internal control. mRNA levels were calculated using the relative comparative ΔΔCT method. 100 µM ATP induced IL-6 mRNA levels at 30 min (p<0.05),1 hr (p<0.001) and 2 hr (p<0.001) compared to DM. 10−6 M NE increased levels at 30 min (p<0.05), 1 hr (p<0.001) and 2 hr (p<0.01) versus DM. ATP and NE synergistically induce IL-6 mRNA at 1 hr (***p<0.001) and 2 hr (**p<0.01) versus additive effect of each agent alone. Results shown are the average of three independent experiments. Error bars represent the mean +/− SD.
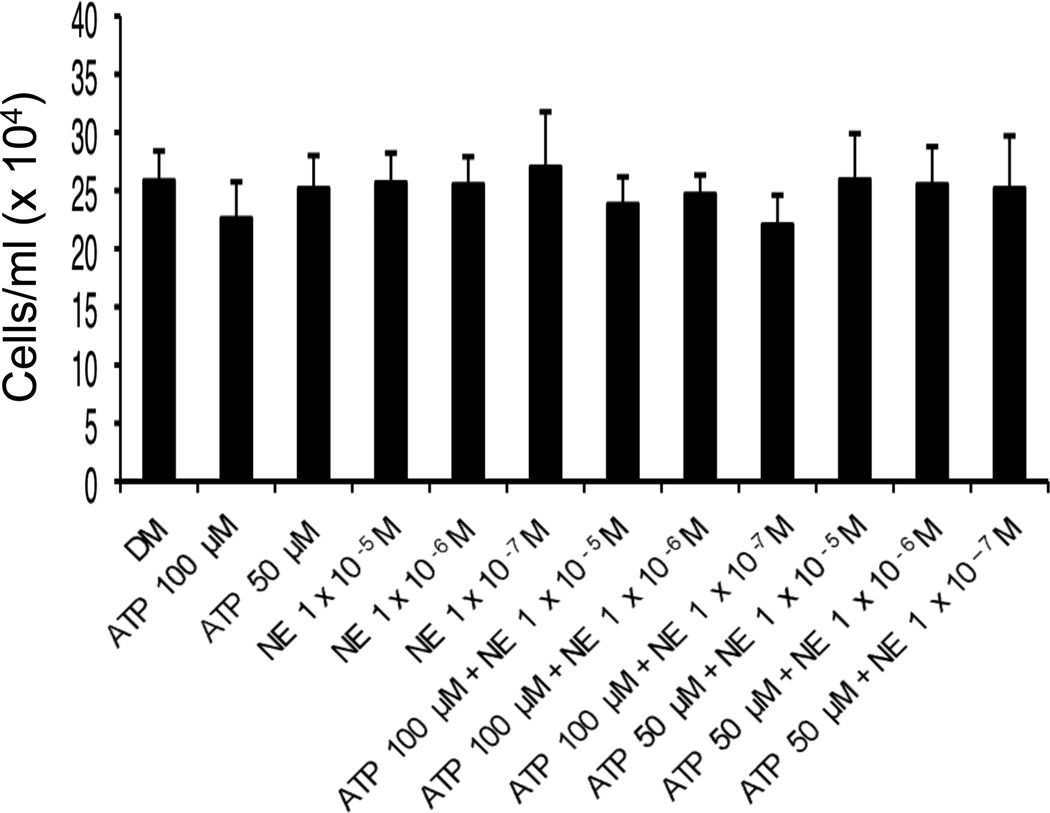
3.4 Neither norepinephrine nor ATP affect HMEC-1 cell viability 24 hrs after treatment
To exclude the possibility that treatment of HMEC-1 cells with the combination of ATP and NE resulted in an increase in cell death and concurrent increase in release of IL-6 protein, cell viability studies were conducted. HMEC-1 cells were treated with 50 µM or 100 µM ATP in the presence or absence of NE (10−5 M, 10−6 M, 10−7 M) for 24 hrs. Cells were harvested by trypsinization and washing from wells. Viable cells were counted by the trypan blue exclusion method. As shown in Figure 4, neither ATP nor NE alone, or in combination, significantly affected HMEC-1 viable cell count after 24 hrs treatment.
Figure 4.
HMEC-1 viable cell counts 24 hrs after indicated treatments. Two hundred-thousand cells/well in a 12-well plate were treated in triplicate with various combinations of ATP and NE for 24 hrs. Supernatants were collected and saved and cells were removed by trypsinazation, plates washed and all liquids were centrifuged. Viable cells were counted by the Trypan blue exclusion method. Results shown are the average of two independent experiments done in triplicate. Error bars represent the mean +/− standard deviation.
3.5 Norepinephrine and ATP also synergize in inducing release of IL-6 by primary human microvascular endothelial cells and is primarily mediated by 2-ARs
Initial experiments employed HMEC-1 cells as a surrogate for primary human cells. We subsequently investigated whether ATP and NE could regulate IL-6 release by neonatal pHDMECs. ATP (50 µM or 100 µM) and NE (1×10−5 M or 1×10−6 M) could induce release of IL-6 from these cells (Figure 5A). In addition, when primary cells were treated with various combinations of ATP and NE, significant synergy was also observed for the release of the IL-6 (Figure 5A). To determine which class of ARs were involved in the synergistic response in neonatal pHDMECs, cells were treated with propranolol (non-specific β-antagonist), ICI 118,151 (specific β2-antagonist) and phentolamine (α-antagonist). As shown in Figure 5B, both propranolol and ICI 118,551 completely abolished the synergistic induction of IL-6, indicating the involvement of β2-ARs, while phentolamine only had a small effect. Controls showed that the β-antagonists had little effect on the ATP induction of IL-6, but blocked its induction by NE alone, as expected. Thus, the synergistic effect of ATP and NE could be reproduced in neonatal pHDMECs and was also mediated primarily by β2-ARs.
Figure 5.
NE and ATP synergistically induce IL-6 release by neonatal pHDMECs primarily through 2-ARs. Supernatants were collected after 8 hrs and analyzed by ELISA. (A) Cells treated with 50 µM or 100 µM ATP alone significantly increased IL-6 release versus DM(p<0.01). Cells treated with 50 µM ATP plus NE (10−5 M or 10−6 M) or 100 µM ATP plus NE (10−5 M or 10−6 M) synergistically induced IL-6 release. ***p<0.001, **p<0.01, *p<0.05 versus additive effect of each agent alone. (B) Cells treated with 100 µM ATP and 10−6 M NE resulted in synergistic release of IL- 6 (p<0.001). Pretreatment with 10−5 M or 10−6 M Prop or 10−5 M ICI abolished this effect (***p<0.001) while pretreatment with phentolamine had no effect. 10−6 M Prop + 10−6 M NE is statistically different from 10−6 M NE alone (***p<0.001) but not from DM and 10−6 M Prop + 50 µM ATP is not different from 50 µM ATP. 10−5 M ICI +10−6 M NE differs from 10−5 M NE (***p<0.001) but not from DM. Each graph represents the average of two experiments. Error bars represent the mean +/-SD.
4. Discussion
ECs play a key role in many immune-mediated disorders and have important functions in cutaneous inflammation. These activities occur, in part, through the release of inflammatory factors and expression of adhesion molecules involved in migration of leukocytes out of the vasculature [2–5, 7]. Because blood vessels are innervated by sympathetic nerves, we have hypothesized that sympathetic nerve transmitters and co-transmitters have important roles in regulating endothelial cell immunologic and inflammatory functions. We have previously reported that ATP and a hydrolysis-resistant, long-lived analog of ATP, ATPγS, enhance secretion of IL-6 and the chemokines CXCL8, CCL2 and CXCL1 by HMEC-1 cells and pHDMECs [54, 55]. Sympathetic nerve influences on expression of inflammatory factors by dermal microvascular endothelial cells may be of particular interest as inflammatory skin diseases including psoriasis, atopic dermatitis, acne and rosacea are all believed to be exacerbated by stress [20, 29–31, 33, 77–79]. We have hypothesized that under conditions of stress, the SNS is activated and sympathetic nerves associated with dermal vessels release ATP, which then enhances the inflammatory functions of endothelial cells, resulting in increased cutaneous inflammation. In this regard, IL-6 may be of particular interest as it has a key role in the differentiation of Th17-type helper lymphocytes [59, 61, 80], believed to have an important role in the pathogenesis of psoriasis [63–66, 68].
IL-6 was originally defined as a B cell differentiation factor but is now known to be a pleiotropic factor with many different activities [81–83]. These include induction of an acute phase response, angiogenic activity and stimulation of myelopoiesis among many other effects [83]. With respect to the immune system, IL-6 has many activities [82–84]. It promotes antibody production, including autoantibody production, and thus plays an important role in autoimmune diseases characterized by humoral mechanisms. It also plays a role in the proliferation and differentiation of T helper cells. IL-6 enhances IL-4 -induced Th2 helper cell differentiation and inhibits IL-12-associated Th1 helper cell differentiation [61, 83]. Of particular interest to the skin, IL-6 plays an important role in the differentiation of Th17 cells [57–59, 61, 62]. Th17 cells most probably have physiologic activities in protection against extracellular organisms [85]. However, they have important functions in the pathophysiology of several autoimmune diseases [63, 86–88] and, notably, psoriasis. Through production of IL-17A, these cells play a key role in inflammation associated with psoriasis [63, 65–72] and, through the elaboration of IL-22, they induce acanthosis of the epidermis [89, 90], characteristic of psoriasis. In the mouse, IL-6 and TGFβ work together to promote the generation of Th17 cells [58, 59, 80]. Interestingly, IL-6-deficient, gene-targeted mice do have some Th17 cells although lesser than control animals. IL-21 may be able to substitute for some of the effects of IL-6 in promoting Th17 cell generation [91]. In humans, IL-6, TGFβ and IL-1β are involved in Th17 cell development [63, 92]. In this regard, IL-6 levels are elevated in lesions of psoriasis [93, 94].
The data presented herein demonstrate a possible novel locus of interaction between the sympathetic nervous system and endothelial cells resulting in enhancement of Th17 responses. The finding that norepinephrine and ATP synergize to induce endothelial cells to produce large amounts of IL-6 suggests a mechanism by which stress may result in exacerbation of psoriasis or other Th17 cell-associated inflammatory skin conditions. In this model, stress-induced activation of the sympathetic nervous system will lead to release of the sympathetic transmitters norepinephrine and ATP by nerve fibers surrounding blood vessels in the skin. Norepinephrine and ATP would then, in turn, bind to receptors on the endothelial cells followed by release of large amounts of IL-6. IL-6 would then function to potentiate the differentiation of Th17 cells. This mechanism may also be operative in draining lymph nodes as lymph nodes are innervated by the sympathetic nervous system [15, 21, 22]. In support of this concept, a recent paper implicated ATP receptor signaling in the skin in Th17 cell responses [95].
Interestingly, there is some precedent for this type of mechanism. Norepinephrine and ATP each stimulate production of IL-6 by thymic epithelial cells and co-stimulation results in an additive effect. It has been hypothesized that the effect of sympathetic co-transmitters on IL-6 synthesis is important for thymocyte differentiation and proliferation within the thymus [56]. Glucocorticoids also are important mediators of stress responses and recently it was reported that dexamethasone enhanced ATP-induced IL-6 secretion by HMEC-1 cells [96].
Our results are important for at least two reasons. First, if our model is correct, release of sympathetic co-transmitters by stress may account for the exacerbation of psoriasis that occurs with stress. Secondly, these results suggest that mechanisms to alleviate stress or novel pharmacologic agents to block the effects of ATP and/ or norepinephrine at the endothelium of dermal vessels may be useful for the therapy of psoriasis. Indeed, because agents can be applied topically to the skin, it may be possible to develop agents that can effectively block norepinephrine and/or ATP effects in the skin without systemic absorption, thus avoiding systemic adverse side effects. Since betablockers reportedly worsen psoriasis [97], our results may seem unexpected. However, failure to find an association of beta-blockers and psoriasis has also been reported [98] and it has been reported that beta-adrenergic agonists induce or worsen pustular psoriasis, concordant with our findings [99,100].
Important future directions include determining more precisely what stimuli induce release of norepinephrine and ATP from sympathetic nerves within the skin and whether other products of nerves, including sensory nerves, may influence release of IL-6 by endothelial cells.
Highlights.
-
*
Norepinephrine and ATP are sympathetic nerve transmitters
-
*
Dermal blood vessels are associated with sympathetic nerves
-
*
Norepinephrine and ATP synergize in inducing IL-6 production by endothelial cells.
-
*
Norepinephrine and ATP similarly synergize in increasing IL-6 mRNA levels.
-
*
These effects require norepinephrine signaling through the 2-adrenergic receptor.
-
*
This pathway may account for stress-induced exacerbation of cutaneous inflammation.
Acknowledgements
A grant from the National Rosacea Society (RDG), a gift from the Jacob L. and Lillian Holtzmann Foundation (RDG), a grant from the Edith C Blum Foundation (RDG), contributions from the Carl and Fay Simons Family Trust (RDG), a grant from the Lewis B. and Dorothy Cullman Foundation (RDG) and NIH grant R01 AR42429 (RDG).
Abbreviations
- EC
endothelial cell
- ATP
adenosine-5’-triphosphate
- NE
norepinephrine
- SNS
sympathetic nervous system
- AR
adrenergic receptor
- pHDMEC
primary human dermal endothelial cells
- ICAM-1
intercellular adhesion molecule 1
- Prop
propranolol
- Sal
salbutamol
- DM
depleted medium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Danese S, Dejana E, Fiocchi C. Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J Immunol. 2007;178:6017–6022. doi: 10.4049/jimmunol.178.10.6017. [DOI] [PubMed] [Google Scholar]
- 2.Swerlick RA, Lawley TJ. Role of microvascular endothelial cells in inflammation. J Invest Dermatol. 1993;100:111S–115S. doi: 10.1111/1523-1747.ep12356595. [DOI] [PubMed] [Google Scholar]
- 3.Krishnaswamy G, Kelley J, Yerra L, Smith JK, Chi DS. Human endothelium as a source of multifunctional cytokines: molecular regulation and possible role in human disease. J Interferon Cytokine Res. 1999;19:91–104. doi: 10.1089/107999099314234. [DOI] [PubMed] [Google Scholar]
- 4.Goebeler M, Yoshimura T, Toksoy A, Ritter U, Brocker EB, Gillitzer R. The chemokine repertoire of human dermal microvascular endothelial cells and its regulation by inflammatory cytokines. J Invest Dermatol. 1997;108:445–451. doi: 10.1111/1523-1747.ep12289711. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Swerlick RA, Sepp N, Bosse D, Ades EW, Lawley TJ. Characterization of expression and modulation of cell adhesion molecules on an immortalized human dermal microvascular endothelial cell line (HMEC-1) J Invest Dermatol. 1994;102:833–837. doi: 10.1111/1523-1747.ep12382086. [DOI] [PubMed] [Google Scholar]
- 6.Lidington EA, Moyes DL, McCormack AM, Rose ML. A comparison of primary endothelial cells and endothelial cell lines for studies of immune interactions. Transpl Immunol. 1999;7:239–246. doi: 10.1016/s0966-3274(99)80008-2. [DOI] [PubMed] [Google Scholar]
- 7.Johnston B, Butcher EC. Chemokines in rapid leukocyte adhesion triggering and migration. Semin Immunol. 2002;14:83–92. doi: 10.1006/smim.2001.0345. [DOI] [PubMed] [Google Scholar]
- 8.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elenkov IJ. Neurohormonal-cytokine interactions: implications for inflammation, common human diseases and well-being. Neurochem Int. 2008;52:40–51. doi: 10.1016/j.neuint.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, et al. Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 2008;252:27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 12.Dhabhar FS. Enhancing versus Suppressive Effects of Stress on Immune Function: Implications for Immunoprotection versus Immunopathology. Allergy Asthma Clin Immunol. 2008;4:2–11. doi: 10.1186/1710-1492-4-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa-Pinto FA, Palermo-Neto J. Neuroimmune interactions in stress. Neuroimmunomodulation. 2010;17:196–199. doi: 10.1159/000258722. [DOI] [PubMed] [Google Scholar]
- 15.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 17.Liezmann C, Klapp B, Peters EM. Stress, atopy and allergy: A re-evaluation from a psychoneuroimmunologic persepective. Dermatoendocrinol. 2011;3:37–40. doi: 10.4161/derm.3.1.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimyai-Asadi A, Usman A. The role of psychological stress in skin disease. J Cutan Med Surg. 2001;5:140–145. doi: 10.1007/BF02737869. [DOI] [PubMed] [Google Scholar]
- 19.Slominski A, Wortsman J, Paus R, Elias PM, Tobin DJ, Feingold KR. Skin as an endocrine organ: implications for its function. Drug Discov Today Dis Mech. 2008;5:137–144. doi: 10.1016/j.ddmec.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall JM, Cruser D, Podawiltz A, Mummert DI, Jones H, Mummert ME. Psychological Stress and the Cutaneous Immune Response: Roles of the HPA Axis and the Sympathetic Nervous System in Atopic Dermatitis and Psoriasis. Dermatol Res Pract. 2012;2012:403908. doi: 10.1155/2012/403908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135:755s–765s. [PubMed] [Google Scholar]
- 22.Felten SY, Felten DL, Bellinger DL, Carlson SL, Ackerman KD, Madden KS, et al. Noradrenergic sympathetic innervation of lymphoid organs. Prog Allergy. 1988;43:14–36. [PubMed] [Google Scholar]
- 23.Felten SY, Madden KS, Bellinger DL, Kruszewska B, Moynihan JA, Felten DL. The role of the sympathetic nervous system in the modulation of immune responses. Adv Pharmacol. 1998;42:583–587. doi: 10.1016/s1054-3589(08)60818-2. [DOI] [PubMed] [Google Scholar]
- 24.Madden KS, Felten SY, Felten DL, Hardy CA, Livnat S. Sympathetic nervous system modulation of the immune system. II. Induction of lymphocyte proliferation and migration in vivo by chemical sympathectomy. J Neuroimmunol. 1994;49:67–75. doi: 10.1016/0165-5728(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 25.Madden KS. Catecholamines, sympathetic innervation, and immunity. Brain Behav Immun. 2003;17(Suppl 1):S5–S10. doi: 10.1016/s0889-1591(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 26.Szelenyi J, Vizi ES. The catecholamine cytokine balance: interaction between the brain and the immune system. Ann N Y Acad Sci. 2007;1113:311–324. doi: 10.1196/annals.1391.026. [DOI] [PubMed] [Google Scholar]
- 27.Pavlovsky L, Friedman A. Pathogenesis of stress-associated skin disorders: exploring the brain-skin axis. Curr Probl Dermatol. 2007;35:136–145. doi: 10.1159/000106420. [DOI] [PubMed] [Google Scholar]
- 28.Pavlovic S, Daniltchenko M, Tobin DJ, Hagen E, Hunt SP, Klapp BF, et al. Further exploring the brain-skin connection: stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. J Invest Dermatol. 2008;128:434–446. doi: 10.1038/sj.jid.5701079. [DOI] [PubMed] [Google Scholar]
- 29.Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681–694. doi: 10.1016/j.det.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Arndt J, Smith N, Tausk F. Stress and atopic dermatitis. Curr Allergy Asthma Rep. 2008;8:312–317. doi: 10.1007/s11882-008-0050-6. [DOI] [PubMed] [Google Scholar]
- 31.Reich A, Wojcik-Maciejewicz A, Slominski AT. Stress and the skin. G Ital Dermatol Venereol. 2010;145:213–219. [PubMed] [Google Scholar]
- 32.Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine circuitry of the 'brain-skin connection'. Trends Immunol. 2006;27:32–39. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697–1704. doi: 10.1038/sj.jid.5700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seiffert K, Granstein RD. Neuroendocrine regulation of skin dendritic cells. Ann N Y Acad Sci. 2006;1088:195–206. doi: 10.1196/annals.1366.011. [DOI] [PubMed] [Google Scholar]
- 35.Tausk F, Christian E, Johansson O, Milgram S. Neurobiology of the skin. In: Fitzpatrick AZE TB, Wolff K, Freedberg IM, Austen KF, editors. Dermatology in General Medicine. New York: McGraw- Hill; 1993. pp. 396–406. [Google Scholar]
- 36.Matze D. Neuroanatomy of the skin. In: Granstein RD, Luger TA, editors. Neuroimmunology of the Skin. Springer: Verlag Berlin; 2009. pp. 3–12. [Google Scholar]
- 37.Graf K, Grafe M, Dummler U, O'Connor A, Regitz-Zagrosek V, Kunkel G, et al. Regulation of beta-adrenergic receptors on endothelial cells in culture. Eur Heart J. 1993;14:173–176. [PubMed] [Google Scholar]
- 38.Howell RE, Albelda SM, Daise ML, Levine EM. Characterization of beta-adrenergic receptors in cultured human and bovine endothelial cells. J Appl Physiol. 1988;65:1251–1257. doi: 10.1152/jappl.1988.65.3.1251. [DOI] [PubMed] [Google Scholar]
- 39.Steinkraus V, Steinfath M, Korner C, Mensing H. Binding of beta-adrenergic receptors in human skin. J Invest Dermatol. 1992;98:475–480. doi: 10.1111/1523-1747.ep12499860. [DOI] [PubMed] [Google Scholar]
- 40.Sivamani RK, Lam ST, Isseroff RR. Beta adrenergic receptors in keratinocytes. Dermatol Clin. 2007;25:643–653. doi: 10.1016/j.det.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McSwigan JD, Hanson DR, Lubiniecki A, Heston LL, Sheppard JR. Down syndrome fibroblasts are hyperresponsive to beta-adrenergic stimulation. Proc Natl Acad Sci U S A. 1981;78:7670–7673. doi: 10.1073/pnas.78.12.7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillbro JM, Marles LK, Hibberts NA, Schallreuter KU. Autocrine catecholamine biosynthesis and the beta-adrenoceptor signal promote pigmentation in human epidermal melanocytes. J Invest Dermatol. 2004;123:346–353. doi: 10.1111/j.0022-202X.2004.23210.x. [DOI] [PubMed] [Google Scholar]
- 43.Muskhelishvili LV, Gedevanishvili MD. Adrenergic receptors in murine epidermis. Tsitologiia. 1976;18:627–629. [PubMed] [Google Scholar]
- 44.Drummond PD, Skipworth S, Finch PM. alpha 1-adrenoceptors in normal and hyperalgesic human skin. Clin Sci (Lond) 1996;91:73–77. doi: 10.1042/cs0910073. [DOI] [PubMed] [Google Scholar]
- 45.Gornikiewicz A, Sautner T, Brostjan C, Schmierer B, Fugger R, Roth E, et al. Catecholamines up-regulate lipopolysaccharide-induced IL-6 production in human microvascular endothelial cells. Faseb J. 2000;14:1093–1100. doi: 10.1096/fasebj.14.9.1093. [DOI] [PubMed] [Google Scholar]
- 46.Burnstock G, Fredholm BB, North RA, Verkhratsky A. The birth and postnatal development of purinergic signalling. Acta Physiol (Oxf) 2010;199:93–147. doi: 10.1111/j.1748-1716.2010.02114.x. [DOI] [PubMed] [Google Scholar]
- 47.Burnstock G, Kennedy C. P2X receptors in health and disease. Adv Pharmacol. 2011;61:333–372. doi: 10.1016/B978-0-12-385526-8.00011-4. [DOI] [PubMed] [Google Scholar]
- 48.Hasko G, Szabo C. Regulation of cytokine and chemokine production by transmitters and cotransmitters of the autonomic nervous system. Biochem Pharmacol. 1998;56:1079–1087. doi: 10.1016/s0006-2952(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 49.Burnstock G. Purinergic cotransmission. Exp Physio. 2009;94:20–24. doi: 10.1113/expphysiol.2008.043620. [DOI] [PubMed] [Google Scholar]
- 50.Burnstock G. Purinergic cotransmission. F1000 Biol Rep. 2009;1:46. doi: 10.3410/B1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 52.Burnstock G. Purinergic signalling: past, present and future. Braz J Med Biol Res. 2009;42:3–8. doi: 10.1590/s0100-879x2008005000037. [DOI] [PubMed] [Google Scholar]
- 53.Ohman J, Erlinge D. The touching story of purinergic signaling in epithelial and endothelial cells. Purinergic Signal. 2012;8:599–608. doi: 10.1007/s11302-012-9316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seiffert K, Ding W, Wagner JA, Granstein RD. ATPgammaS enhances the production of inflammatory mediators by a human dermal endothelial cell line via purinergic receptor signaling. J Invest Dermatol. 2006;126:1017–1027. doi: 10.1038/sj.jid.5700135. [DOI] [PubMed] [Google Scholar]
- 55.Bender A, Zapolanski T, Watkins S, Khosraviani A, Seiffert K, Ding W, et al. Tetracycline suppresses ATP gamma S-induced CXCL8 and CXCL1 production by the human dermal microvascular endothelial cell-1 (HMEC-1) cell line and primary human dermal microvascular endothelial cells. Exp Dermatol. 2008;17:752–760. doi: 10.1111/j.1600-0625.2008.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Patay B, Kurz B, Mentlein R. Effect of transmitters and co-transmitters of the sympathetic nervous system on interleukin-6 synthesis in thymic epithelial cells. Neuroimmunomodulation. 1999;6:45–50. doi: 10.1159/000026363. [DOI] [PubMed] [Google Scholar]
- 57.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 59.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 60.Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183:3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 62.Benwell RK, Lee DR. Essential and synergistic roles of IL1 and IL6 in human Th17 differentiation directed by TLR ligand-activated dendritic cells. Clin Immunol. 2010;134:178–187. doi: 10.1016/j.clim.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 63.Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 Cells: Biology, Pathogenesis of Autoimmune and Inflammatory Diseases, and Therapeutic Strategies. Am J Pathol. 2012;181:8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 64.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 65.Coimbra S, Figueiredo A, Castro E, Rocha-Pereira P, Santos-Silva A. The roles of cells and cytokines in the pathogenesis of psoriasis. Int J Dermatol. 2012;51:389–395. doi: 10.1111/j.1365-4632.2011.05154.x. [DOI] [PubMed] [Google Scholar]
- 66.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 69.Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127:1110–1118. doi: 10.1016/j.jaci.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 70.Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part II: immune cell subsets and therapeutic concepts. J Allergy Clin Immunol. 2011;127:1420–1432. doi: 10.1016/j.jaci.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 71.Raychaudhuri SP. Role of IL-17 in Psoriasis and Psoriatic Arthritis. Clin Rev Allergy Immunol. 2013;2:183–193. doi: 10.1007/s12016-012-8307-1. [DOI] [PubMed] [Google Scholar]
- 72.Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, et al. The Emerging Role of IL-17 in the Pathogenesis of Psoriasis: Preclinical and Clinical Findings. J Invest Dermatol. 2013;133:17–26. doi: 10.1038/jid.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, et al. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 74.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 75.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 76.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 77.Tausk F, Elenkov I, Moynihan J. Psychoneuroimmunology. Dermatol Ther. 2008;21:22–31. doi: 10.1111/j.1529-8019.2008.00166.x. [DOI] [PubMed] [Google Scholar]
- 78.Schmid-Ott G, Jaeger B, Boehm T, Langer K, Stephan M, Raap U, et al. Immunological effects of stress in psoriasis. Br J Dermato. 2009;160:782–785. doi: 10.1111/j.1365-2133.2008.09013.x. [DOI] [PubMed] [Google Scholar]
- 79.Suarez AL, Feramisco JD, Koo J, Steinhoff M. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Derm Venereol. 2012;92:7–15. doi: 10.2340/00015555-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 81.Hirano T. Interleukin 6 and its receptor: ten years later. Int Rev Immunol. 1998;16:249–284. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 82.Hirano T. Interleukin 6 in autoimmune and inflammatory diseases: a personal memoir. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:717–730. doi: 10.2183/pjab.86.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22:347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 84.Paquet P, Pierard GE. Interleukin-6 and the skin. Int Arch Allergy Immunol. 1996;109:308–317. doi: 10.1159/000237257. [DOI] [PubMed] [Google Scholar]
- 85.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oukka M. Th17 cells in immunity and autoimmunity. Ann Rheum Dis. 2008;67(Suppl 3):iii26–iii29. doi: 10.1136/ard.2008.098004. [DOI] [PubMed] [Google Scholar]
- 87.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. The phenotype of human Th17 cells and their precursors, the cytokines that mediate their differentiation and the role of Th17 cells in inflammation. Int Immunol. 2008;20:1361–1368. doi: 10.1093/intimm/dxn106. [DOI] [PubMed] [Google Scholar]
- 88.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Type 17 T helper cells-origins, features and possible roles in rheumatic disease. Nat Rev Rheumatol. 2009;5:325–331. doi: 10.1038/nrrheum.2009.80. [DOI] [PubMed] [Google Scholar]
- 89.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin- 22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 90.Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard FX, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407–415. doi: 10.1111/j.1365-2249.2007.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neuner P, Urbanski A, Trautinger F, Moller A, Kirnbauer R, Kapp A, et al. Increased IL-6 production by monocytes and keratinocytes in patients with psoriasis. J Invest Dermatol. 1991;97:27–33. doi: 10.1111/1523-1747.ep12477880. [DOI] [PubMed] [Google Scholar]
- 94.Grossman RM, Krueger J, Yourish D, Granelli-Piperno A, Murphy DP, May LT, et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci U S A. 1989;86:6367–6371. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Killeen ME, Ferris L, Kupetsky EA, Falo L, Jr, Mathers AR. Signaling through purinergic receptors for ATP induces human cutaneous innate and adaptive Th17 responses: implications in the pathogenesis of psoriasis. J Immunol. 2013;190:4324–4336. doi: 10.4049/jimmunol.1202045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ding Y, Gao ZG, Jacobson KA, Suffredini AF. Dexamethasone enhances ATP-induced inflammatory responses in endothelial cells. J Pharmacol Exp Ther. 2010;335:693–702. doi: 10.1124/jpet.110.171975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Basavaraj KH, Ashok NM, Rashmi R, Praveen TK. The role of drugs in the induction and/or exacerbation of psoriasis. Int J Dermatol. 2010;49:1351–1361. doi: 10.1111/j.1365-4632.2010.04570.x. [DOI] [PubMed] [Google Scholar]
- 98.Brauchli YB, Jick SS, Curtin F, Meier CR. Association between beta-blockers, other antihypertensive drugs and psoriasis: population-based case-control study. Br J Dermatol. 2008;158:1299–1307. doi: 10.1111/j.1365-2133.2008.08563.x. [DOI] [PubMed] [Google Scholar]
- 99.D'Incan M, Fabricio L, Souteyrand P. Ritodrine-induced pustular eruption in a pregnant woman with psoriasis. J Eur Acad Dermatol Venereol. 1998;11:91–93. doi: 10.1111/j.1468-3083.1998.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 100.Kuwabara Y, Sato A, Abe H, Abe S, Kawai N, Takeshita T. Ritodrine-induced pustular eruptions distinctly resembling impetigo herpetiformis. J Nippon Med Sch. 2011;78:329–333. doi: 10.1272/jnms.78.329. [DOI] [PubMed] [Google Scholar]