Abstract
Multipotent P19CL6 cells differentiate into cardiac myocytes or neural lineages when stimulated with dimethyl sulfoxide (DMSO) or retinoic acid (RA), respectively. Expression of the transcription factor Tbx6 was found to increase during cardiac myocyte differentiation and to decrease during neural differentiation. Overexpression of Tbx6 was not sufficient to drive P19CL6 cells to a cardiac myocyte fate or to accelerate DMSO-induced differentiation. In contrast, knockdown of Tbx6 dramatically inhibited DMSO-induced differentiation of P19CL6 cells to cardiac myocytes, as evidenced by the loss of striated muscle-specific markers and spontaneous beating. Tbx6 knockdown was also accompanied by almost complete loss of Nkx2.5, a transcription factor involved in the specification of the cardiac myocyte lineage, indicating that Nkx2.5 is downstream of Tbx6. In distinction to its positive role in cardiac myocyte differentiation, Tbx6 knockdown augmented RA-induced differentiation of P19CL6 cells to both neurons and glia, and accelerated the rate of neurite formation. Conversely, Tbx6 overexpression attenuated differentiation to neural lineages. Thus, in the P19CL6 model, Tbx6 is required for cardiac myocyte differentiation and represses neural differentiation. We propose a model in which Tbx6 is a part of a molecular switch that modulates divergent differentiation programs within a single progenitor cell.
Keywords: Tbx6, Cardiac myocyte, Neuron/glial cells, Differentiation, P19CL6 cells
1. Introduction
Stem cell therapies hold particular promise for cardiac and neurological disease. To exploit their therapeutic potential, it is essential to understand the transcriptional programs that regulate the differentiation of stem cells to various specialized cell types. A lack of appropriate and reliable cell culture models that recapitulate early steps in specification has been a major obstacle in investigating early cell fate decisions in cardiac myocyte and neuronal progenitors. P19 mouse embryonal carcinoma stem cells have been widely used for the studies of embryonic cell fate decisions. In response to various stimuli, these cells differentiate into cell types of the three germ layers (McBurney, 1993; van der Heyden and Defize, 2003). P19CL6 cells, a subclone of this line, have maintained multi-potency, but are capable of differentiating with high efficiency into cardiac myocytes upon dimethyl sulfoxide (DMSO) stimulation and neurons and glial cells (hereafter referred to as neural cells) upon retinoic acid (RA) stimulation (Habara-Ohkubo, 1996).
Our previous microarray studies using DMSO-induced differentiation of P19CL6 cells identified Tbx6 as a gene whose expression commences prior to commitment of these cells to the cardiac myocyte lineage (Peng et al., 2002). Tbx6 is a member of the evolutionarily conserved T-box family of transcription factors that are essential regulators of normal embryonic development (Naiche et al., 2005). Abnormal expression or function of various T-box genes has been implicated in human congenital malformations such as DiGeorge/velocardiofacial syndrome (Tbx1), ulnar–mammary syndrome (Tbx3), and Holt–Oram syndrome (Tbx5) (Naiche et al., 2005; Plageman and Yutzey, 2005). Previous studies suggest that Tbx6 is essential for the specification of the posterior paraxial mesoderm where, in its absence, myogenic cells differentiate along the default neural pathway resulting in the replacement of posterior somites by two ectopic neural tubes (Chapman and Papaioannou, 1998). Furthermore, Tbx6 is essential in left–right body axis determination in the mouse (Hadjantonakis et al., 2008). While a cardiac pheno-type has not been described in Tbx6-deficient mice, congenital heart defects, such as ventricular septal defects and hypoplastic chambers, are a significant part of the pathology resulting from mutations in other T-box genes (Chapman and Papaioannou, 1998; Plageman and Yutzey, 2005). Furthermore, Drosophila lacking the Tbx6 homologue Dorsocross have no dorsal vessel (Drosophila equivalent of the heart), indicating that Tbx6 is crucial for the specification of cardiac progenitor cells in the fly (Plageman and Yutzey, 2005; Reim and Frasch, 2005). Collectively, these data suggest that Tbx6 could play an important role in the specification of cardiac myocytes and neural cells.
In the present study, we investigate the role of Tbx6 as a regulator of cell fate decisions in multipotent P19CL6 cells. We show that Tbx6 is necessary, but not sufficient, for DMSO-induced differentiation of P19CL6 into cardiac myocytes. Moreover, the data provide genetic evidence that Nkx2.5, a transcription factor involved in specification of the cardiac myocyte lineage (Harvey, 1996; Schwartz and Olson, 1999; Prall et al., 2002), is downstream of Tbx6. In contrast to its role in cardiac myocyte differentiation, Tbx6 suppresses neural differentiation. Thus, Tbx6 plays a role in the specification of cardiac myocyte and neural lineages in P19CL6 cells and may function as a molecular switch that regulates the differentiation of the same multipotent cell into cardiac myocytes rather than neurons or glia.
2. Materials and methods
2.1. Cell lines and differentiation protocols
P19CL6 and P19CL6-MLC-2v-GFP cells were obtained as previously described (Peng et al., 2002; Moore et al., 2004). For cardiac myocyte differentiation, P19CL6 and P19CL6-MLC-2v-GFP cell lines were plated at a density of 3 × 105 per 10 cm dish and induced to differentiate with 1% DMSO as previously described (Peng et al., 2002). The duration of DMSO treatment for each experiment is as specified. Cells were harvested at the conclusion of DMSO treatment unless noted otherwise. For neural differentiation, cells were plated at a density of 1 × 106 per 10 cm dish and induced to differentiate with 2 μM all-trans RA (Sigma) as previously described (Liour et al., 2000; Staines et al., 1994). After 4 days of RA treatment, cells were trypsinized, diluted 1:10, and replated on new 10 cm culture dishes (modified from Liour et al., 2000; Staines et al., 1994). The time of harvest for each experiment is as specified. The day of the addition of DMSO or RA was designated as day 0.
2.2. Cloning, constructs, and stable cell lines
Tbx6 short hairpin RNA (shRNA) and scrambled shRNA constructs were obtained from Open Biosystems (Cat. no. RHS1764-9102328 and RHS1703, respectively). The scrambled shRNA lacks homology to known mammalian genes. Full length murine Tbx6 (Accession NM_011538) was cloned into the pEF6/V5-His TOPO TA (Invitrogen) using following primers: forward: 5′-CCACCATGTACCATCCACGA GAG-3′ and reverse: 5′-GTACATTGGCTTGATCCCATG-3′. P19CL6-MLC-2v-GFP cells were transfected using Effectene reagent (Qiagen) according to manufacturer's instructions. Stable transfectants were selected for 2 weeks, isolated, and expanded in medium containing 2 μg/ml puromycin (for shRNA constructs) or 5 μg/ml blasticidin (for constructs in pEF6/V5-His TOPO TA). Stable transfectants expressing empty pEF6/V5-His TOPO TA were used as a negative control. For both knockdown and overexpression experiments, two independent clones were studied. Nkx2.5 was recovered from pEF1/His B-Nkx2.5-ER (gift of Dr. Chang-Fu Peng, Albert Einstein College of Medicine) and subcloned into pBABE hygro (obtained from http://www.addgene.org; Addgene plasmid 1765). Nkx2.5 retrovirus was produced in Phoenix cells (http://www.stanford.edu/group/nolan/retroviral_systems/phx.html).
2.3. Semi-quantitative RT-PCR and quantitative real-time RT-PCR (qRT-PCR)
RNA was isolated from cells using TRIzol reagent (Invitrogen). Samples were treated with DNase I (1.0 U/μl, Invitrogen) to minimize genomic DNA contamination and subsequently reverse transcribed to cDNA using SuperScript III First-Strand Synthesis System (Invitrogen) according to manufacturer's instructions. Each PCR primer pair spans an intron to differentiate between the amplification of cDNA and contaminating genomic DNA. BLAST searches for each primer sequence revealed no homology to other genes. The following primers were used for semi-quantitative RT-PCR: β-actin (638 bp product): forward: 5′-TGACGGGGTTCACCCACACTGTGCCCATCTA-3′ and reverse: 5′-CTAGAAGCATTTGTGGTGGACGATGGAGGG-3′; and Tbx6 (320 bp product): forward: 5′-CCTGACTCTCCTGCCACTG-3′; reverse: 5′-CCTCTTCACACGGGCATCC-3′. The intensities of Tbx6 and β-Actin PCR products were assessed at various cycle numbers using densitometry. The relative abundance of Tbx6 mRNA was normalized to that of β-actin. The following primers, designed with the Light Cycler Probe design 2 program (Roche), were used for qRT-PCR: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (220 bp product); forward: 5′-CCTGCACCACCAACTGCTTAG-3′ and reverse: 5′-AGCTCTGGGATGACCTTGCC-3′; Tbx6 (as specified above); and Nkx2.5 (222 bp product): forward: 5′-TCTCCGATCCATCCCACTTTATTG-3′; reverse: 5′-TTGCGTTACGCACTCACTTTAATG-3′. qRT-PCR was performed with a LightCycler FastStart DNA Master Plus SYBR Green I kit (Roche) using 4 μl of cDNA as template per each real-time reaction. Negative internal controls included the omission of reverse transcriptase or cDNA. The relative abundance of each mRNA was normalized to that of GAPDH mRNA. The fold-change of each mRNA was calculated using the 2 –ΔΔCt method as previously described (Livak and Schmittgen, 2001) with data from at least three independent experiments. Agarose gel electrophoresis was used to confirm the correct molecular size of the PCR products.
2.4. Western blotting
Cell lysates were collected with RIPA buffer as previously described (Jasmin et al., 2004). Following centrifugation at 14,000 × g, the protein concentrations of the retained supernatants were determined using the Bradford Protein Assay Reagent (Bio-Rad Laboratories) per manufacturer's instructions. Western blotting was performed as previously described (Ashton et al., 1999) using following antibodies: rabbit polyclonal anti-Tbx6 (1:1000, Invitrogen); rabbit polyclonal anti-GFAP (1:1000, Chemicon); rabbit polyclonal anti-GFP (1:2000, Abcam); goat polyclonal anti-Nkx2.5 (1:100, Santa Cruz Biotechnologies), mouse monoclonal anti-MAP2 (1:1000, Chemicon), and mouse monoclonal anti-GAPDH (1:5000, Abcam). The relative abundance of proteins was determined by densitometry using NIH Image J software, and levels of each protein were normalized to those of GAPDH.
2.5. Microscopic analysis and movies
An inverted microscope (Nikon Eclipse TE2000-S) was used to observe neurite formation, spontaneous beating, and GFP fluorescence. Movies were acquired with a Coolpix 995 camera (Nikon), and post-acquisition image processing was performed using Photoshop (Adobe).
2.6. Statistical analysis
Quantitative data are presented as mean ± SD of experiments. The number of independent replicates is specified for each experiment. Data were analyzed by paired two-tail Student's t test or ANOVA followed by Newman–Keuls multiple comparison test. P < 0.05 was considered statistically significant.
3. Results
3.1. Tbx6 levels increase during DMSO-induced differentiation of P19CL6 cells into cardiac myocytes
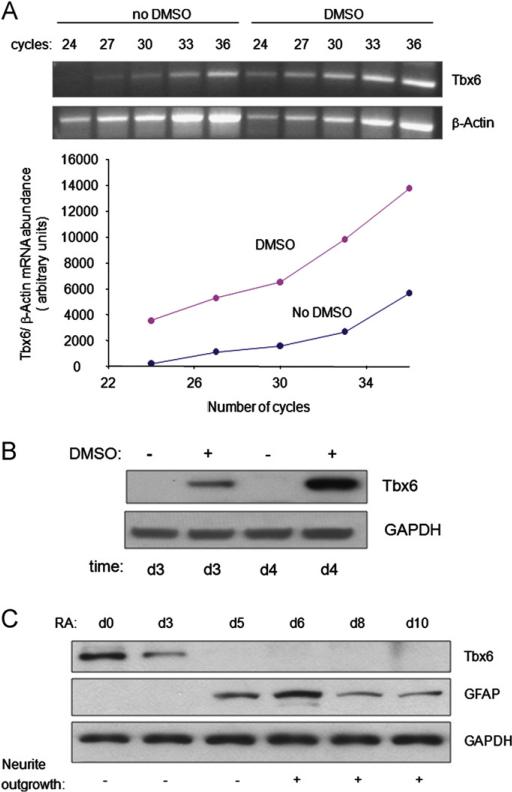
In our previous study, we showed that 4 days of DMSO stimulation is sufficient to commit P19CL6 cells to a cardiac myocyte fate. In addition, our microarray analysis of this process identified Tbx6 as a gene whose expression is induced prior to this commitment point (Peng et al., 2002). To validate these microarray results, we quantified Tbx6 mRNA and protein levels in P19CL6 cells over a time course of DMSO-induced differentiation. The abundance of Tbx6 mRNA was 32-fold higher in P19CL6 cells treated with DMSO for 6 days as compared with cells treated with media alone (Fig. 1A). Induction of Tbx6 gene expression was accompanied by marked increases in Tbx6 protein as assessed at days 3 and 4 of treatment with DMSO (Fig. 1B). Following 6 days of DMSO treatment, Tbx6 protein levels declined, and became undetectable by day 8 (data not shown). In contrast, very low levels of Tbx6 protein were present in cells not treated with DMSO. Thus, the abundance of Tbx6 mRNA and protein increases during the DMSO-induced differentiation of P19CL6 cells to cardiac myocytes. Moreover, these increases begin before the commitment point, suggesting that Tbx6 may be a regulator of cardiac myocyte differentiation.
Fig. 1.
The abundance of Tbx6 mRNA and protein during differentiation of P19CL6 cells into cardiac myocytes or neural cells. (A) Tbx6 mRNA levels increase during DMSO-induced cardiac myocyte differentiation. Cells were cultured with or without DMSO for 6 days and Tbx6 and β-actin mRNA levels were assessed by semi-quantitative RT-PCR following the indicated number of amplification cycles. The abundance of Tbx6 mRNA has been normalized to that of β-actin mRNA. Similar results were obtained from three independent experiments. (B) Tbx6 protein levels increase during DMSO-induced cardiac myocyte differentiation. Cells were cultured with or without DMSO for 3 or 4 days (d) as indicated. Tbx6 protein levels were assessed by Western blot. Results are the representative of three independent experiments. (C) Tbx6 protein levels decrease during RA-induced neural differentiation. Cells were treated with RA for the times indicated. Levels of Tbx6 and GFAP, a glial marker, were assessed by Western blot. Neurite outgrowth was observed by microscopy. Western blot is the representative of three independent differentiation assays.
3.2. Tbx6 levels decline during RA-induced differentiation of P19CL6 cells into neural cells
We assessed whether the already low baseline levels of Tbx6 in P19CL6 cells decrease further during their RA-triggered differentiation into neurons and glia. Tbx6 protein levels decreased in P19CL6 cells following 2 days of RA treatment and became undetectable by day 5 (Fig. 1C and data not shown). In contrast, glial fibrillar acidic protein (GFAP), a glial marker (Liour et al., 2000), first became detectable at day 5 coincident with the absence of Tbx6 (Fig. 1C). In addition, neurite outgrowth became apparent at day 6 (Fig. 1C), consistent with previous observations in P19 cells (Liour et al., 2000). Thus, Tbx6 protein levels decline during RA-induced differentiation of P19CL6 cells to neurons and glia.
3.3. Tbx6 overexpression alone is insufficient to promote differentiation of P19CL6 cells into cardiac myocytes
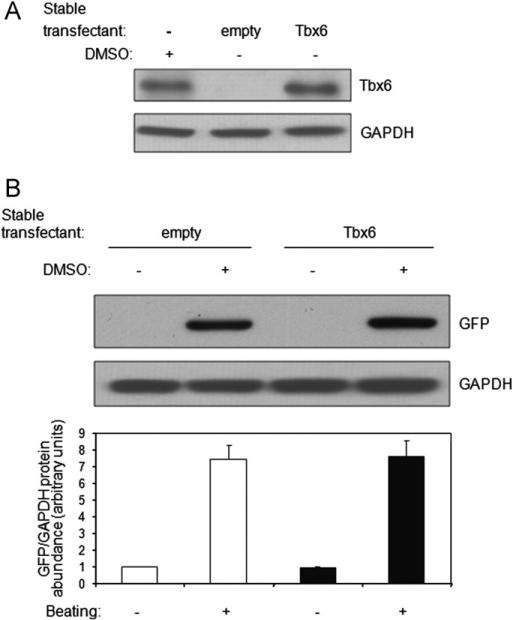
Since Tbx6 is induced by DMSO, we investigated the ability of Tbx6, by itself, to direct cardiac myocyte differentiation. For this and subsequent experiments, we employed a P19CL6 reporter cell line (P19CL6-MLC-2v-GFP) that stably expresses Green Fluorescent Protein (GFP) under the transcriptional control of the rat ventricular myosin light chain-2 promoter (MLC-2v) (Moore et al., 2004) to identify differentiated ventricular cardiac myocytes. Tbx6 was stably expressed in these cells, and two independent clones were identified that contained levels of Tbx6 protein under non-differentiating conditions (no DMSO) that were comparable to endogenous Tbx6 levels following 4 days of DMSO treatment (Fig. 2A, compare lanes 1 and 3). To test the sufficiency of Tbx6 to direct P19CL6 cells to differentiate into cardiac myocytes, P19CL6-MLC-2v-GFP cells stably transfected with empty vector or Tbx6 were cultured without DMSO. GFP expression, an indicator of the activation of the MLC-2v promoter, and spontaneous beating were not detected in cells overexpressing Tbx6 after 10 days (Fig. 2B, lanes 1 and 3), or even after 15 days in culture (data not shown). In contrast, after only 10 days of DMSO treatment, P19CL6-MLC-2v-GFP cells stably transfected with either empty vector or Tbx6 exhibited GFP expression and beating (Fig. 2B, lanes 2 and 4). Moreover, there were no differences in the magnitude of DMSO-stimulated GFP expression (P NS) and the time of onset of beating between empty vector or Tbx6 transfectants. Similar results were obtained with the second independent clone overexpressing Tbx6 (data not shown). Our results demonstrate that Tbx6 is not sufficient to drive cardiac myocyte differentiation in the absence of DMSO. In addition, with DMSO stimulation, Tbx6 did not increase the magnitude or accelerate the time course of this process (data not shown).
Fig. 2.
Tbx6 overexpression does not promote cardiac myocyte differentiation. (A) Tbx6 levels in Tbx6 transfectants. P19CL6-MLC-2v-GFP cells were stably transfected with Tbx6 or empty vector or left untransfected. Tbx6 levels were assessed by Western blot. Empty denotes P19CL6-MLC-2v-GFP cells stably transfected with empty vector. Similar results were obtained in two independent experiments. (B) Tbx6 overexpression is not sufficient to induce cardiac myocyte differentiation in the absence of DMSO and does not alter the magnitude of DMSO-induced differentiation. P19CL6-MLC-2v-GFP cells stably transfected with empty vector or Tbx6 were cultured with or without DMSO for 10 days. GFP levels were assessed by Western blot and quantified by densitometry. GFP expression, driven by the striated muscle-specific MLC-2v promoter, serves as a marker of striated muscle differentiation. Graph shows abundance (mean ± SD) of GFP protein normalized to that of GAPDH for three independent experiments (P NS for Tbx6 transfectants vs. empty vector transfectants treated with DMSO). Spontaneous beating was scored using microscopy.
3.4. Tbx6 is required for DMSO-induced differentiation of P19CL6 cells into cardiac myocytes
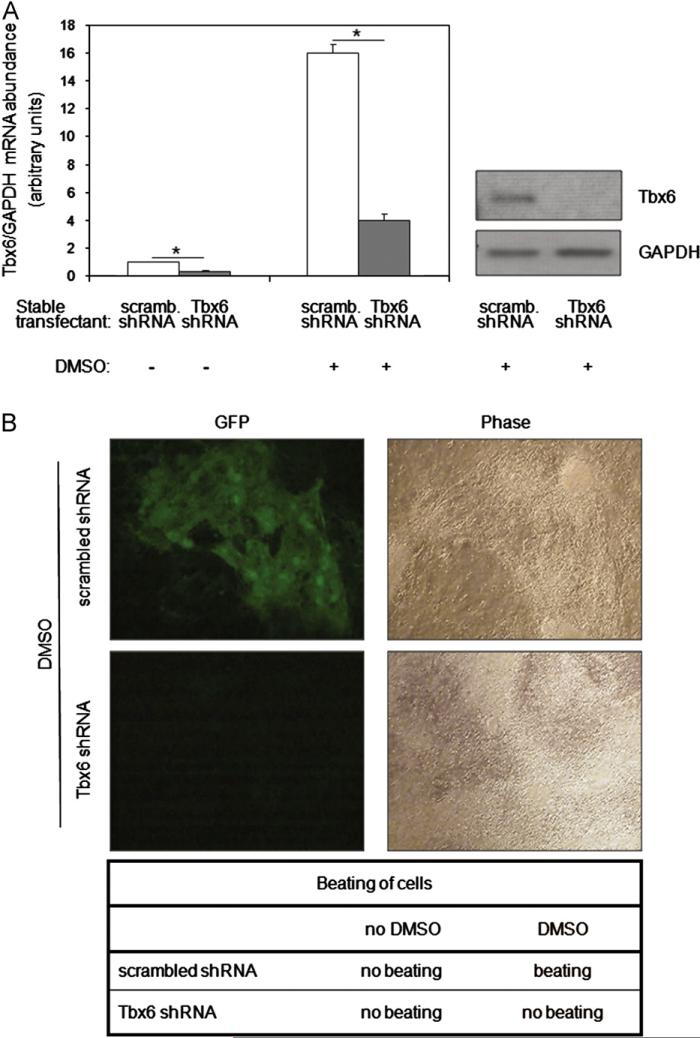
To examine the necessity of Tbx6 for DMSO-induced differentiation of P19CL6 cells into cardiac myocytes, Tbx6 was knocked down by stably transfecting P19CL6-MLC-2v-GFP cells with a specific shRNA construct against Tbx6, and two independent clones were studied. A scrambled shRNA without homology to known mammalian genes was used as the control. Quantitative real-time PCR (qRT-PCR) demonstrated that Tbx6 mRNA levels were reduced 4-fold by Tbx6 shRNA in the absence or presence of DMSO stimulation (P < 0.01, Fig. 3A, left panel). Moreover, Tbx6 protein levels, which were increased markedly by DMSO in scrambled shRNA transfectants, remained undetectable in Tbx6 shRNA transfectants (Fig. 3A, right panel). Together these results show that Tbx6 knockdown is efficient and maintained during DMSO-induced differentiation.
Fig. 3.
Tbx6 is essential for DMSO-induced cardiac myocyte differentiation. (A) Knockdown of basal and DMSO-induced levels of Tbx6 in P19CL6-MLC-2v-GFP cells. Scrambled (scramb) shRNA or Tbx6 shRNA stable transfectants were cultured with or without DMSO for 6 days. Levels of Tbx6 and GAPDH mRNA were assessed by qRTPCR, and the abundance of Tbx6 mRNA normalized to that of GAPDH mRNA (displayed in left panel as mean ± SD). *P < 0.01 for Tbx6 shRNA vs. scrambled shRNA. Tbx6 protein levels were assessed at the same time point by Western blot (right panel). Results are the representative of three independent experiments. (B) Tbx6 knockdown ablates MLC-2v promoter activity and spontaneous beating. Scrambled shRNA and Tbx6 shRNA stable transfectants were cultured with DMSO for 17 days following which the expression of MLC-2v-driven GFP was assessed by fluorescence microscopy. The presence or absence of beating is indicated below the figure (also see movies in Supplementary information). The onset of beating in scrambled shRNA with DMSO treatment was detected at day 10. Similar results were obtained in the four independent experiments.
To test whether Tbx6 is required for cardiac myocyte differentiation, scrambled and Tbx6 shRNA stable transfectants were treated with DMSO. While scrambled shRNA transfectants expressed GFP and initiated spontaneous beating at day 10 of treatment as expected, Tbx6 shRNA transfectants failed to undergo differentiation as evidenced by the absence of GFP fluorescence and spontaneous beating (data not shown). To rule out the possibility that differentiation was merely delayed by Tbx6 knockdown, we extended the treatment period to 17 days. The rationale for this experiment was that, although in wild type P19CL6 cells, GFP fluorescence and spontaneous beating first become apparent after 10 days of DMSO treatment, these parameters continue to increase over the next 7 days. Despite the extended period of DMSO treatment, we found that GFP expression and spontaneous beating remained absent in Tbx6 shRNA transfectants (Figs. 3B and 4A and Supplemental Movies 1–4). Similar results were obtained with the second independent knockdown clone (data not shown). These results indicate that Tbx6 is required for the DMSO-induced differentiation of P19CL6 cells into cardiac myocytes.
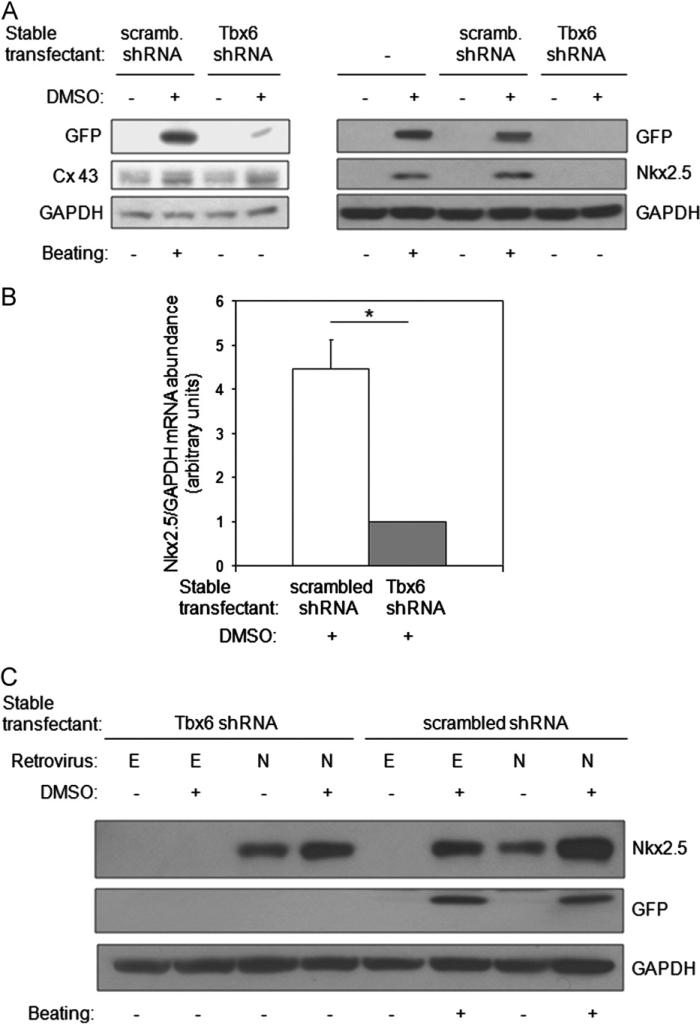
Fig. 4.
Tbx6 is necessary for the activation of Nkx2.5 expression during DMSO-induced cardiac myocyte differentiation. (A) Tbx6 knockdown decreases protein levels of Nkx2.5, but not Connexin43 (Cx43). Untransfected P19CL6-MLC-2v-GFP cells or cells stably expressing scrambled shRNA or Tbx6 shRNA were cultured with or without DMSO for 10 days. The abundance of the indicated proteins was assessed by Western blot and spontaneous beating by direct observation. Results are the representative of three independent experiments. (B) Tbx6 knockdown decreases the induction of Nkx2.5 mRNA by DMSO. P19CL6-MLC-2v-GFP cells stably expressing scrambled shRNA or Tbx6shRNA were treated with or without DMSO for 4 days and harvested at day 6. Nkx2.5 and GAPDH mRNA levels were assessed by qRT-PCR. Results are expressed as mean ± SD. Data are from three independent experiments. *P < 0.05. (C) Nkx2.5 overexpression fails to rescue defective cardiac myocyte differentiation resulting from Tbx6 knockdown. P19CL6-MLC-2v-GFP cells stably expressing Tbx6 shRNA or scrambled shRNA were cultured with or without DMSO for 4 days. Following this, they were infected with a retrovirus constitutively expressing Nkx2.5 (labeled N) or a retrovirus containing empty vector (labeled E). Cells were observed for the onset of beating and harvested at day 10. The abundance of the indicated proteins was assessed by Western blot. Data are the representative of three independent experiments.
3.5. Knockdown of Tbx6 markedly decreases DMSO-induced Nkx2.5 mRNA and protein
Given the necessity of Tbx6 for the differentiation of P19CL6 into cardiac myocytes and the fact that Tbx6 is a transcription factor, we explored potential candidates that might mediate its effects. Because P19CL6-MLC-2v-GFP Tbx6 shRNA transfectants do not exhibit spontaneous beating following DMSO treatment, we asked whether knockdown of Tbx6 affects the expression of Connexin 43, a gap junction protein critical for the coupling of cardiac myocytes (Eckardt et al., 2006; Gros et al., 2004). In response to DMSO treatment, Connexin 43 levels increased mildly and to a similar extent in both scrambled shRNA and Tbx6 shRNA transfectants (Fig. 4A, left panel). These data show that, while Connexin 43 levels are mildly increased by DMSO treatment, these increases appear to be independent of Tbx6.
The transcription factor tinman is required for formation of the dorsal vessel in Drosophila (Azpiazu and Frasch, 1993; Bodmer, 1993). Complete loss of Nkx2.5, the mammalian homolog of tinman, causes a fatal defect in cardiac looping (Harvey, 1996; Schwartz and Olson, 1999; Prall et al., 2002). As previously shown in P19CL6 cells (Monzen et al., 1999), Nkx2.5 protein levels were markedly induced by DMSO in P19CL6-MLC-2v-GFP cells that were untransfected or stably transfected with scrambled shRNA (Fig. 4A, right panel). In contrast, Nkx2.5 protein levels did not increase in response to DMSO in Tbx6 shRNA stable transfectants. To further explore why Tbx6 knockdown abrogated DMSO-induced increases in Nkx2.5, we examined the effect of Tbx6 knockdown on Nkx2.5 mRNA levels. RNA was analyzed at day 6 of DMSO treatment because Nkx2.5 mRNA levels increase at this time (Monzen et al., 1999). Analysis by qRT-PCR demonstrated that Nkx2.5 mRNA levels were 4.5-fold lower in Tbx6 shRNA transfectants as compared with scrambled shRNA transfectants (P < 0.01, Fig. 4B). The results demonstrate that Tbx6 is required for DMSO-induced increases in Nkx2.5 mRNA and protein.
3.6. Suppression of cardiac myocyte differentiation by Tbx6 knockdown is not rescued by Nkx2.5
We next investigated whether adding back Nkx2.5 would rescue the absence of P19CL6 differentiation into cardiac myocytes observed with Tbx6 knockdown. Nkx2.5 retrovirus was generated and used to transduce P19CL6-MLC-2v-GFP cells already stably transfected with scrambled or Tbx6 shRNA (Fig. 4C). Retrovirus was administered at day 4 of DMSO treatment before endogenous Nkx2.5 expression was induced, and cells were cultured until day 10. While GFP expression and spontaneous beating were present in DMSO-treated scrambled shRNA transfectants (lanes 6 and 8), they were absent from DMSO-treated Tbx6 shRNA transfectants (lanes 2 and 4) even if expressing exogenous Nkx2.5 (lane 4). Thus, despite being downstream of Tbx6, Nkx2.5 is unable to rescue inhibition of cardiac myocyte differentiation by Tbx6 knockdown.
3.7. Tbx6 overexpression decreases and delays neural differentiation of P19CL6 cells
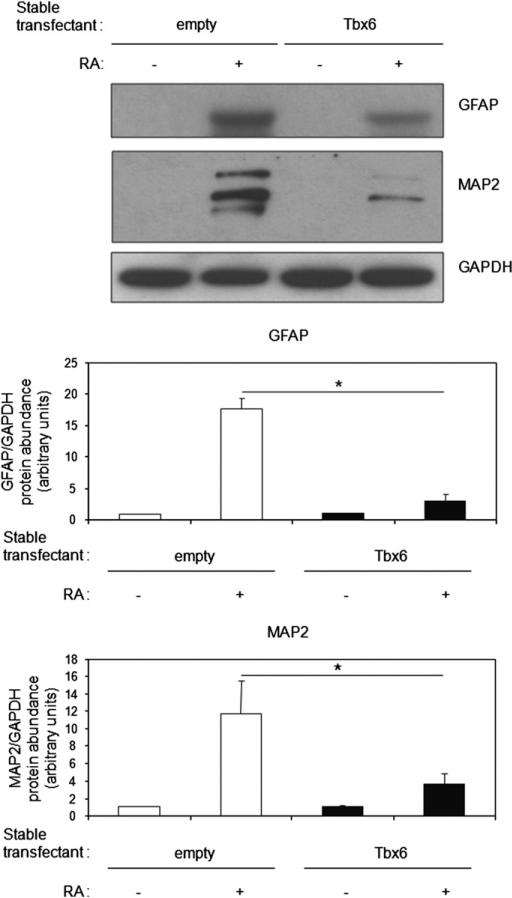
Tbx6 protein levels decrease during RA-induced differentiation of P19CL6 cells into neurons and glia (Fig. 1C). This suggests the hypothesis that, in contrast to its role in cardiac myocyte differentiation, decreases in Tbx6 abundance may be necessary for neural differentiation. To assess this possibility, we used P19CL6-MLC-2v-GFP cells that had been stably transfected with Tbx6 or empty vector. Two independent clones were studied. Tbx6 overexpression inhibited RA-induced increases in GFAP, a glial marker, and Microtubule Associated Protein (MAP2), a neuronal marker (Liour et al., 2000), by 6-fold and 4-fold, respectively (P < 0.01, Fig. 5). Moreover, neurite outgrowth was delayed until day 8 in Tbx6 transfectants, while it was evident at day 6 in empty vector transfectants (Table 1). Similar results were obtained with the second independent clone overexpressing Tbx6 (data not shown). These results indicate that Tbx6 is a repressor of neural differentiation. Moreover, they demonstrate that RA-stimulated decreases in Tbx6 abundance are necessary for neural differentiation to proceed.
Fig. 5.
Tbx6 overexpression inhibits RA-induced glial and neuronal differentiation. P19CL6-MLC-2v-GFP cells stably transfected with empty vector or Tbx6 were cultured with or without RA for 4 days and harvested on day 8. Levels of GFAP (glial marker) and MAP2 (neuronal marker) were assessed by Western blot. Mean ± SD of protein levels from densitometric analysis are as indicated. Data from three independent experiments (*P < 0.01 for Tbx6 transfectants vs. empty vector transfectants treated with RA).
Table 1.
Neurite outgrowth in RA-treated P19CL6 cell lines.
| RA | Neurite outgrowth |
||||||
|---|---|---|---|---|---|---|---|
| d2 | d3 | d5 | d6 | d8 | d10 | d12 | |
| Empty vector | – | – | – | + | + | + | + |
| Tbx6 overexpression | – | – | – | – | + | + | + |
| scrambled shRNA | – | – | – | + | + | + | + |
| Tbx6 shRNA | – | + | + | + | + | + | + |
P19CL6-MLC-2v-GFP cells stably transfected with empty vector, Tbx6 expression construct, scrambled shRNA, or Tbx6 shRNA were treated with RA for 4 days (d), and neurite outgrowth was assessed on the days indicated by light microscopy.
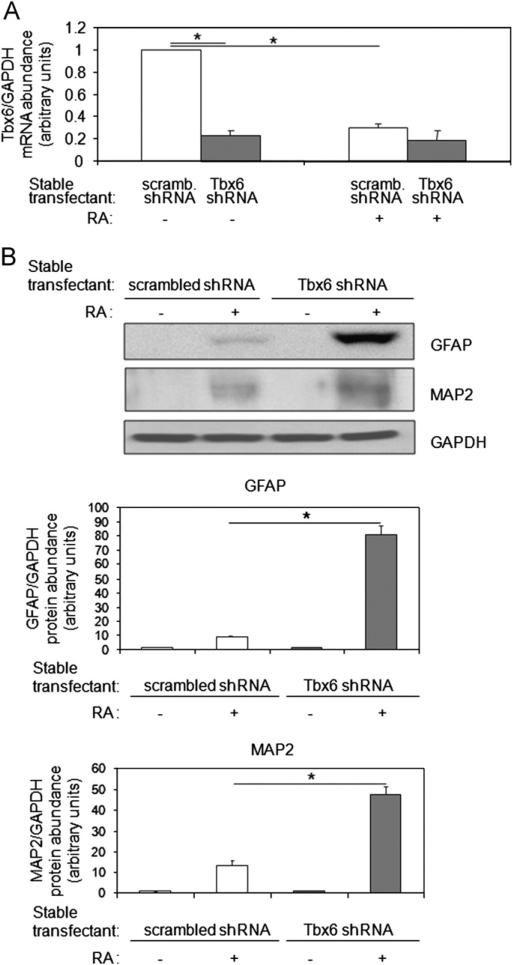
3.8. Tbx6 knockdown augments and accelerates neural differentiation
Given that Tbx6 overexpression suppresses neural differentiation, we asked whether Tbx6 deficiency would promote this process. To assess this, we evaluated neural differentiation in P19CL6-MLC-2v-GFP cells in which Tbx6 had been knocked down with shRNA. Two independent clones were studied. In the absence of RA treatment, the abundance of Tbx6 transcripts were 4-fold lower in Tbx6 shRNA stable transfectants as compared with scrambled shRNA stable transfectants (P < 0.01, Fig. 6A, lanes 2 vs. 1). Reduction of Tbx6 levels in the absence of RA, however, was not sufficient to trigger neural differentiation (Fig. 6B, lanes 3 vs. 1). Treatment with RA for 4 days decreased levels of Tbx6 transcripts 3-fold in scrambled shRNA transfectants (P < 0.01, Fig. 6A, lanes 3 vs. 1) such that they reached levels similar to those in Tbx6 shRNA transfectants (P NS, Fig. 6A, lanes 4 vs. 3). In response to RA, the levels of GFAP and MAP2 were 10-fold and 4-fold higher, respectively, in Tbx6 shRNA transfectants as compared with scrambled shRNA transfectants (P < 0.01, Fig. 6B, lanes 4 vs. 2). Moreover, Tbx6 knockdown accelerated the onset of RA-stimulated neurite outgrowth from day 6 to day 3 (Table 1). Similar results were obtained with the second independent knockdown clone (data not shown). Thus, while RA treatment for several days leads to decreases in Tbx6 levels, depletion of Tbx6 prior to RA treatment further facilitates differentiation of P19Cl6 cells into neurons and glia.
Fig. 6.
Tbx6 knockdown augments RA-induced glial and neuronal differentiation. (A) Tbx6 knockdown decreases Tbx6 mRNA levels. P19CL6-MLC-2v-GFP cells stably expressing scrambled shRNA or Tbx6 shRNA were cultured with or without RA for 4 days and Tbx6 mRNA abundance assessed by qRT-PCR. Quantitative data from three independent experiments. (B) Effect of Tbx6 knockdown on RA-induced GFAP and MAP2 expression. Scrambled shRNA and Tbx6 shRNA stable transfectants were cultured with or without RA for 4 days and harvested on day 8. Levels of GFAP (glial marker) and MAP2 (neuronal marker) were assessed by Western blot. Mean ± SD of protein levels from densitometric analysis are indicated. Data from three independent experiments (*P < 0.01).
4. Discussion
Using the multipotent P19CL6 model, we demonstrate here that Tbx6 is a determinant of the fate decision towards the cardiac myocyte lineage while suppressing neural differentiation. Consistent with this role, Tbx6 abundance increases during DMSO-induced differentiation of P19CL6 cells into cardiac myocytes and peaks co-incident with the commitment point of this differentiation process. Moreover, Tbx6 is essential for cardiac myocyte differentiation in this model. Conversely, Tbx6 levels decrease during RA-induced differentiation of P19CL6 cells to neurons and glia, and maintenance of Tbx6 levels blocks neural differentiation. These data suggest that Tbx6 is part of a molecular switch that directs this multipotent progenitor cell towards cardiac myocyte rather than neural lineages.
Overexpression studies have shown that Tbx5 can accelerate DMSO-induced differentiation of P19CL6 cells into cardiac myocytes, although the applicability of this finding requires further exploration because Tbx5 is not normally expressed in this cell type (Fijnvandraat et al., 2003; Hiroi et al., 2001). Similarly, overexpression of combinations of Tbx5/GATA4/Bap60c and Tbx5/GATA4/MEF2c have been shown to reprogram mesenchymal cells or cardiac fibroblasts, respectively, to cardiac myocytes (Takeuchi and Bruneau, 2009; Ieda et al., 2010). In contrast to these overexpression studies, genes that are necessary for cardiac myocyte differentiation have eluded detection. Although multiple genes have been found that play key roles in cardiac morphogenesis, defects in cardiac myocyte specification has not been observed (Cripps and Olson, 2002; Harvey, 2002; Plageman and Yutzey, 2005). For example, within the T-box gene family, mutations in Tbx1 and Tbx5 cause abnormalities in cardiac morpho-genesis but do not disrupt cardiac myocyte differentiation (Naiche et al., 2005; Plageman and Yutzey, 2005). In contrast, our findings indicate that Tbx6 is necessary, but not sufficient, for DMSO-induced differentiation of P19CL6 into cardiac myocytes. This result is consistent with previous work showing that inactivation of Dorsocross, the Drosophila homolog of Tbx6, results in failure of cardioblasts to form (Plageman and Yutzey, 2005; Reim and Frasch, 2005). Thus, in these two paradigms, Tbx6 plays a role in the specification of multipotent mammalian cells to the cardiac myocyte lineage. The lack of a cardiac phenotype in Tbx6 mutant mice may result from redundancy with other T-box genes, although it remains possible that the P19CL6 system does not faithfully recapitulate all aspects of cardiac myocyte differentiation in vivo.
Our results show that the knockdown of Tbx6 markedly decreased Nkx2.5 mRNA, which correlated with severely reduced Nkx2.5 protein levels. These findings place Nkx2.5 downstream of Tbx6. In addition, they raise the possibility that the Nkx2.5 gene is a transcriptional target of Tbx6, but further work is needed to test whether the effects of Tbx6 on Nkx2.5 are direct or indirect. Interestingly, overexpression of Nkx2.5 failed to rescue the Tbx6 knockdown phenotype, suggesting that additional genes are involved in mediating the effects of Tbx6 in this model. Identification of the transcriptional targets of Tbx6 will be required to understand how this protein modulates cardiac myocyte differentiation.
BMP4 (Bone Morphogenetic Protein 4) is known to induce Tbx6 expression in P19 cells to inhibit neural differentiation (Finley et al., 1999). Thus, a mechanism of Tbx6 regulation during cardiac myocyte differentiation might also involve signaling through BMPs. Consistent with this, BMP signaling is required for the induction of Nkx2.5 expression during cardiac myocyte differentiation in the P19 model (Jamali et al., 2001). The fact that Tbx6 is not sufficient to drive or accelerate cardiac differentiation in the absence of DMSO stimulation suggests that other signaling cascade(s) must also be activated by DMSO to trigger this process.
We have demonstrated that Tbx6 functions as a repressor of both neuron and glial differentiation in the P19CL6 model. Our results are consistent with the enhanced neural differentiation seen in Tbx6 null mice (Chapman and Papaioannou, 1998). Tbx6 might inhibit neural differentiation by inducing inhibitors of neurogenesis and/or repressing activators of this process. Other T-box genes have been shown to inhibit expression of proneurogenic factors. In particular, it has been demonstrated that Tbx1 suppresses the expression of neurogenin 1, a basic helix–loop–helix transcription factor with important functions in neurogenesis (Raft et al., 2004). RA decreases endogenous levels of Tbx6, and our Tbx6 overexpression experiments indicate that removal of this barrier is necessary for neural differentiation to proceed. However, although knockdown of Tbx6 accelerates neural differentiation, the requirement for RA in this process suggests the involvement of additional pathways.
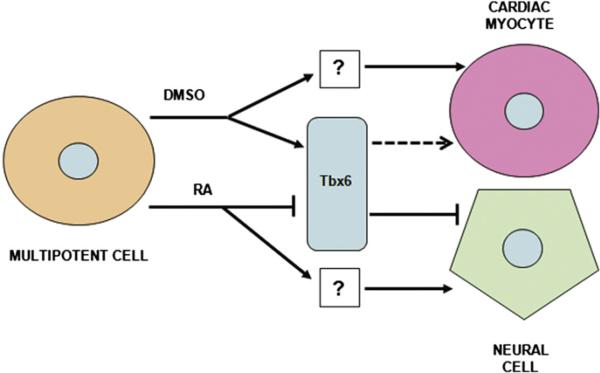
In summary, we show that Tbx6 modulates two differentiation pathways within a single progenitor cell. DMSO increases Tbx6 abundance in multipotent P19CL6 cells, and Tbx6 is necessary for the DMSO-induced differentiation of these cells into cardiac myocytes. Conversely, RA decreases Tbx6 levels in P19CL6 cells, and reduction of Tbx6 abundance is necessary to allow neural differentiation. Together, these findings indicate that Tbx6 is an important determinant of cell fate in this system and may function as a molecular switch in the decision of multipotent cells to adopt a cardiac myocyte vs. neural fate (Fig. 7). The applicability of this paradigm remains to be tested in embryonic and induced pluripotent stem cell lines and in vivo. This role for Tbx6 might not be unique to the P19CL6 model as induction of Tbx6 expression has also been recognized during the differentiation of murine embryonic stem cells to cardiac myocytes (Menard et al., 2005; Stefanovic and Puceat, 2007). An enhanced knowledge of these mechanisms will deepen our understanding of how progenitor cells are directed to differentiate into specific cell types.
Fig. 7.
Proposed model for Tbx6 regulation of cardiac and neural differentiation programs. DMSO-induced increases in Tbx6 abundance are necessary for P19CL6 cells to differentiate into cardiac myocytes. However, Tbx6 alone is not sufficient for this process (dashed arrow). This implies the existence of Tbx6-independent actions of DMSO that are also necessary, but not sufficient, for cardiac myocyte differentiation. In contrast, RA-stimulated decreases in Tbx6 levels are necessary, but not sufficient, for P19CL6 cells to differentiate into neurons and glia. This suggests Tbx6-independent actions of RA that are also necessary, but not sufficient, for neural differentiation. In this model, Tbx6 functions as a molecular switch that regulates fate decisions of a single progenitor cell. Arrows or flat-ended lines represent activation/increased abundance or inhibition/decreased abundance, respectively.
Supplementary Material
Acknowledgments
R.N.K. was supported by NIH R01 Grants HL60665, HL61550, and HL80607 and the Dr. Gerald and Myra Dorros Chair in Cardiovascular Disease of the Albert Einstein College of Medicine. We thank the Wilf Family for their generosity and support. Data in this manuscript are from a thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate Division of Medical Sciences of the Albert Einstein College of Medicine of Yeshiva University.
Footnotes
Appendix A. Supporting information
Movies showing that Tbx6 knockdown inhibits DMSO-induced differentiation of P19CL6 cells into cardiac myocytes and spontaneous beating. The movie panels correspond directly with those in Fig. 3B. P19CL6-MLC-2v-GFP cells stably transfected with scrambled shRNA or Tbx6 shRNA were cultured with DMSO for 17 days following which the expression of MLC-2v-driven GFP was assessed by fluorescence microscopy and the presence or absence of beating by phase and fluorescence microscopy. Similar results were obtained in two independent experiments. Movie 1: scrambled shRNA, fluorescence (DSCN2173). Movie 2: scrambled shRNA, phase (DSCN2174). Movie 3: Tbx6 shRNA, fluorescence (DSCN2181). Movie 4: Tbx6 shRNA, phase (DSCN2182).
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.diff.2012.04.007.
References
- Ashton AW, Yokota R, John G, Zhao S, Suadicani SO, Spray DC, Ware JA. Inhibition of endothelial cell migration, intercellular communication, and vascular tube formation by thromboxane A(2). The Journal of Biological Chemistry. 1999;274:35562–35570. doi: 10.1074/jbc.274.50.35562. [DOI] [PubMed] [Google Scholar]
- Azpiazu N, Frasch M. Tinman and bagpipe: two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes and Development. 1993;7:1325–1340. doi: 10.1101/gad.7.7b.1325. [DOI] [PubMed] [Google Scholar]
- Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development (Cambridge, England) 1993;118:719–729. doi: 10.1242/dev.118.3.719. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Papaioannou VE. Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature. 1998;391:695–697. doi: 10.1038/35624. [DOI] [PubMed] [Google Scholar]
- Cripps RM, Olson EN. Control of cardiac development by an evolutionarily conserved transcriptional network. Developmental Biology. 2002;246:14–28. doi: 10.1006/dbio.2002.0666. [DOI] [PubMed] [Google Scholar]
- Eckardt D, Kirchhoff S, Kim JS, Degen J, Theis M, Ott T, Wiesmann F, Doevendans PA, Lamers WH, de Bakker JM, van Rijen HV, Schneider MD, Willecke K. Cardiomyocyte-restricted deletion of connexin43 during mouse development. Journal of Molecular and Cellular Cardiology. 2006;41:963–971. doi: 10.1016/j.yjmcc.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Fijnvandraat AC, Lekanne Deprez RH, Christoffels VM, Ruijter JM, Moorman AF. TBX5 overexpression stimulates differentiation of chamber myocardium in P19C16 embryonic carcinoma cells. Journal of Muscle Research and Cell Motility. 2003;24:211–218. doi: 10.1023/a:1026063409656. [DOI] [PubMed] [Google Scholar]
- Finley MF, Devata S, Huettner JE. BMP-4 inhibits neural differentiation of murine embryonic stem cells. Journal of Neurobiology. 1999;40:271–287. [PubMed] [Google Scholar]
- Gros D, Dupays L, Alcolea S, Meysen S, Miquerol L, Theveniau-Ruissy M. Genetically modified mice: tools to decode the functions of connexins in the heart-new models for cardiovascular research. Cardiovascular Research. 2004;62:299–308. doi: 10.1016/j.cardiores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Habara-Ohkubo A. Differentiation of beating cardiac muscle cells from a derivative of P19 embryonal carcinoma cells. Cell Structure and Function. 1996;21:101–110. doi: 10.1247/csf.21.101. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Pisano E, Papaioannou VE. Tbx6 regulates left/right patterning in mouse embryos through effects on nodal cilia and perinodal signaling. PLoS One. 2008;3:e2511. doi: 10.1371/journal.pone.0002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RP. NK-2 homeobox genes and heart development. Developmental Biology. 1996;178:203–216. doi: 10.1006/dbio.1996.0212. [DOI] [PubMed] [Google Scholar]
- Harvey RP. Patterning the vertebrate heart. Nature Reviews. 2002;3:544–556. doi: 10.1038/nrg843. [DOI] [PubMed] [Google Scholar]
- Hiroi Y, Kudoh S, Monzen K, Ikeda Y, Yazaki Y, Nagai R, Komuro I. Tbx5 associates with Nkx2-5 and synergistically promotes cardiomyocyte differentiation. Nature Genetics. 2001;28:276–280. doi: 10.1038/90123. < /http://www.stanford.edu/group/nolan/retroviral_systems/phx.htmlS.>. [DOI] [PubMed] [Google Scholar]
- Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali M, Karamboulas C, Rogerson PJ, Skerjanc IS. BMP signaling regulates Nkx2-5 activity during cardiomyogenesis. FEBS Letters. 2001;509:126–130. doi: 10.1016/s0014-5793(01)03151-9. [DOI] [PubMed] [Google Scholar]
- Jasmin JF, Mercier I, Hnasko R, Cheung MW, Tanowitz HB, Dupuis J, Lisanti MP. Lung remodeling and pulmonary hypertension after myocardial infarction: pathogenic role of reduced caveolin expression. Cardiovascular Research. 2004;63:747–755. doi: 10.1016/j.cardiores.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Liour SS, Kapitonov D, Yu RK. Expression of gangliosides in neuronal development of P19 embryonal carcinoma stem cells. Journal of Neuroscience Research. 2000;62:363–373. doi: 10.1002/1097-4547(20001101)62:3<363::AID-JNR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McBurney MW. P19 embryonal carcinoma cells. International Journal of Developmental Biology. 1993;37:135–140. [PubMed] [Google Scholar]
- Menard C, Hagege AA, Agbulut O, Barro M, Morichetti MC, Brasselet C, Bel A, Messas E, Bissery A, Bruneval P, Desnos M, Puceat M, Menasche P. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study. Lancet. 2005;366:1005–1012. doi: 10.1016/S0140-6736(05)67380-1. [DOI] [PubMed] [Google Scholar]
- Monzen K, Shiojima I, Hiroi Y, Kudoh S, Oka T, Takimoto E, Hayashi D, Hosoda T, Habara-Ohkubo A, Nakaoka T, Fujita T, Yazaki Y, Komuro I. Bone morphogenetic proteins induce cardiomyocyte differentiation through the mitogen-activated protein kinase kinase kinase TAK1 and cardiac transcription factors Csx/Nkx-2.5 and GATA-4. Molecular and Cellular Biology. 1999;19:7096–7105. doi: 10.1128/mcb.19.10.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JC, Spijker R, Martens AC, de Boer T, Rook MB, van der Heyden MA, Tertoolen LG, Mummery CL. A P19Cl6 GFP reporter line to quantify cardiomyocyte differentiation of stem cells. International Journal of Developmental Biology. 2004;48:47–55. doi: 10.1387/ijdb.15005574. [DOI] [PubMed] [Google Scholar]
- Naiche LA, Harrelson Z, Kelly RG, Papaioannou VE. T-box genes in vertebrate development. Annual Review of Genetics. 2005;39:219–239. doi: 10.1146/annurev.genet.39.073003.105925. [DOI] [PubMed] [Google Scholar]
- Peng CF, Wei Y, Levsky JM, McDonald TV, Childs G, Kitsis RN. Microarray analysis of global changes in gene expression during cardiac myocyte differentiation. Physiological Genomics. 2002;9:145–155. doi: 10.1152/physiolgenomics.00027.2002. [DOI] [PubMed] [Google Scholar]
- Plageman TF, Jr., Yutzey KE. T-box genes and heart development: putting the “T” in heart. Developmental Dynamics. 2005;232:11–20. doi: 10.1002/dvdy.20201. [DOI] [PubMed] [Google Scholar]
- Prall OW, Elliott DA, Harvey RP. Developmental paradigms in heart disease: insights from tinman. Annals of Medicine. 2002;34:148–156. [PubMed] [Google Scholar]
- Raft S, Nowotschin S, Liao J, Morrow BE. Suppression of neural fate and control of inner ear morphogenesis by Tbx1. Development (Cambridge, England) 2004;131:1801–1812. doi: 10.1242/dev.01067. [DOI] [PubMed] [Google Scholar]
- Reim I, Frasch M. The Dorsocross T-box genes are key components of the regulatory network controlling early cardiogenesis in Drosophila. Development (Cambridge, England) 2005;132:4911–4925. doi: 10.1242/dev.02077. [DOI] [PubMed] [Google Scholar]
- Schwartz RJ, Olson EN. Building the heart piece by piece: modularity of cis-elements regulating Nkx2-5 transcription. Development (Cambridge, United Kingdom) 1999;126:4187–4192. doi: 10.1242/dev.126.19.4187. [DOI] [PubMed] [Google Scholar]
- Staines WA, Morassutti DJ, Reuhl KR, Ally AI, McBurney MW. Neurons derived from P19 embryonal carcinoma cells have varied morphologies and neurotransmitters. Neuroscience. 1994;58:735–751. doi: 10.1016/0306-4522(94)90451-0. [DOI] [PubMed] [Google Scholar]
- Stefanovic S, Puceat M. Oct-3/4: not just a gatekeeper of pluripotency for embryonic stem cell, a cell fate instructor through a gene dosage effect. Cell Cycle. 2007;6:8–10. doi: 10.4161/cc.6.1.3633. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–711. doi: 10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heyden MA, Defize LH. Twenty one years of P19 cells: what an embryonal carcinoma cell line taught us about cardiomyocyte differentiation. Cardiovascular Research. 2003;58:292–302. doi: 10.1016/s0008-6363(02)00771-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.