To the Editor
The connection between insulin resistance (IR) and heart failure (HF) is well recognized (1). Insulin resistance has repeatedly been demonstrated to be a strong predictor of the development of HF and to predict a poor prognosis in patients with HF. The mechanism by which IR contributes to the pathogenesis of HF is unclear, but it likely involves the balance between the utilization of free fatty acids (FFAs) and glucose as an energy substrate in the myocardium. Glucose is a more efficient fuel source than FFAs and is therefore preferentially used under conditions of stress, such as HF. In states of IR, this compensatory switch is inhibited, leading to decreased cardiac efficiency (1).
HF patients without diabetes may demonstrate improvements in myocardial glucose uptake (MGU) in response to standard therapies such as neurohormonal blockade and cardiac resynchronization therapy. MGU may also be improved by treatment with metabolic modulators. Interventions that improve insulin sensitivity (e.g., diet modification, insulin-sensitizing agents, gene overexpression/underexpression) seem to protect against myocardial injury in animal models (2). Consequently, there is growing interest in the role of pharmacologic modulation of myocardial FFAs and glucose utilization as an adjunct therapy for selected patients with HF (1).
We propose that patients with HF and IR may demonstrate improvement in MGU after treatment with insulin-sensitizing agents. We describe the findings of a pilot study to measure MGU alterations using serial fluorodeoxyglucose (FDG) positron emission tomography (PET) before and after treatment with rosiglitazone, a peroxisome proliferator-activated receptor gamma agonist. Our goal was to demonstrate that alteration of whole-body insulin sensitivity can result in quantifiable improvements in MGU, even in the absence of frank diabetes mellitus (DM).
Patients with nonischemic dilated cardiomyopathy and New York Heart Association functional class I to II HF who had been on a stable medication and device regimen for at least 3 months were eligible for screening. Fasting insulin and glucose levels were used to calculate the Homeostasis Model Assessment for IR for eligible patients. Patients underwent a 6-min walk test, fasting lipid studies, and an FDG-PET study to assess MGU at baseline and after a 4-week course of rosiglitazone, 4 mg/day.
The FDG-PET/computed tomography (CT) scans were performed using a Discovery LS PET/CT unit (GE Medical Systems, Milwaukee, Wisconsin). Patients fasted for at least 6 h before imaging, and their blood glucose levels were <150 mg/dl at the time of the study. Four multislice helical noncontrast CT images were obtained over the thorax to be used for attenuation correction purposes and anatomic localization. Subjects then received a 50-g oral glucose load and 20-mCi FDG injection, followed by serial PET acquisition at 0, 29, 39, 49, and 59 min. The PET scan was corrected using segmented attenuation data of the conventional transmission scan. PET images were reconstructed with a standard iterative algorithm. All images were reformatted into axial, coronal, and sagittal views and viewed with the software provided by the manufacturer (eNTEGRA, GE Medical Systems, Haifa, Israel).
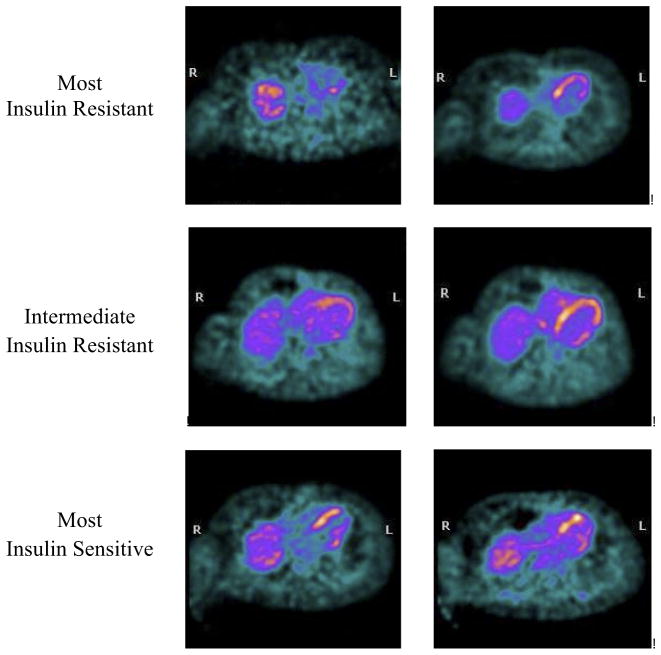
Seven patients enrolled in the protocol and received baseline studies. Three subjects withdrew during the treatment phase; 2 withdrew because of fluid retention and 1 because of lightheadedness. Four subjects completed the full course of treatment, and 3 subjects had baseline and follow-up PET scans of sufficient quality for analysis, whereas the follow-up scan for 1 subject was corrupted and uninterpretable. All patients were on standard HF medications including beta-blockers and angiotensin-converting enzyme inhibitors. Homeostasis Model Assessment for IR values of the 3 subjects with complete imaging were 6.67, 1.78, and 0.64. Two subjects (data unavailable for subject #1) demonstrated a confirmed decrease in Homeostasis Model Assessment for IR after treatment, corresponding with improved insulin sensitivity. Although all 3 subjects demonstrated an increase in MGU by FDG-PET, the increase was much more dramatic with increasing levels of baseline IR (Fig. 1). There were no significant changes in 6-min walk distance or weight in any of the subjects.
Figure 1. Myocardial Glucose Uptake Before and After Treatment With Rosiglitazone in Individual Patients.
Note how the glucose uptake increases in all subjects, but much more dramatically with increasing baseline insulin resistance. In the most insulin-resistant subject, no appreciable myocardial glucose uptake was present at all in the baseline images.
FDG-PET studies in humans with DM have demonstrated impaired MGU in the setting of normal myocardial systolic function. Although this impairment was ameliorated by treatment with rosiglitazone, studies using insulin secretagogues and metformin to improve MGU have shown mixed results (4). Peroxisome proliferator-activated receptor gamma receptors are predominantly found in adipose tissue and are most commonly associated with modulation of whole-body insulin sensitivity and a decrease in circulating FFAs. Improvement in MGU in response to rosiglitazone may be related to suppression of FFA levels, given that there is little peroxisome proliferator-activated receptor gamma expression in the myocardium. Although this has been studied previously in patients with type 2 DM, to our knowledge, our study is the first demonstration of improvement in MGU with an insulin-sensitizing agent in patients with HF in the absence of DM.
Use of thiazolidinediones in HF has been limited by the side effect of fluid retention, and 2 of 7 of our subjects withdrew because of fluid retention. Our study size was also limited by our decision to end enrollment of new subjects in August 2007 when the U.S. Food and Drug Administration decided to add a black-box warning for the use of rosiglitazone in HF in light of concerns about the safety of thiazolidinediones in the presence of heart disease (5). Consequently, other drugs may be better suited for further study of metabolic modulation in HF. Trimetazidine is an antianginal agent that promotes glucose metabolism by inhibiting the final step of FFA β-oxidation, and it has been shown in a placebo-controlled, crossover study (6) to improve left ventricular function and high-energy phosphate levels in heart failure. Glucagon-like peptide 1, which stimulates insulin secretion and insulin sensitivity, has been shown to improve quality of life and measures of left ventricular function, suggesting that the glucagon-like peptide 1 receptor agonists (e.g., exenatide) and the dipeptidyl-peptidase IV inhibitors (e.g., sitagliptin) may also merit further study as adjunct therapies for HF in the setting of IR.
Our results suggest that MGU in patients with nonischemic dilated cardiomyopathy may be improved through modulation of whole-body insulin sensitivity, even in the absence of DM, and that patients with higher degrees of IR may derive more benefit from such therapy than insulin-sensitive patients.
Acknowledgments
Please note: The authors thank Dr. David Dick in the Stanford Cyclotron/Radiochemistry Facility and Dr. Andrei Iagaru in the Department of Nuclear Medicine for their invaluable assistance in execution of this study. Dr. Kao received funding from the National Institutes of Health Training grant 2 T15 LM007033-24. Dr. Witteles was supported for this study by a research grant from the Heart Failure Society of America. Dr. Wu received funding from the National Institutes of Health L30 HL085899.
This is a commentary on article Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol. 2008;51(2):93-102.
References
- 1.Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy. J Am Coll Cardiol. 2008;51:93–102. doi: 10.1016/j.jacc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan J, Mazumder PK, Hu P, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–9. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 3.Ohtake T, Yokoyama I, Watanabe T, Momse T, Serezawa T. Myocardial glucose metabolism in noninsulin-dependent diabetes mellitus patients evaluated by FDG-PET. J Nucl Med. 1995;36:456–63. [PubMed] [Google Scholar]
- 4.Hallsten K, Virtanen KA, Lonnqvistt F, et al. Enhancement of insulin-simulated myocardial glucose uptake in patents with type 2 diabetes treated with rosiglitazone. Diabet Med. 2004;21:1280–7. doi: 10.1111/j.1464-5491.2004.01332.x. [DOI] [PubMed] [Google Scholar]
- 5.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 6.Fragasso G, Perseghin G, De Cobelli F, et al. Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J. 2006;27:942–8. doi: 10.1093/eurheartj/ehi816. [DOI] [PubMed] [Google Scholar]