Abstract
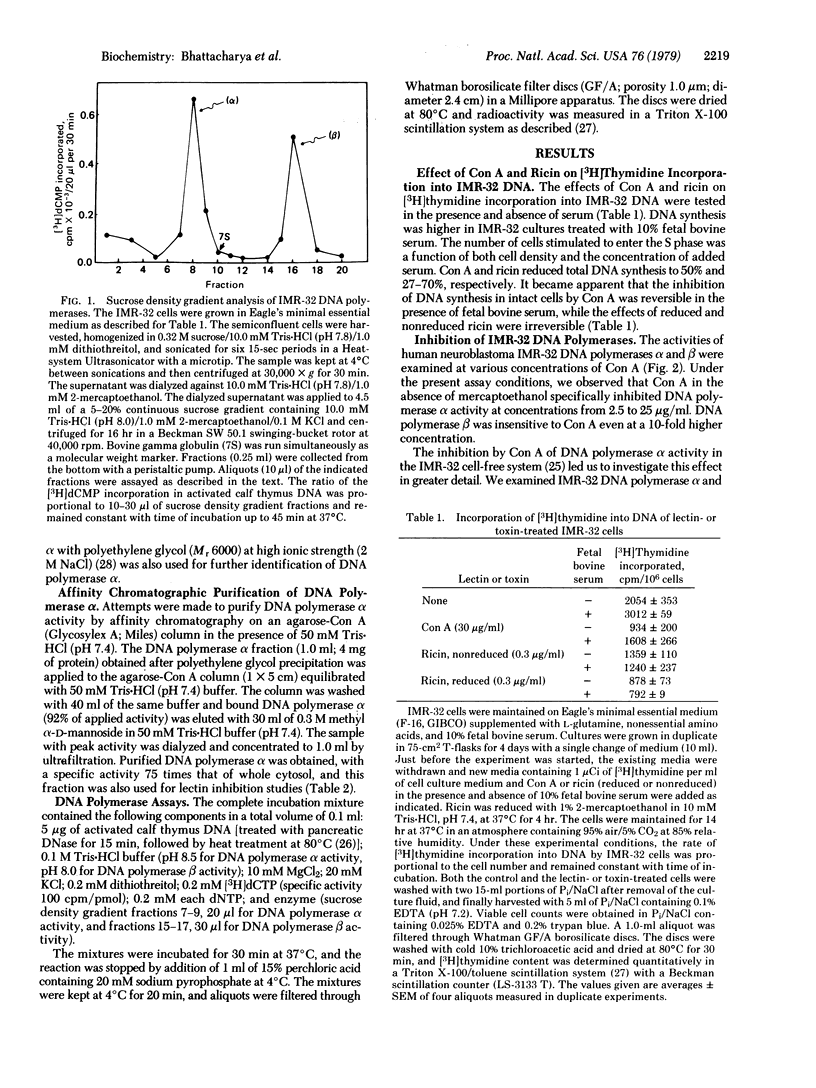
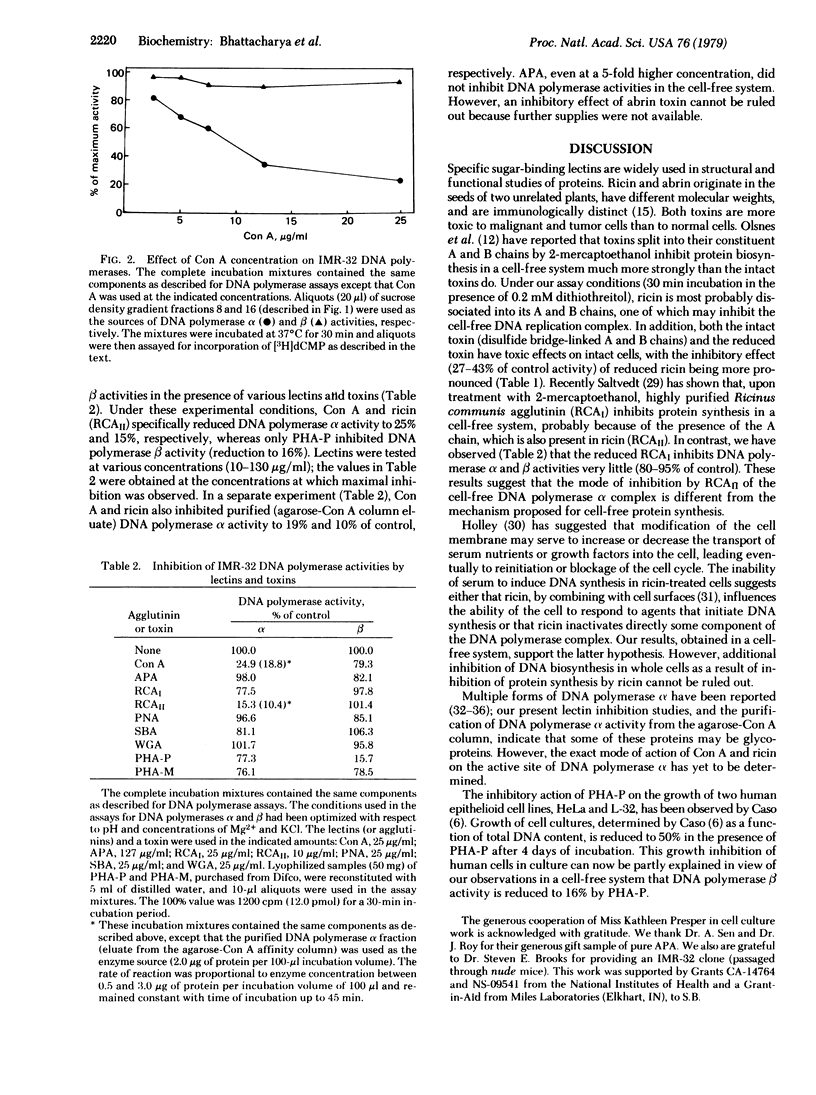
The effects of concanavalin A and ricin (RCAII, Mr 65,000) on [3H]thymidine incorporation into human neuroblastoma IMR-32 DNA showed reduction of total DNA synthesis to 50% and 70% of control, respectively. Two DNA polymerase (DNA nucleotidyltransferase, EC 2.7.7.7.) activities (alpha and beta) involved in the biosynthesis in vitro of DNA were separated by sucrose density gradient centrifugation from IMR-32 cell homogenate. The DNA polymerase alpha activity was also purified by selective precipitation with polyethylene glycol (Mr 6000) followed by agarose-concanavalin A column chromatography. The activities of both DNA polymerases were examined at various concentrations of mutagenic and nonmutagenic plant agglutinins and the toxin ricin. Concanavalin A and ricin specifically inhibited DNA polymerase alpha activity (activity reduced to 19% and 10%, respectively), whereas DNA polymerase beta activity was inhibited (reduced to 16%) by red kidney bean agglutinin (PHA-P).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abell C. W., Kamp C. W., Johnson L. D. Effects of phytohemagglutinin and isoproterenol on DNA synthesis in lymphocytes from normal donors and patients with chronic lymphocytic leukemia. Cancer Res. 1970 Mar;30(3):717–723. [PubMed] [Google Scholar]
- Agarwal S. S., Loeb L. A. Studies on the induction of DNA polymerase during transformation of human lymphocytes. Cancer Res. 1972 Jan;32(1):107–113. [PubMed] [Google Scholar]
- Bhattacharya P., Basu S. DNA polymerase activities in differentiating mouse neuroblastoma N-18 cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1289–1293. doi: 10.1073/pnas.75.3.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P., Moskal J. R., Basu S. Embryonic chicken brain and mouse neuroblastoma cells N1E-115 and N-18 contain an inhibitor of acid deoxyribonuclease. Proc Natl Acad Sci U S A. 1977 Mar;74(3):842–845. doi: 10.1073/pnas.74.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd W. C. Lectins. Ann N Y Acad Sci. 1970 Feb 13;169(1):168–190. doi: 10.1111/j.1749-6632.1970.tb55984.x. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Noonan K. D. Restoration of normal growth by covering of agglutinin sites on tumour cell surface. Nature. 1970 Nov 7;228(5271):512–515. doi: 10.1038/228512a0. [DOI] [PubMed] [Google Scholar]
- Burns M. V., Lozzio B. B. Inhibition of marrow granulocytic colony formation by phytohemagglutinin. J Cell Physiol. 1973 Oct;82(2):277–283. doi: 10.1002/jcp.1040820216. [DOI] [PubMed] [Google Scholar]
- Caso L. V. The effects of phytohemagglutinin on the growth and histochemical properties of mammalian cells in tissue culture. Anat Rec. 1968 Dec;162(4):459–466. doi: 10.1002/ar.1091620407. [DOI] [PubMed] [Google Scholar]
- Dent P. B. Inhibition by phytohemagglutinin of DNA synthesis in cultured mouse lymphomas. J Natl Cancer Inst. 1971 Apr;46(4):763–773. [PubMed] [Google Scholar]
- Duguet M., Méchali M., Rossignol J. M. A rapid technique to separate DNA polymerase-alpha and -beta activity from a cytosol extract. Anal Biochem. 1978 Aug 1;88(2):399–405. doi: 10.1016/0003-2697(78)90437-2. [DOI] [PubMed] [Google Scholar]
- Gail M. H., Boone C. W. Cell-substrate adhesivity. A determinant of cell motility. Exp Cell Res. 1972 Jan;70(1):33–40. doi: 10.1016/0014-4827(72)90178-4. [DOI] [PubMed] [Google Scholar]
- Holley R. W. A unifying hypothesis concerning the nature of malignant growth. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2840–2841. doi: 10.1073/pnas.69.10.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A. M., Johnston I. R. DNA polymerases of eukaryotes. FEBS Lett. 1975 Dec 15;60(2):233–243. doi: 10.1016/0014-5793(75)80721-6. [DOI] [PubMed] [Google Scholar]
- Inbar M., Ben-Bassat H., Sachs L. Inhibition of ascites tumor development by concanavalin A. Int J Cancer. 1972 Jan 15;9(1):143–149. doi: 10.1002/ijc.2910090117. [DOI] [PubMed] [Google Scholar]
- Lin J. Y., Tserng K. Y., Chen C. C., Lin L. T., Tung T. C. Abrin and ricin: new anti-tumour substances. Nature. 1970 Jul 18;227(5255):292–293. doi: 10.1038/227292a0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Coleman M. S. Induction of spleen cell growth and DNA polymerase activity by Corynebacterium parvum. Cancer Res. 1978 Jun;38(6):1617–1620. [PubMed] [Google Scholar]
- Matsukage A., Bohn E. W., Wilson S. H. Multiple forms of DNA polymerase in mouse myeloma. Proc Natl Acad Sci U S A. 1974 Feb;71(2):578–582. doi: 10.1073/pnas.71.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOWELL P. C. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960 May;20:462–466. [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J., Etzler M. E. Characterization of two plant lectins from Ricinus communis and their quantitative interaction with a murine lymphoma. Biochemistry. 1974 Jan 1;13(1):196–204. doi: 10.1021/bi00698a029. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Lacorbiere M., Hunter T. R. Mechanism of cell entry and toxicity of an affinity- purified lectin from Ricinus communis and its differential effects on normal and virus-transformed fibroblasts. Cancer Res. 1975 Jan;35(1):144–155. [PubMed] [Google Scholar]
- Nicolson G. L. The interactions of lectins with animal cell surfaces. Int Rev Cytol. 1974;39:89–190. doi: 10.1016/s0074-7696(08)60939-0. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Treatment of abrin and ricin with -mercaptoethanol opposite effects on their toxicity in mice and their ability to inhibit protein synthesis in a cell-free system. FEBS Lett. 1972 Nov 15;28(1):48–50. doi: 10.1016/0014-5793(72)80674-4. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Refsnes K., Pihl A. Mechanism of action of the toxic lectins abrin and ricin. Nature. 1974 Jun 14;249(458):627–631. doi: 10.1038/249627a0. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Saltvedt E., Pihl A. Isolation and comparison of galactose-binding lectins from Abrus precatorius and Ricinus communis. J Biol Chem. 1974 Feb 10;249(3):803–810. [PubMed] [Google Scholar]
- Ralph P., Nakoinz I. Inhibitory effects of lectins and lymphocyte mitogens on murine lymphomas and myelomas. J Natl Cancer Inst. 1973 Sep;51(3):883–890. doi: 10.1093/jnci/51.3.883. [DOI] [PubMed] [Google Scholar]
- Refsnes K., Olsnes S., Pihl A. On the toxic proteins abrin and ricin. Studies of their binding to and entry into Ehrlich ascites cells. J Biol Chem. 1974 Jun 10;249(11):3557–3562. [PubMed] [Google Scholar]
- Saltvedt E. Structure and toxicity of pure ricinus agglutinin. Biochim Biophys Acta. 1976 Dec 21;451(2):536–548. doi: 10.1016/0304-4165(76)90149-5. [DOI] [PubMed] [Google Scholar]
- Shoham J., Inbar M., Sachs L. Differential toxicity on normal and transformed cells in vitro and inhibition of tumour development in vivo by concanavalin A. Nature. 1970 Sep 19;227(5264):1244–1246. doi: 10.1038/2271244a0. [DOI] [PubMed] [Google Scholar]
- Spadari S., Villani G., Hardt N. DNA polymerase alpha, beta and gamma activities in rabbit spleen cell populations stimulated by various doses of concanavalin A. Exp Cell Res. 1978 Apr;113(1):57–62. doi: 10.1016/0014-4827(78)90087-3. [DOI] [PubMed] [Google Scholar]
- Toyoshima S., Fukuda M., Osawa T. Chemical nature of the receptor site for various phytomitogens. Biochemistry. 1972 Oct 10;11(21):4000–4005. doi: 10.1021/bi00771a025. [DOI] [PubMed] [Google Scholar]
- Weissbach A. Eukaryotic DNA polymerases. Annu Rev Biochem. 1977;46:25–47. doi: 10.1146/annurev.bi.46.070177.000325. [DOI] [PubMed] [Google Scholar]



