Abstract
Interleukin-1 (IL-1), a key molecule in systemic immune responses in health and disease, has analogous roles in the brain where it may contribute to neuronal degeneration. Numerous findings suggest that this is the case. For example, IL-1 overexpression in the brain of Alzheimer patients relates directly to the development and progression of the cardinal neuropathological changes of Alzheimer’s disease, i.e., the genesis and accumulation of β-amyloid (Aβ) plaques and the formation and accumulation of neurofibrillary tangles in neurons, both of which contribute to neuronal dysfunction and demise. Several genetic studies show that inheritance of a specific IL-1A gene polymorphism increases risk for development of Alzheimer’s disease by as much as sixfold. Moreover, this increased risk is associated with earlier age of onset of the disease. Homozygosity for this polymorphism in combination with another in the IL-1B gene further increases risk.
Keywords: brain, neurodegenerative disease, Down’s syndrome, head injury, aging, epilepsy, genetic risk, immunogenetics
INTRODUCTION
More than a decade ago, we proposed that immune response-generating cytokines are driving forces in pathogenic processes in the brain in Alzheimer’s disease. This idea was a departure from accepted ideas about Alzheimer pathogenesis, and our early demonstration of interleukin-1 (IL-1) overexpression in Alzheimer brain and very early on in the life of those with Down’s syndrome [1] was the first evidence supporting this controversial new idea. We also proposed that this idea is not confined to Alzheimer’s disease and Down’s syndrome, a recognized precursor for Alzheimer’s disease, but rather may be a generalization that applies to other conditions exhibiting IL-1 overexpression and neuronal injury and loss. In support of this, subsequent studies have shown IL-1 overexpression within hours after head injury [2], in chronic, intractable epilepsy [3], and in AIDS [4]; each of these conditions has been associated with Alzheimer’s disease itself or precocious development of Alzheimer pathology [2, 5, 6]. Recent microarray studies, assessing gene expression of many immune and Alzheimer-related, cytokines, show selective overexpression of IL-1 and S100B in Alzheimer’s disease [7]. These and other findings provided the rationale for recent genetic studies, which show, in the main, that specific polymorphisms in IL-1 genes increase risk for Alzheimer’s disease [8–13], lending importance to what we propose as a new area for exploration: the immunogenetics of Alzheimer’s disease.
This review focuses on three lines of investigation delineating the contribution of IL-1 to Alzheimer pathogenesis. The first is the identification and characterization of IL-1 temporal and spatial overexpression patterns and their relation to Alzheimer neuropathological changes. The second is the definition of the molecular events involved in IL-1-driven cascades and their potential to initiate and propagate the neuropathological changes of Alzheimer’s disease. The third is the identification of Alzheimer’s disease risk associated with polymorphisms within the two genes (IL-1A and IL-1B) that encode the two isoforms of IL-1, IL-1α and IL-1β, respectively.
IL-1 OVEREXPRESSION IN ALZHEIMER’S DISEASE
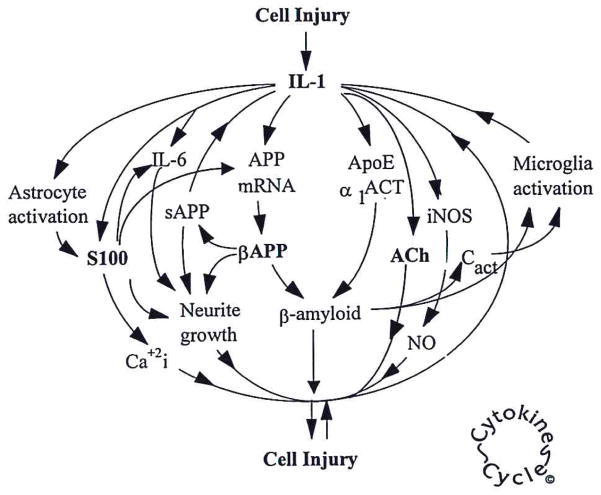
In Alzheimer’s disease, IL-1 overexpression is differentially distributed to the activated (enlarged) microglia found associated with β-amyloid (Aβ) plaques and immediately adjacent to neurons, in particular those bearing tangles [14, 15]. This and numerous subsequent findings have supported a central role for IL-1 in Alzheimer pathogenesis. These include observations that IL-1 is excessively expressed in Alzheimer brain tissue [1, 16], that excessive expression of IL-1 is related to the pathological involvement in a given brain region, and that IL-1 overexpression is correlated with the spread of Alzheimer pathology across cortical regions. Human postmortem studies, of course, cannot provide definitive proof that IL-1-mediated events, shown experimentally, occur in the development of Alzheimer’s disease, Down’s with Alzheimer’s disease, head injury, or in precocious development of Alzheimer changes in epilepsy or AIDS. However, such human studies actually demonstrate that experimental results are relevant to human disease. These human studies, together with the established functions of IL-1 (Fig. 1), strongly implicate IL-1 in neuropathogenesis.
Fig. 1.
Depiction of the ways in which IL-1 may be induced and amplified by and contribute to cellular injury. NO, nitric oxide; iNOS, inducible NO synthase. © University of Arkansas for Medical Sciences.
POTENTIAL IL-1-INDUCED CASCADES IN ALZHEIMER PATHOGENESIS
Down’s syndrome is a natural model of Alzheimer’s disease as virtually all Down’s patients develop marked Alzheimer-type neuropathological changes in middle age. IL-1 is overexpressed early in Down’s syndrome, decades before the appearance of the florid Alzheimer-type, neuropathological changes [1]—the Aβ plaques and the neurofibrillary tangles—that characterize Down’s syndrome at middle age [17]. As early as the second trimester, activated microglia of Down’s fetuses dramatically overexpress IL-1; this overexpression continues throughout life with the inevitable appearance decades later of Alzheimer-like changes that include activated microglia colocalizing with Aβ plaques and tangles [1]. These findings, together with IL-1 functions established in vitro and in animal experiments, suggest that early and persistent overexpression of IL-1 in Down’s syndrome may explain, in part, the early and excessive synthesis (i.e., greater than the 1.5× that is expected from gene loading), translation [18], and processing [19] of the precursor of the principle protein in plaques, Aβ precursor protein (βAPP) in neurons [20]. By analogy, we have proposed that in Alzheimer’s disease itself, the pathology is initiated and driven, in part, by early and sustained overexpression of IL-1 with consequent overexpression of products of IL-1-driven cascades. It may also explain the intense, early, and persistent activation of astrocytes [21] with accompanying overexpression of astrocytic S100B [22], a cytokine known to promote dystrophic growth of neuronal processes [23]. In Alzheimer’s disease, astrocyte activation with overexpression of S100B correlates with the number of neuritic plaques in a given region [24] as well as with the density of dystrophic, neuronal processes in individual neuritic plaques [25]. Taken together, such events are consistent with our idea that microglial activation with overexpression of IL-1 is an early, important event in the generation of the neuritic Aβ plaques, which themselves are associated with neuronal dysfunction and loss. The latter is based on findings showing that neuronal DNA damage worsens with plaque progression from amyloid deposits to the neuritic plaques diagnostic of Alzheimer’s disease [26]. In the dense core end-stage plaque, there are few associated microglia, astrocytes, or neurons. All of the remaining plaque-associated neurons show evidence of DNA damage. We have shown that neuronal damage is associated with microglial activation and overexpression of IL-1 [27, 28], providing feedback from damaged neurons that may act as a continuing driving force for amplification of IL-1-driven cascades. All such cascades shown in Figure 1 can result in neurodegeneration. The basis for the idea that these cascades may be important in the human brain and lead to neurodegenerative events lies in the existence of analogous IL-1-driven cascades in the periphery [29–31] and the production of IL-1 by glial cells of a type that respond to injury in the brain [32]. The finding that IL-1 activates astrocytes [21] and induces overexpression [22] of the neurite growth-promoting cytokine S100B [23] suggests a link whereby IL-1 could contribute to the proliferation of dystrophic neurites and thus to the conversion of Aβ deposits into the more complex neuritic Aβ plaques diagnostic of Alzheimer’s disease. In addition, S100B overexpression may contribute to the initial deposition of Aβ, as S100B induces the increased synthesis of its precursor, βAPP [33].
IL-1 induction of excessive expression of the βAPP [34] provides a further link between the overexpression of IL-1 in Alzheimer’s disease and its principal neuropathological feature, deposition of Aβ. The importance of IL-1 regulation of βAPP expression was subsequently expanded to include regulation of its translation [18] and processing into amyloidogenic secreted fragments (sAPP) [19]. These results suggest that IL-1 overexpression is an important factor in driving Aβ production, deposition, and thus plaque formation, as well as through astrocyte activation and overexpression of S100B to the growth of dystrophic neurites and formation of neurofibrillary tangles.
INTERLEUKIN-1 AND NEURONAL DYSFUNCTION
The overexpression of IL-1 observed in Alzheimer’s disease could potentially contribute directly to the neuronal dysfunction and loss seminal to the disease. With regard to direct toxicity, elevating concentrations of IL-1 in vitro are toxic to neuronal explant cultures [35]. As for dysfunction, experimental elevation of IL-1 in the brain in vivo [36] induces the overexpression and phosphorylation of neurofilament proteins and of the microtubule-associated protein tau and the paired helical filaments, which are present in the neurofibrillary tangles of Alzheimer’s disease. Moreover, elevation of IL-1 in vivo is associated with excessive growth of dystrophic neurites [36]. Activated microglia overexpressing IL-1 and activated astrocytes overexpressing S100B are intimately associated with neurons bearing neurofibrillary tangles in Alzheimer’s disease [15]. Such neurons in Alzheimer brains also overexpress mitogen-activated protein kinase-p38, which phosphorylates tau at the sites that are phosphorylated in neurofibrillary tangles [16].
A further role for IL-1 overexpression in neuronal dysfunction may arise from IL-1 effects on cholinergic systems, as the cholinergic decline characteristic of Alzheimer’s disease is thought to contribute to memory loss. IL-1 released from activated microglia directly induces increases in the acetylcholine-degrading enzyme, acetylcholinesterase, production, and activity in neurons [28]. In experiments showing this IL-1-induced increase, microglia were activated in response to neuronal release of sAPP. Both toxic and subtoxic stress to neurons results in synthesis and release of sAPP, which in turn activates microglia and induces excessive microglial expression of IL-1 [27]. These findings suggest that the increased concentration of IL-1 in Alzheimer brain may contribute to the observed decreases in tissue acetylcholine levels by increasing synthesis and activity of neuronal acetylcholinesterase.
The increased tissue levels of IL-1 and the neuronal dysfunction in Alzheimer’s disease may be attributable in part to increased production and activity of the IL-1β-converting enzyme (ICE), which converts pro-IL-1β to mature IL-1β. ICE activity and expression are increased in Alzheimer’s disease, and this increase is related to neuronal DNA damage as well as to compromised, neuronal function [37, 38]. Overexpression of ICE by plaque- and neuron-associated microglia may contribute to neuronal DNA damage associated with neuritic Aβ plaque progression [26] and with neurons bearing neurofibrillary tangles in Alzheimer’s disease [39].
IL-1 AND Aβ PLAQUE FORMATION AND PROGRESSION
The overexpression of IL-1 by plaque-associated, activated microglia dramatically increases with progression of plaques from non-neuritic, diffuse Aβ deposits to the neuritic Aβ plaques characteristic of Alzheimer’s disease [14]. As discussed above, this conversion from diffuse deposits to the more complex, neuritic plaque may be, in part, a result of IL-1 promotion of astrocytic expression of S100B [40, 41]. IL-1 also induces astrocytic expression of other plaque-associated proteins that may contribute to this progression. These include IL-6 [42], α1-antichymotrypsin, apolipoprotein E [43], and some complement proteins [44]. These findings suggest that IL-1 contributes to plaque progression through increased synthesis of astrocyte-derived proteins as well as through IL-1 effects on synthesis and processing of βAPP, which may directly contribute to the genesis and deposition of amyloidogenic fragments.
IL-1 GENETICS IN ALZHEIMER PATHOGENESIS
The potential role of IL-1 as a key orchestrating cytokine in Alzheimer pathogenesis focused interest on the possibility that polymorphisms in IL-1 might confer risk for development of Alzheimer’s disease. A polymorphism in the promoter region of the IL-1α gene as well as one in the coding region of the IL-1β gene have been shown to confer increased risk for peripheral immune diseases, and both polymorphisms are associated with overexpression of IL-1 [45, 46]. Five groups have now shown that the polymorphism in the IL-1α gene increases risk for Alzheimer’s disease [8–12]. Patients homozygous for this IL-1α polymorphism carry at least three times the risk of developing Alzheimer’s disease. In addition, homozygosity for the IL-1α gene polymorphism was associated with earlier onset of disease, lowering the average age of onset from 68–70 years to 61 years [9]. Furthermore, homozygosity for both of these polymorphisms was associated with a tenfold increased risk of developing Alzheimer’s disease [8]. This increased risk is independent of the ApoE genotype, another risk-conferring polymorphism for Alzheimer’s disease. That these polymorphisms increase expression of IL-1 suggests that they increase risk for Alzheimer’s disease by increasing the gain of an IL-1-driven cycle such as we propose in the cytokine cycle [47], acting through IL-1-mediated cascades that favor plaque formation and progression, dystrophic neurite proliferation, and neuronal dysfunction and loss. Neurodegenerative consequences of IL-1 overexpression, in turn, engender further activation of microglia through stress-induced overexpression of neuronal βAPP and Aβ deposition, thus inducing self-amplification of the cycle, a requisite for cycles that give rise to and maintain the progression of degenerative diseases.
A ROLE FOR IL-1 IN HEAD INJURY AND ITS RISK FOR LATER DEVELOPMENT OF ALZHEIMER’S DISEASE
Epidemiological observations implicate a previous head injury as a significant environmental risk factor for the subsequent development of Alzheimer’s disease [48, 49], and this is now supported by a variety of neuropathological findings. Pathological changes that are similar to those seen in Alzheimer’s disease, i.e., large numbers of diffuse Aβ plaques and numerous neurofibrillary tangles, are present in boxers who develop dementia pugilistica (“punch-drunk syndrome”) [50]. Diffuse Aβ plaques are even found in the brains of approximately 30% of individuals who die shortly after a single, severe head injury [51, 52]. Genetic analyses of the latter head injury patients with Aβ show an overrepresentation of the ApoE ε4 allele [53], suggesting a synergism between head injury and ApoE genotype in conferring increased risk for Alzheimer’s disease [54].
The mechanisms underlying the association between head injury and Alzheimer’s disease are not clear. However, in head-injured patients [55] and in experimental models of head injury [56, 57], there is increased, acute expression of βAPP, suggesting that head injury may initiate prolonged overexpression of βAPP. In addition, activated microglia overexpressing IL-1 are intimately associated with the neurons that are overexpressing βAPP in such head-injured patients [2]. This overexpression of IL-1 and βAPP may ultimately lead to the development of Alzheimer-type, neuropathological changes, as predicted by the increased risk for development of Alzheimer’s disease in such patients through actions illustrated in the cytokine cycle. These studies demonstrate fundamental molecular similarities between acute-phase responses to head injury and those responses characteristic of Alzheimer’s disease. Acute head injury may thus be viewed as a natural model [58] for the early (acute) stages of the later (chronic) inflammatory processes thought to underlie Alzheimer’s disease.
A ROLE FOR IL-1 IN PROGRESSIVE NEURODEGENERATION WITH AGING
Alzheimer’s disease is not an inevitable consequence of aging, but aging may, like head trauma, represent another priming process. The characteristic laminar distribution pattern of microglia expressing IL-1 observed in normal brain mirrors that of Aβ plaques in Alzheimer’s disease [59], suggesting that age-related activation of these microglia with overexpression of IL-1 could be such a priming process. Neurologically intact patients do show a progressive, age-associated increase in brain expression of IL-1 and IL-1 mRNA [60] as well as of S100B and S100B mRNA [61]. These increases in expression of IL-1 and S100B, both of which make multiple contributions to the neurodegenerative cascades we propose, may act synergistically with additional risk factors such as head injury and inheritance of genetic risk factors in order to precipitate the neurodegenerative changes characteristic of Alzheimer’s disease.
IL-1-MEDIATED CHANGES IN EPILEPSY AND PRECOCIOUS DEVELOPMENT OF SENILE CHANGES
Chronic, intractable epilepsy is not an established risk factor for the later development of Alzheimer’s disease, but epileptic patients do show accelerated appearance of Alzheimer-type “senile” changes [5]. These Alzheimer-type histological changes are accompanied by expression of Alzheimer-associated proteins in the brain of epileptic patients. We have shown overexpression of microglial IL-1, astrocytic S100B, and neuronal βAPP in resected temporal lobe tissue from patients with intractable, complex, partial seizures [3, 62]. The neuronal βAPP overexpression is particularly dramatic, appearing in fields of cortical neurons. Neuronal βAPP overexpression in epilepsy is correlated with increased tissue levels of βAPP, increased numbers of activated microglia overexpressing IL-1, and localization of these activated microglia adjacent to neurons overexpressing βAPP. As is the case with head injury [53], the accelerated Alzheimer-type changes seen in epilepsy are more common and more pronounced in patients carrying the Alzheimer-associated ApoE ε4 allele [63]. Chronic microglial activation and IL-1 overexpression may thus provide a pathogenic explanation for the increased incidence of “senile” changes observed in patients with chronic epilepsy.
SUMMARY
It is now an accepted clinical tenet that immune-system imbalances, perhaps as a result of interactions of multiple cytokines, cause or contribute to many diseases and age-related changes. IL-1 is a preeminent factor in immune function, as a regulator of intricately controlled, normal immune responses and as a participant in degenerative events arising from inappropriate activation of the immune system. The association of polymorphisms in IL-1 genes with risk for several diseases further underscores a role for IL-1 in pathology [64–71]. Similar to observations from systemic diseases, several brain degenerative conditions involve overexpression of IL-1. Alzheimer’s disease, in particular, shows overexpression of IL-1 that conelates with the neuronal dysfunction and loss associated with plaque and tangle formation. Again, similar to the case with systemic diseases, several studies have demonstrated a positive association between inheritance of specific polymorphisms in IL-1 genes and increased risk for Alzheimer’s disease [8–12]. The overexpression of IL-1 that occurs in systemic and neural diseases amplifies established IL-1-driven cascades, which, because of the excessive expression of IL-1, become degenerative and self-propagating. Such a process is illustrated in the cytokine cycle depicted in Figure 1.
Acknowledgments
This work was supported in part by National Institutes of Health grant AG12411. We thank the donors and our technical staff who made this work possible and Ms. Pam Free for secretarial support.
References
- 1.Griffin WST, Stanley LC, Ling C, White L, Macleod V, Perrot LJ, White CL, III, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin WST, Sheng JG, Gentleman SM, Graham DI, Mrak RE, Roberts GW. Microglial interleukin-1α expression in human head injury: correlations with neuronal and neuritic β-amyloid precursor protein expression. Neurosci Lett. 1994;176:133–136. doi: 10.1016/0304-3940(94)90066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheng JG, Boop FA, Mrak RE, Griffin WST. Increased neuronal β-amyloid precursor protein expression in human temporal lobe epilepsy: association with interleukin-1 alpha immunoreactivity. J Neurochem. 1994;63:1872–1879. doi: 10.1046/j.1471-4159.1994.63051872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanley LC, Mrak RE, Woody RC, Perrot LJ, Zhang SX, Marshak DR, Nelson SJ, Griffin WST. Glial cytokines as neuropathogenic factors in HIV infection: pathogenic similarities to Alzheimer’s disease. J Neuropathol Exp Neurol. 1994;53:231–238. doi: 10.1097/00005072-199405000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Mackenzie IRA, Miller LA. Senile plaques in temporal lobe epilepsy. Acta Neuropathol. 1994;87:504–510. doi: 10.1007/BF00294177. [DOI] [PubMed] [Google Scholar]
- 6.Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry. 1998;65:29–33. doi: 10.1136/jnnp.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loring JF, Wen X, Lee JM, Seilhamer J, Somogyi R. A gene expression profile of Alzheimer’s disease. DNA Cell Biol. 2001;20:683–695. doi: 10.1089/10445490152717541. [DOI] [PubMed] [Google Scholar]
- 8.Nicoll JAR, Mrak RE, Graham DI, Stewart J, Wilcock G, MacGowan S, Esiri MM, Murray LS, Dewar D, Love S, Moss T, Griffin WST. Association of inlerleukin-1 gene polymorphisms with Alzheimer’s disease. Ann Neurol. 2000;47:365–368. [PMC free article] [PubMed] [Google Scholar]
- 9.Grimaldi LME, Casadei VM, Ferri C, Veglia F, Licastro F, Annoni G, Biunno I, De Bellis G, Sorbi S, Mariani C, Griffin WST, Franceschi M. Association of early onset Alzheimer’s disease with an interleukin-1α gene polymorphism. Ann Neurol. 2000;47:361–365. [PubMed] [Google Scholar]
- 10.Du Y, Dodel RC, Eastwood BJ, Bales KR, Gao F, Lohmüller F, Müller U, Kurz A, Zimmer R, Evans RM, Hake A, Gasser T, Oertel WH, Griffin WST, Paul SM, Farlow MR. Association of an interleukin 1α polymorphism with Alzheimer’s disease. Neurology. 2000;55:480–483. doi: 10.1212/wnl.55.4.480. [DOI] [PubMed] [Google Scholar]
- 11.Rebeck GW. Confirmation of the genetic association of IL-1α with early onset sporadic Alzheimer’s disease. Neurosci Lett. 2000;293:75–77. doi: 10.1016/s0304-3940(00)01487-7. [DOI] [PubMed] [Google Scholar]
- 12.Hedley R, Hallmayer J, Groth DM, Brooks WS, Gandy SE, Martins RN. Association of interleukin-1 polymorphisms with Alzheimer’s disease in an Australian population. Ann Neurol. 2002;51:795–797. doi: 10.1002/ana.10196. [DOI] [PubMed] [Google Scholar]
- 13.Minster RL, DeKosky ST, Ganguli M, Belle S, Kamboh MI. Genetic association studies of interleukin-1 (IL-1α and IL-1β) and interleukin-1 receptor antagonist genes and the risk of Alzheimer’s disease. Ann Neurol. 2000;48:817–819. [PubMed] [Google Scholar]
- 14.Griffin WST, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer’s disease. J Neuropathol Exp Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Sheng JG, Mrak RE, Griffin WST. Glial-neuronal interactions in Alzheimer’s disease: progressive association of IL-1α+ microglia and S100β+ astrocytes with neurofibrillary tangle stages. J Neuropathol Exp Neurol. 1997;56:285–290. [PubMed] [Google Scholar]
- 16.Sheng JG, Jones RA, Zhou XQ, McGinness JM, Van Eldik LJ, Mrak RE, Griffin WST. Interleukin-1 promotion of MAPK-p38 overexpression in experimental animals and in Alzheimer’s disease: potential significance for tau protein phosphorylation. Neuroehem Int. 2001;39:341–348. doi: 10.1016/s0197-0186(01)00041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- 18.Rogers JT, Leiter LM, McPhee J, Cahill CM, Zhan SS, Potter H, Nilsson LN. Translation of the Alzheimer amyloid precursor protein mRNA is up-regulated by interleukin-1 through 5′-untranslated region sequences. J Biol Chem. 1999;274:6421–6431. doi: 10.1074/jbc.274.10.6421. [DOI] [PubMed] [Google Scholar]
- 19.Buxbaum JD, Oishi M, Chen HI, Pinkas-Kramarski R, Jaffe EA, Gandy SE, Greengard P. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer β/A4 amyloid protein precursor. Proc Natl Acad Sci USA. 1992;89:10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forloni G, Demicheli F, Giorgi S, Bendotti C, Angeretti N. Expression of amyloid precursor protein mRNAs in endothelial, neuronal and glial cells: modulation by interleukin-1. Brain Res Mol Brain Res. 1992;16:128–134. doi: 10.1016/0169-328x(92)90202-m. [DOI] [PubMed] [Google Scholar]
- 21.Giulian D, Woodward J, Young DG, Krebs JF, Lachman LB. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci. 1988;8:2485–2490. doi: 10.1523/JNEUROSCI.08-07-02485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng JG, Ito K, Skinner RD, Mrak RE, Rovnaghi CR, Van Eldik LJ, Griffin WST. In vivo and in vitro evidence supporting a role for the inflammatory cytokine interleukin-1 as a driving force in Alzheimer pathogenesis. Neurobiol Aging. 1996;17:761–766. doi: 10.1016/0197-4580(96)00104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kligman D, Marshak DR. Purification and characterization of a neurite extension factor from bovine brain. Proc Natl Acad Sci USA. 1985;82:7136–7139. doi: 10.1073/pnas.82.20.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheng JG, Mrak RE, Griffin WST. S100β protein expression in Alzheimer’s disease: potential role in the pathogenesis of neuritic plaques. J Neurosci Res. 1994;39:398–404. doi: 10.1002/jnr.490390406. [DOI] [PubMed] [Google Scholar]
- 25.Mrak RE, Sheng JG, Griffin WST. Correlation of astrocytic S100β expression with dystrophic neurites in amyloid plaques of Alzheimer’s disease. J Neuropathol Exp Neurol. 1996;55:273–279. doi: 10.1097/00005072-199603000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheng JG, Zhou XQ, Mrak RE, Griffin WST. Progressive neuronal injury associated with amyloid plaque formation in Alzheimer disease. J Neuropathol Exp Neurol. 1998;57:714–717. doi: 10.1097/00005072-199807000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barger SW, Harmon AD. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;28:878–881. doi: 10.1038/42257. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Liu L, Kang J, Sheng JG, Barger SW, Mrak RE, Griffin WST. Neuronal-glial interactions mediated by interleukin-1 enhance neuronal acetylcholinesterase activity and mRNA expression. J Neurosci. 2000;20:149–155. doi: 10.1523/JNEUROSCI.20-01-00149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lachman LB, Page SO, Metzgar RS. Purification of human interleukin 1. J Supramol Struct. 1980;13:457–466. doi: 10.1002/jss.400130405. [DOI] [PubMed] [Google Scholar]
- 30.Smith KA, Lachman LB, Oppenheim JJ, Favata MF. The functional relationship of the interleukins. J Exp Med. 1980;151:1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duff G. Immune diseases. Many roles for interleukin-1. Nature. 1985;313:352–353. doi: 10.1038/313352a0. [DOI] [PubMed] [Google Scholar]
- 32.Fontana A, Kristensen F, Dubs R, Gemsa D, Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982;129:2413–2419. [PubMed] [Google Scholar]
- 33.Li Y, Wang J, Sheng JG, Liu L, Barger SW, Jones RA, Van Eldik LJ, Mrak RE, Griffin WST. S100β increases levels of β-amyloid precursor protein and its encoding mRNA in rat neuronal cultures. J Neuroehem. 1998;71:1421–1428. doi: 10.1046/j.1471-4159.1998.71041421.x. [DOI] [PubMed] [Google Scholar]
- 34.Goldgaber D, Harris HW, Hla T, Maciag T, Donnelly RG, Jacobsen JS, Vitek MP, Gajdusek DC. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci USA. 1989;86:7606–7610. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brenneman DE, Page SW, Schultzberg M, Thomas FH, Zelazowski P, Burnet P, Avidor R, Sternberg EM. A decomposition product of a contaminant implicated in L-tryptophan eosinophilia myalgia syndrome affects spinal cord neuronal cell death and survival through stereospecific, maturation and partly interleukin-1-dependent mechanisms. J Pharmacol Exp Ther. 1992;266:1029–1035. [PubMed] [Google Scholar]
- 36.Sheng JG, Zhu SG, Griffin WST, Mrak RE. Interleukin-1 promotes expression and phosphorylation of neurofilament and tau protein in vivo. Exp Neurol. 2000;163:388–391. doi: 10.1006/exnr.2000.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu SG, Sheng JG, Jones RA, Brewer MM, Zhou XQ, Mrak RE, Griffin WST. Increased interleukin-1β converting enzyme expression and activity in Alzheimer disease. J Neuropathol Exp Neurobiol. 1999;58:582–587. doi: 10.1097/00005072-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Chan SL, Griffin WST, Mattson MP. Evidence for Caspase-mediated cleavage of AMPA receptor subunits in neuronal apoptosis and Alzheimer’s disease. J Neurosci Res. 1999;57:315–323. doi: 10.1002/(SICI)1097-4547(19990801)57:3<315::AID-JNR3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 39.Sheng JG, Zhou XQ, Mrak RE, Griffin WST. Progressive neuronal injury associated with neurofibrillary tangle formation in Alzheimer’s disease. J Neuropathol Exp Neurol. 1998;57:323–328. doi: 10.1097/00005072-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Marshak DR, Pesce SA, Stanley LC, Griffin WST. Increased S100β neurotrophic activity in Alzheimer disease temporal lobe. Neurobiol Aging. 1991;13:1–7. doi: 10.1016/0197-4580(92)90002-f. [DOI] [PubMed] [Google Scholar]
- 41.Sheng JG, Mrak RE, Griffin WST. Interleukin-1β expression in brain regions in Alzheimer’s disease: correlation with neuritic plaque distribution. Neuropathol Appl Neurobiol. 1995;21:290–301. doi: 10.1111/j.1365-2990.1995.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 42.Bauer J, Strauss S, Schreiter-Gasser U, Ganter U, Schlegel P, Witt I, Volk B, Berger M. Interleukin-6 and alpha2-macroglobulin indicate an acute-phase state in Alzheimer’s disease cortices. FEBS Lett. 1991;285:111–114. doi: 10.1016/0014-5793(91)80737-n. [DOI] [PubMed] [Google Scholar]
- 43.Das S, Potter H. Expression of the Alzheimer amyloid-promoting factors α1-antichymotrypsin and apolipoprotein E is induced in astrocytes by IL-1. Neuron. 1995;14:447–456. doi: 10.1016/0896-6273(95)90300-3. [DOI] [PubMed] [Google Scholar]
- 44.Barnum SR, Jones JL. Differential regulation of C3 gene expression in human astroglioma cells by interferon-gamma and interleukin-1 beta. Neurosci Lett. 1995;197:121–124. doi: 10.1016/0304-3940(95)11923-k. [DOI] [PubMed] [Google Scholar]
- 45.McDowell TL, Symons JA, Ploski R, Forre O, Duff GW. A genetic association between juvenile rheumatoid arthritis and a novel interleukin-1 alpha polymorphism. Arthritis Rheum. 1995;38:221–228. doi: 10.1002/art.1780380210. [DOI] [PubMed] [Google Scholar]
- 46.Pociot F, Molvig J, Wogensen L, Worsaae H, Nerup JA. TaqI polymorphism in the human interleukin-1β (IL-1β) gene correlates with IL-1 beta secretion in vitro. Eur J Clin Investig. 1992;22:396–402. doi: 10.1111/j.1365-2362.1992.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 47.Griffin WST, Sheng JG, Royston MC, Gentleman SM, McKenzie JE, Graham DI, Roberts GW, Mrak RE. Glial-neuronal interactions in Alzheimer’s disease: the potential role of a ‘cytokine cycle’ in disease progression. Brain Pathol. 1998;8:65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayeux R, Ottman R, Tang MX, Noboa-Bauza L, Marder K, Gurland B, Stern Y. Genetic susceptibility and head injury as risk factors for Alzheimer’s disease among community-dwelling elderly persons and their first-degree relatives. Ann Neurol. 1993;33:494–501. doi: 10.1002/ana.410330513. [DOI] [PubMed] [Google Scholar]
- 49.Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, Jorm AF, Kokmen E, Kondo K, Rocca WA. Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20:S28–S35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- 50.Roberts GW, Allsop D, Bruton CJ. The occult aftermath of boxing. J Neurol Neurosurg Psychiatry. 1990;53:373–378. doi: 10.1136/jnnp.53.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts GW, Gentleman SM, Lynch A, Graham DI. βA-4 Amyloid protein deposition in brain after head trauma. Lancet. 1991;338:1422–1423. doi: 10.1016/0140-6736(91)92724-g. [DOI] [PubMed] [Google Scholar]
- 52.Roberts GW, Gentleman SM, Lynch A, Murray L, Landon M, Graham DI. β-Amyloid protein deposition in the brain following severe head injury: implications for the pathogenesis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1994;7:419–425. doi: 10.1136/jnnp.57.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicoll JAR, Roberts GW, Graham DI. Apolipoprotein E ε4 allele is associated with deposition of amyloid β-protein following head injury. Nat Med. 1995;1:135–137. doi: 10.1038/nm0295-135. [DOI] [PubMed] [Google Scholar]
- 54.Mayeux R, Ottman R, Maestre G, Ngai C, Tang MX, Ginsberg H, Chun M, Tycko B, Shelanski M. Synergistic effects of traumatic head injury and apolipoprotein-E in patients with Alzheimer’s disease. Neurology. 1995;45:555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 55.McKenzie JE, Gentleman SM, Roberts GW, Graham DI, Royston MC. Increased numbers of βAPP-immunoreactive neurons in the entorhinal cortex after head injury. Neuroreport. 1994;6:161–164. doi: 10.1097/00001756-199412300-00041. [DOI] [PubMed] [Google Scholar]
- 56.Abe K, Tanzi RE, Kogure K. Selective induction of Kunitz-type protease inhibitor domain containing β-APP mRNA after persistent focal ischaemia in rat cerebral cortex. Neurosci Lett. 1991;125:172–174. doi: 10.1016/0304-3940(91)90020-t. [DOI] [PubMed] [Google Scholar]
- 57.Pluta R, Kida E, Lossinsky AS, Golabak AA, Mossakowski MJ, Wisniewski HM. Complete cerebral ischemia with short-term survival in rats induced by cardiac arrest. Extracellular accumulation of Alzheimer’s β-amyloid protein precursor in the brain. Brain Res. 1994;649:323–328. doi: 10.1016/0006-8993(94)91081-2. [DOI] [PubMed] [Google Scholar]
- 58.Gentleman SM, Graham DI, Roberts GW. Molecular pathology of head trauma: altered βAPP metabolism and the aetiology of Alzheimer’s disease. Prog Brain Res. 1993;96:237–246. doi: 10.1016/s0079-6123(08)63270-7. [DOI] [PubMed] [Google Scholar]
- 59.Sheng JG, Mrak RE, Griffin WST. Distribution of interleukin-1-immunoreactive microglia in cerebral cortical layers: implications for neuritic plaque formation in Alzheimer’s disease. Neuropathol Appl Neurobiol. 1998;24:278–283. doi: 10.1046/j.1365-2990.1998.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheng JG, Mrak RE, Griffin WST. Enlarged and phagocytic, but not primed, IL-1α+ microglia increase with age in normal human brain. Acta Neuropathol. 1998;94:1–5. doi: 10.1007/s004010050792. [DOI] [PubMed] [Google Scholar]
- 61.Sheng JG, Mrak RE, Rovnaghi CR, Kozlowska E, Van Eldik LJ, Griffin WST. Human brain S100β and S100β mRNA expression increases with age: pathogenic implications for Alzheimer’s disease. Neurobiol Aging. 1996;17:359–363. doi: 10.1016/0197-4580(96)00037-1. [DOI] [PubMed] [Google Scholar]
- 62.Griffin WST, Yeralan O, Boop F, Rovnaghi CR, Sheng JG, Mrak RE, Van Eldik LJ. Overexpression of the neurotrophic cytokine S100β in human temporal lobe epilepsy. J Neurochem. 1995;65:228–233. doi: 10.1046/j.1471-4159.1995.65010228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gouras GK, Relkin NR, Sweeney D, Munoz DG, Mackenzie IR, Gandy S. Increased apolipoprotein E epsilon 4 in epilepsy with senile plaques. Ann Neurol. 1997;41:402–404. doi: 10.1002/ana.410410317. [DOI] [PubMed] [Google Scholar]
- 64.McDowell TL, Symons JA, Ploski R, Forre O, Duff GW. A genetic association between juvenile rheumatoid arthritis and a novel interleukin-1α polymorphism. Arthritis Rheum. 1995;38:221–228. doi: 10.1002/art.1780380210. [DOI] [PubMed] [Google Scholar]
- 65.Momiyama Y, Hirano R, Taniguchi H, Nakamura H, Ohsuzu F. Effects of interleukin-1 gene polymorphisms on the development of coronary artery disease associated with Chlamydia pneumoniae infection. J Am Coll Cardiol. 2001;38:712–717. doi: 10.1016/s0735-1097(01)01438-3. [DOI] [PubMed] [Google Scholar]
- 66.Kornman KS, Crane A, Wang H-Y, di Giovine FS, Newman MG, Pirk FW, Wilson TG, Jr, Higginbottom FL, Duff GW. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol. 1997;24:72–77. doi: 10.1111/j.1600-051x.1997.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 67.Donaldson P, Agarwal K, Craggs A, Craig W, James O, Jones D. HLA and interleukin 1 gene polymorphisms in primary biliary cirrhosis: associations with disease progression and disease susceptibility. Gut. 2001;48:397–402. doi: 10.1136/gut.48.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kato S, Onda M, Yamada S, Matsuda N, Tokunaga A, Matsukura N. Association of the interleukin-1 beta genetic polymorphism and gastric cancer risk in Japanese. J Gastroenterol. 2001;36:696–699. doi: 10.1007/s005350170033. [DOI] [PubMed] [Google Scholar]
- 69.Nemetz A, Toth M, Garcia-Gonzalez MA, Zagoni T, Feher J, Pena AS, Tulassay Z. Allelic variation at the interleukin 1beta gene is associated with decreased bone mass in patients with inflammatory bowel diseases. Gut. 2001;49:644–649. doi: 10.1136/gut.49.5.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng C, Huang DR, Bergenbrant S, Sundblad A, Osterborg A, Bjorkholm M, Holm G, Yi Q. Interleukin 6, tumour necrosis factor alpha, interleukin 1beta and interleukin 1 receptor antagonist promoter or coding gene polymorphisms in multiple myeloma. Br J Haematol. 2000;109:39–45. doi: 10.1046/j.1365-2141.2000.01963.x. [DOI] [PubMed] [Google Scholar]
- 71.Wilkinson RJ, Patel P, Llewelyn M, Hirsch CS, Pasvol G, Snounou G, Davidson RN, Toossi Z. Influence of polymorphism in the genes for the interleukin (IL)-1 receptor antagonist and IL-1beta on tuberculosis. J Exp Med. 1999;189:1863–1874. doi: 10.1084/jem.189.12.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]