Abstract
Do cancer cells escape their confinement of their original habitat in the primary tumor or are they forced out by ecological changes in their home niche? Describing metastasis in terms of a simple one-way migration of cells from the primary to target organs is an insufficient concept to cover the nuances of cancer spread. A diaspora is the scattering of people away from an established homeland. To date, “diaspora” has been a uniquely human term utilized by social scientists, however, the application of the diaspora concept to metastasis may yield new biological insights as well as therapeutic paradigms. The diaspora paradigm takes into account and models several variables: the quality of the primary tumor microenvironment, the fitness of individual cancer cell migrants as well as migrant populations, the rate of bidirectional migration of cancer and host cells between cancer sites, and the quality of the target microenvironments to establish metastatic sites. Ecological scientific principles can be applied to the cancer diaspora to develop new therapeutic strategies. For example, ecological traps, habitats that lead to the extinction of a species, can be developed to attract cancer cells to a place where they can be better exposed to treatments or to cells of the immune system for improved antigen presentation. Merging the social science concept of diaspora with ecological and population sciences concepts can inform the cancer field to understand the biology of tumorigenesis and metastasis and inspire new ideas for therapy.
Metastasis viewed in terms of migration
Do cancer cells escape their original habitat in the primary tumor or are they forced out by ecological changes in their home niche? The latter process can be considered a diaspora and has many analogies in population and social sciences that could yield new insights into metastasis. In 1829, Récamier recognized that cancer can spread from a primary tumor and coined the term “metastasis” from the Greek “methistemi”, meaning to change or displace (1). Paget's “seed and soil” theory explained the non-random pattern of cancer metastasis in 1889 when he postulated that factors within the metastatic site promoted growth in the same way that fertile soil allows the successful growth of seeds (2). In a complementary hypothesis, James Ewing proposed in 1928 that cancer cells were directed to that site by the direction of lymphatic and circulatory systems (3).
From the perspective of species migration, both Paget's and Ewing's theories are correct. Migration is subdivided into emigration (the act of leaving), migration (the act of travelling), and immigration (the process of arriving) (4). In the paradigm of migration, Ewing focused on the migration step and Paget focused on immigration (2-4). Ewing's theory accounts for the migration of prostate cancer cells to the lumbar vertebrae via Batson's plexus of draining lymph nodes and Paget's theory helps explain the organ specificity of prostate cancer metastases to bone. Fidler further refined the seed/soil hypothesis in 2003 to take into account the emigration step (5). First, primary tumors contain heterogeneous subpopulations of cells with different angiogenic, invasive and metastatic properties (properties that promote emigration). The metastatic process is then selective for cells that can successfully survive migration to the distal target organ. The successful proliferation of metastatic cells depends on the ability of these cells to interact and utilize the soil of the new microenvironment (5).
Diasporas
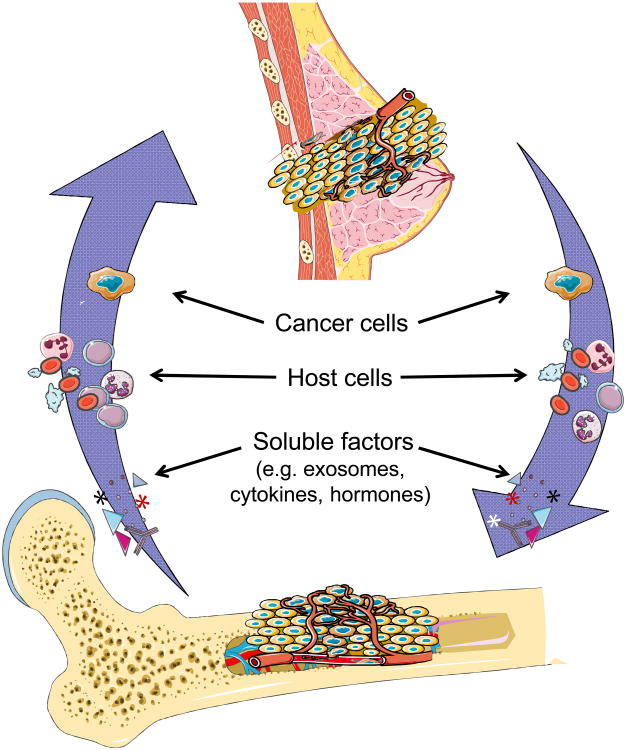
Describing metastasis in terms of migration alone is insufficient to cover the nuances of cancer spread (Figure 1). A diaspora, from the Greek diasporá, meaning “scattering” or “dispersion”, is the movement, migration, or scattering of people away from an established homeland. To date, “diaspora” has been applied exclusively to describe human behavior. In ancient Greece, a diaspora referred to citizens of a conquering city-state who colonized a conquered land to assimilate the territory into the empire (6). Subsequent to the Bibles translation into Greek, “Diaspora” referre d to Jewish peoples exiled from Israel by the Babylonians in 587 BCE and by the Romans from Judea in 70 CE (7). Since that time, diaspora has come to refer to mass-dispersions, usually involuntary, of people with common roots.
Figure 1. The Cancer Diaspora.
Describing metastasis in terms of migration alone is insufficient to cover the nuances of cancer spread. The diaspora paradigm takes into account and models several variables: the quality of the primary tumor microenvironment, the fitness of individual cancer cell migrants as well as migrant populations, the bidirectional movement of cancer and host cells between cancer sites (including between primary and metastases as well as between metastases), and the quality of the target microenvironments to establish metastatic sites.
Diasporas share several common features that differentiate them from migrations. First, dispersal occurs to more than one destination. By definition, a diaspora implies a scattering rather than a simple displacement from one place to another (8-11). This dispersal to multiple destinations is a necessary step that eventually leads to the formation of links between the various populations of the diaspora. In population studies, a group is only considered a diaspora if it first exits from a homeland such that sub-groups eventually exist across multiple national boundaries. Second, the scattered populations must continue to retain a relationship with the homeland. A diaspora exhibits ancestral memory – the dispersed population does not assimilate completely into the new environment, keeping a unique identity that has roots in their original home. This results in a continued unique identity for the dispersed population and allows the diaspora to exist over multiple generations and exist over time. (9-12).
Metastasis viewed as a diaspora
Metastasis is not a simple one-way migration, but rather a dynamic dispersal of cells that are inherently linked to the primary tumor (Table 1). While migrants are usually attracted to a new country and can come from single or multiple origins, a diaspora is dispersed, usually expelled, from a single origin. At least early in tumorigenesis, before the establishment of multiple distant deposits of cancer cells, metastases arise from a single site of origin, the primary tumor (13). Several types of diaspora have been described based on why the population scattered (14,15). Which peoples moved and what segments of society left? Were they expelled, taken captive, or did they leave voluntarily? Did they leave as a group all at once or as individuals over a protracted period of time (15)? These emigrations have usually, but not always, been the result of harsh economic conditions or other similar hardships (4,9). Migratory diasporas may arise when individuals transit in and out of a host country but in doing so, institutions and networks become established in the hostlands, e.g. trading posts (15). It is becoming clearer that cancers utilize the “trading post” diaspora analogy by forming pre-metastatic niches (16-18). Primary tumors, driven perhaps by hypoxic conditions, orchestrate the formation of pre-metastatic niches in part by the secretion of a variety of cytokines, growth factors, and exosomes to promote mobilization and recruitment of bone marrow derived cells to future metastatic sites (19-23).
Table 1.
A comparison of migrants, diaspora, and cancer metastases.
| Social Demography | Cancer Demography | |
|---|---|---|
| Migrant Communities | Diaspora Communities | Cancer Metastasis |
| Moved from a single or multiple primary homelands | Dispersed from a single homeland | Dispersed from a primary cancer |
| Attracted to new country | Pushed from homeland | Hypoxia and lack of nutrients causes pressure to leave primary |
| Host country may or may not be receptive | Host country may or may not be receptive | Target organ receptive |
| Group maintains collective memory of their homeland and culture | Group maintains collective memory of their homeland and culture | Pathologists can identify where a cancer cell originated |
| Often assimilate into the new homeland | They wish to survive as a distinct community | Metastases as distinct masses |
| Relationship with host country is complicated and uneasy but improves over time | Relationship with host country is complicated and uneasy | Immune system tries to destroy the cancer cells |
| May or may not be tied to the homeland by exchange of resources | They are tied to their homeland at many levels – exchange of resources (economic, sociopolitical) | Multiple cell type trafficking, trafficking of resources/info |
The paradigm of the imperial diaspora also can be applied to metastasis. As in the ancient Greece city-state example, diasporas originated as conquests, often resulting in subjugation of an indigenous population. Similarly, metastasis is generally considered to be an active process of cells leaving the primary tumor site (as opposed to passive sloughing of cells into the circulation), to colonize a target organ (19-23). In an imperial diaspora, the homeland is responsible for helping to maintain an interdependent network of the diaspora. In turn, established colonies not only send resources back to the home country, but often send future generations back to the homeland for education, training, and cultural indoctrination. The exchange of resources between dispersed communities and the home country is a key aspect defining a diaspora (24-26). This exchange of resources is often defined by the diaspora communities sending economic support to the home country as well as supporting the interests of the home country with the host countries on sociopolitical levels.
It appears that multiple host cells, including hematopoietic stem cells, mesenchymal stem cells, endothelial progenitors, cancer- associated fibroblasts, and inflammatory mononuclear cells (T-, B-, and monocytes) traffic freely between tumor sites (27-32). Furthermore, it has been demonstrated in experimental systems that cancer cells traffic between tumor sites within a host. Massagué et al demonstrated that metastasis is a multidirectional process whereby cancer cells seed distant sites and return to the primary tumor itself (33-35). This self-seeding, at least in preclinical models, functions to facilitate and accelerate tumor growth, angiogenesis, and recruitment of stromal cells to the tumor microenvironments. Whether bidirectional tumor movement does occur remains hotly debated. Indirect evidence for the concept occurring in patients can be inferred from work on disseminated tumor cells (DTCs) in breast cancer patients. Pantel and colleagues have demonstrated that the presence of DTCs in bone marrow at initial diagnosis predicts local and metastatic relapse (36-39). These investigators have detected circulating tumor cells (CTCs) in early stage breast cancer patients months after primary tumor resection, suggesting that CTCs are derived from occult micrometastases (39).
Another critical aspect to understand the dynamics of diaspora development is the area that becomes the new home for the displaced population. For a displaced people, the ability and willingness for the host country to allow immigration is a key step in the formation of the diaspora. This is dependent on physical characteristics (i.e., is there room, ability for the population to thrive economically) as well as socio-political characteristics (i.e., willingness of the host community to accept the displaced peoples)(24-26). This is analogous to the favorable soil described by Paget (2, 5).
Boundary maintenance is another important criterion of a diaspora (24). Both migrant and diaspora communities maintain a collective memory of their homelands as reflected by continuing to celebrate their ethnic roots. The diaspora community, however, must maintain an identity within the host country over time. In contrast to many migrant populations, a diaspora group does not assimilate into the host population but maintains a distinct identity as so much as is possible. Cancer metastases are easily recognized in the target organ, most often observed as masses with distinct boundaries (40). This boundary maintenance blurs in migrant populations. The relationship of migrant populations with the host country tends to improve over time as the migrants assimilate into the host communities. The diaspora relationship with the host community tends to remain complicated and often uneasy and better reflects the relationships of a metastatic deposit with the target organ. The metastatic deposits maintains its identity and is in tension with the host immune and other defense mechanisms which strive to limit growth (41,42).
Diasporas, metastases and ecosystems
Broadening the concept of diaspora and simultaneously applying ecological scientific principles facilitates a broader understanding of the dispersal of cancer cells (43-45). For example, a metapopulation consists of a group of separated populations of the same species that interact at some level. Metapopulation dynamics can be applied to any species in fragmented habitats; much like a diaspora from a single point of origin to multiple host countries. While cancer metastases may be thought of as metapopulations, they are actually metacommunities. Metastases are made up of multiple species of tumor and host cells interacting dynamically. Metacommunities are a network of local communities that are linked through multiple potentially interacting species or populations. Metapopulation and metacommunity dynamics allow us to study not only the growth and interactions of individual populations within different sites, but also the interactions and connectivity between sites (46).
Understanding the dispersion of metastatic cancer cells to sites throughout the body from a site of origin may use the theoretical ecology model of source sink dynamics to describe how variation in habitats (both homeland [the “source” of species] and hostland [the “sink” or habitat to which species migrates]) affect population growth or decline in metapopulations (47). Source-sink dynamics can help model how individual populations flourish or decline among different patches of habitat, e.g., a high quality habitat that on average allows the population to increase or a low quality habitat that, on its own, would not be able to support a population (referred to as a sink). Habitat quality is quite analogous to the concept of fertile or poor soil as delineated by Paget (2). Source-sink models also take into account how population numbers vary– that an excess of individuals often move from a source (primary tumor) and can continue to supply other sites (new seeds) (33-35).
The rate of by which a cancer establishes its diaspora is therefore dependent on several variables (Table 2): 1) the quality of the primary (homeland or origin) microenvironment – the more likely the microenvironment is oxygenated and with a sufficient source of nutrients, the less likely cancer cells will be driven to engage cellular programs that promote extravasation/emigration; 2) the rate of passive and active emigration. Emigrant cancer cell populations can be divided into cells that passively slough into the circulation and cells that actively extravasate into nerves, lymphatics, or blood. It is likely that passive emigrants may not have all of the machinery necessary to survive to successfully migrate. Cells that have been forced to undergo an epithelial to mesenchymal transition (EMT) in response to a hypoxic environment are much more likely to survive a migration; 3) the quality and number of the target organ (hostland) microenvironments. The quality of the soil is well documented as a critical component of successful metastasis.
Table 2.
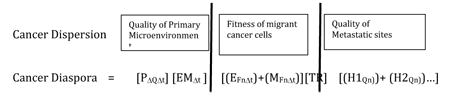
Development of equations may help to define the rate and success or failure of a cancer diaspora.
|
|---|
| Primary Microenvironment (Homeland) factors: |
| OΔQΔt = the quality (Q) of the primary cancer site (P). Cancer cells in a highly vascularized environment with rich nutrients are presumed to be less likely to undergo an epithelial to mesenchymal transition (EMT) and leave the primary. |
| EMΔt= generation of cancer cells (tumor cell heterogeneity) over time (Δt). Epithelioid cancer cells (E) are less likely to be able to metastasize as compared to mesenchymal cancer cells (M). |
| Migration factors: |
| EFnΔt = the number (n) and fitness (F) of passively shed cancer cells (E) over time (Δt). This represents the likelihood that a cancer cell passively shed into the circulation will survive transport to a target organ. It is likely that the fitness of a passively shed cell is less than a cell that actively exits the primary tumor through the lymphatics, nerves, or circulation. |
| MFnΔt = the number (n) and fitness (F) of actively emigrant cancer cells (M) over time (Δt). This represents the likelihood that a cancer cell that actively extravasates into the circulation will survive transport to a target organ. Fitness depends on many variables, including EMT state, ability to secrete MMPs, ability to avoid anoikis, etc. |
| TR = transit resistance factors. This represents factors in the circulation that inhibit migration, including shear forces and the host immune response that destroy cancer cells. |
| Target organ (hostland) factors: |
| HQn = the quality (Q) of the target organ or hostland sites (H1, H2…). Migrating cancer cells will land in multiple sites (n) within different target organs in order to immigrate. Success depends on the quality of the soil of each of these microenvironments. Prostate cancer cells, for example, are more likely to flourish in a high quality bone microenvironment then a low quality habitat such as the lung microenvironment. |
Previously, it was thought that cancer cells could not differentiate between different target organs and the process of seeding was thought to be agnostic i.e., just as many cells would land in the lung as the bone depending on blood flow, etc (48). New data suggests that this is most likely untrue on both the seed and the soil sides of the metastasis argument. First, cancer cells can carry receptors for antigens on particular cell types, i.e, prostate cancer cells have a high level of annexin II which allows binding to osteoblasts in the endosteal niche of the bone marrow (19, 49). Second, the elegant work of Wang and colleagues have suggested that bone marrow derived mesenchymal cells organize pre-metastatic niches (trading posts in the diaspora analogy) that provide a better soil than may actually attract cancer cells, allowing them to distinguish between high and low quality habitats (18). The source-sink model implies and models that some habitat patches may be more important to the long-term survival of the population and can be modeled by their demographic parameters or BIDE rates (birth, immigration, death, emigration) (47).
Utilizing cancer diaspora - ecosystem concepts to target metastasis
Viewing cancer within the context of ecology can lead to therapeutic paradigms based on network disruption across the scale of ecosystems from the cell through to the host patient (46). The diaspora concept adds to this analogy by specifically expanding this therapeutic paradigm as cancer cells metastasize and establish metacommunities. One potential therapeutic strategy would be to create ecological traps (50-52). Ecological traps are poor-quality habitats that are highly attractive to wildlife species based on environmental cues that typically signify a high quality habitat (50). This type of cue is analogous to signals which cancer cells receives to settle in a specific target organ. Van der Sanden and colleagues have suggested that neurotransmitters could be used to attract glioma cells to settle in a location where treatments (e.g. drugs, radiation, surgery) can be effectively or safely concentrated (near the surface, away from sensitive tissues, within surgically implanted tissue or material). Instead of preventing metastasis, such ‘attracticides’ serve as “guides” to faciliate cancer migration toward poor quality habitats where cancer cells can be eliminated (50).
Using traps to treat cancer is analogous to altering the ecological landscape of interactions in the body in ways in which the cells are unable to anticipate or react to and that are ultimately invisible to them. Corollaries of this strategy include placing familiar ‘cues’ in places that lead to the extinction of cancer cells, or masking attractive cues in favorable microenvironments. For glioma cells a reservoir of neurotropic chemokines could be used to attract cancer cells to an area where they could be radiated (51). For prostate cancer cells, a reservoir of SDF-1 could be temporarily inserted intravenously that attracts the cells to a one-way trap (53). An ecological trap could also be constructed to expose cancer cells to cells of the immune system, leading to increased antigen presentations (52), or disrupt the ability of metastasizing cells to recruit appropriate host cells.
Our ability to manipulate the primary tumor or disseminated tumor cells in a diaspora remains limited. It would be ideal to develop strategies to halt the emigration of tumor cells from the primary tumor (homeland) or to mask the attractiveness of the target organs (hostlands). Perhaps we could even use this strategy to ‘recall’ disseminated tumor cells to the primary ‘homeland” to facilitate a more focused and strategic therapy. The technology to create artificial tissue traps or synthetic microenvironments already does exist (52). Another approach to killing cancer cells may be to force a diaspora from their metastatic sites where they may be protected from therapeutic intervention. For example, Shiozawa and colleagues have demonstrated that prostate cancer cells can be mobilized out of the bone marrow microenvironment and into the unprotected circulation where they may be easier to target (49). Recognizing that genetic diversity among tumor cells to respond to different attractants is most likely, the creation of multiple evolutionary traps within a device providing multiple chemokines and/or metastatic niches should be considered (20-23, 50). The recent discovery that cancer cells continue to circulate after they metastasize suggests that this approach may offer therapeutic benefit even in patients with advanced disease (33-39). Together, these concepts demonstrate that merging the social science concept of diaspora with ecological and population sciences concepts can inform the cancer field to understand the biology of tumorigenesis and metastasis and inspire new ideas for therapy.
Statement of Translational Relevance.
Metastases remain the major reason for the morbidity and mortality associated with cancer death. Describing metastasis as a simple migration of cells from the primary to target organs is insufficient to understand the nuances of cancer spread. To date, “diaspora”, the scattering of people away from an established homeland, has been applied exclusively to describe human behavior. The application of the diaspora concept can also be applied to metastasis, which together with ecological principles may help to develop new treatment strategies. For example, ecological traps, habitats that lead to species extinction, can be developed to attract cancer cells to a place where they can be better exposed to treatments or to cells of the immune system for improved antigen presentation. Merging the social science concept of diaspora with ecological and population sciences concepts can inform the cancer field to understand the biology of tumorigenesis and metastasis and inspire new ideas for therapy.
Acknowledgments
This work supported by NCI U54CA143803 (KJP,DSC), CA163124, CA093900 (KJP, RST) and CA143055 (KJP).
Contributor Information
Bruce A. Robertson, Email: broberts@bard.edu.
Donald S. Coffey, Email: dcoffey@jhmi.edu.
Russell S. Taichman, Email: rtaich@umich.edu.
References
- 1.Androutsos G, Karamanou M, Stamboulis E, Tsoucalas G, Kousoulis AA, Mandelenaki D. Joseph-Claude-Anthelme Récamier (1774-1852): forerunner in surgical oncology. J BUON. 2011;16(3):572–6. [PubMed] [Google Scholar]
- 2.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. [PubMed] [Google Scholar]
- 3.Ewing J. Neoplastics. 3rd. Philadelphia: Saunders; 1928. Metastasis; pp. 77–89. [Google Scholar]
- 4.Pienta KJ, Loberg R. The “emigration, migration, and immigration” of prostate cancer. Clin Prostate Cancer. 2005;4:24–30. doi: 10.3816/cgc.2005.n.008. [DOI] [PubMed] [Google Scholar]
- 5.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 6.Tetlow EM. Women, Crime, and Punishment in Ancient Law and Society. Continuum International Publishing Group; 2005. pp. 1–2. [Google Scholar]
- 7.Kantor M. The Jewish time line encyclopedia: a year-by-year history from Creation to the Present. Jason Aronson, Inc; Northvale NJ: 1992. pp. 105–106. [Google Scholar]
- 8.Cohen R. The Diaspora of a Diaspora: The Case of the Caribbean. Social Science Information. 1992;31:159–69. [Google Scholar]
- 9.Butler KD. Defining Diaspora, Refining a Discourse. Diaspora: A Journal of Transnational Studies. 2001;10:2189–219. [Google Scholar]
- 10.Brubaker R. The ‘diaspora’ diaspora. Ethnic and Racial Studies. 2005;28:1–19. [Google Scholar]
- 11.Anderson BR. Imagined communities: reflections on the origin and spread of nationalism. Vol. 224 London: Verso; 1991. [Google Scholar]
- 12.Safran W. Diasporas in Modern Societies: Myths of Homeland and Return Diaspora: A Journal of Transnational Studies. 1991;1:83–99. [Google Scholar]
- 13.Wu C, Wyatt AW, Lapuk AV, McPherson A, McConeghy BJ, Bell RH, Volik SV, et al. Integrated genome and transcriptome sequencing identifies a novel form of hybrid and aggressive prostate cancer. J Pathol. 2012;227(1):53–61. doi: 10.1002/path.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinkerhoff JM. Creating an enabling environment for diasporas' participation in homeland development. Int Migr. 2012;50(1):75–95. doi: 10.1111/j.1468-2435.2009.00542.x. [DOI] [PubMed] [Google Scholar]
- 15.Curtin PD. Cross-Cultural Trade in World History. Cambridge: Cambridge UP; 1984. [Google Scholar]
- 16.Sceneay J, Smyth MJ, Möller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013 May 1; doi: 10.1007/s10555-013-9420-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Irmisch A, Huelsken J. Metastasis: New insights into organ-specific extravasation and metastatic niches. Exp Cell Res. 2013 Feb 21; doi: 10.1016/j.yexcr.2013.02.012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Barcellos-Hoff MH, Lyden D, Wang TC. The evolution of the cancer niche during multistage carcinogenesis. Nat Rev Cancer. 2013 Jun 13; doi: 10.1038/nrc3536. [DOI] [PubMed] [Google Scholar]
- 19.Patel LR, Camacho DF, Shiozawa Y, Pienta KJ, Taichman RS. Mechanisms of cancer cell metastasis to the bone: a multistep process. Future Oncol. 2011;7(11):1285–97. doi: 10.2217/fon.11.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sleeman J, Steeg PS. Cancer metastasis as a therapeutic target. Eur J Cancer. 2010;46(7):1177–80. doi: 10.1016/j.ejca.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taddei ML, Giannoni E, Comito G, Chiarugi P. Microenvironment and tumor cell plasticity: An easy way out. Cancer Lett. 2013 Jan 31; doi: 10.1016/j.canlet.2013.01.042. EPub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi P, Gilkes DM, Wong CC, Kshitiz, Luo W, Zhang H, Wei H, et al. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Invest. 2013 Feb;123(1):189–205. doi: 10.1172/JCI64993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semenza GL. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol Med. 2012;18(9):534–43. doi: 10.1016/j.molmed.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong JA. Mobilized and proletarian diasporas. American Political Science Review. 1976;70(2):393–408. [Google Scholar]
- 25.Alba RD, Nee V. Rethinking assimilation theory for a new era of immigration. International Migration Review. 1997;31(4):826–74. [PubMed] [Google Scholar]
- 26.Brubaker R. The return of assimilation? Ethnic and Racial Studies. 2001;24(4):531–48. [Google Scholar]
- 27.Langley RR, Fidler IJ. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011 Jan;128(11):2527–35. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2011 Nov 19; doi: 10.1007/s10555-011-9340-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Mareel M, Oliveira MJ, Madani I. Cancer invasion and metastasis: interacting ecosystems. Virchows Arch. 2009;454:599–622. doi: 10.1007/s00428-009-0784-0. [DOI] [PubMed] [Google Scholar]
- 30.Grisendi G, Bussolari R, Veronesi E, Piccinno S, Burns JS, De Santis G, et al. Understanding tumor-stroma interplays for targeted therapies by armed mesenchymal stromal progenitors: the Mesenkillers. Am J Cancer Res. 2011;1:787–805. [PMC free article] [PubMed] [Google Scholar]
- 31.Allen M, Louise Jones J. Jekyll and Hyde: the role of the microenvironment on the progression of cancer. J Pathol. 2011;223:162–76. doi: 10.1002/path.2803. [DOI] [PubMed] [Google Scholar]
- 32.Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, Berry JE, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Comen E, Norton L, Massagué J. Clinical implications of cancer self-seeding. Nat Rev Clin Oncol. 2011;8(6):369–77. doi: 10.1038/nrclinonc.2011.64. [DOI] [PubMed] [Google Scholar]
- 34.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–26. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comen E, Norton L. Self-seeding in cancer. Recent Results Cancer Res. 2012;195:13–23. doi: 10.1007/978-3-642-28160-0_2. [DOI] [PubMed] [Google Scholar]
- 36.Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353(8):793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 37.Kang Y, Pantel K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell. 2013;23(5):573–81. doi: 10.1016/j.ccr.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8(5):329–40. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 39.Müller V, Stahmann N, Riethdorf S, Rau T, Zabel T, Goetz A, et al. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res. 2005;11(10):3678–85. doi: 10.1158/1078-0432.CCR-04-2469. [DOI] [PubMed] [Google Scholar]
- 40.Mehra R, Kumar-Sinha C, Shankar S, Lonigro RJ, Jing X, Philips NE, et al. Characterization of bone metastases from rapid autopsies of prostate cancer patients. Clin Cancer Res. 2011;17(12):3924–32. doi: 10.1158/1078-0432.CCR-10-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rutkowski MR, Stephen TL, Conejo-Garcia JR. Anti-tumor immunity: myeloid leukocytes control the immune landscape. Cell Immunol. 2012;278(1-2):21–6. doi: 10.1016/j.cellimm.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zigler M, Shir A, Levitzki A. Targeted cancer immunotherapy. Curr Opin Pharmacol. 2013 May 3; doi: 10.1016/j.coph.2013.04.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Chen KW, Pienta KJ. Modeling invasion of metastasizing cancer cells to bone marrow utilizing ecological principles. Theor Biol Med Model. 2011;8:36. doi: 10.1186/1742-4682-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pienta KJ, McGregor N, Axelrod R, Axelrod DE. Ecological therapy for cancer: defining tumors using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Transl Oncol. 2008;1(4):158–64. doi: 10.1593/tlo.08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Axelrod R, Axelrod DE, Pienta KJ. Evolution of cooperation among tumor cells. Proc Natl Acad Sci U S A. 2006;103(36):13474–9. doi: 10.1073/pnas.0606053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camacho DF, Pienta KJ. Disrupting the networks of cancer. Clin Cancer Res. 2012;18(10):2801–8. doi: 10.1158/1078-0432.CCR-12-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pulliam HR. Sources, sinks, and population regulation. The American Naturalist. 1988;132(5):652–661. [Google Scholar]
- 48.Weiss L, Bronk J, Pickren JW, et al. Metastatic patterns and target organ arterial blood flow. Invasion Metastasis. 1981;(1):126–135. [PubMed] [Google Scholar]
- 49.Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121(4):1298–312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robertson BA, Rehage JS, Sih A. Ecological novelty and the emergence of evolutionary traps. Trends Ecol Evol. 2013 Jun 4; doi: 10.1016/j.tree.2013.04.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 51.van der Sanden B, Appaix F, Berger F, Selek L, Issartel JP, Wion D. Translation of the ecological trap concept to glioma therapy: the cancer cell trap concept. Future Oncol. 2013;(6):817–24. doi: 10.2217/fon.13.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li WA, Mooney DJ. Materials based tumor immunotherapy vaccines. Curr OpinImmunol. 2013;(2):238–45. doi: 10.1016/j.coi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J, Loberg R, Taichman RS. The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Metastasis Rev. 2006;(4):573–87. doi: 10.1007/s10555-006-9019-x. [DOI] [PubMed] [Google Scholar]