Abstract
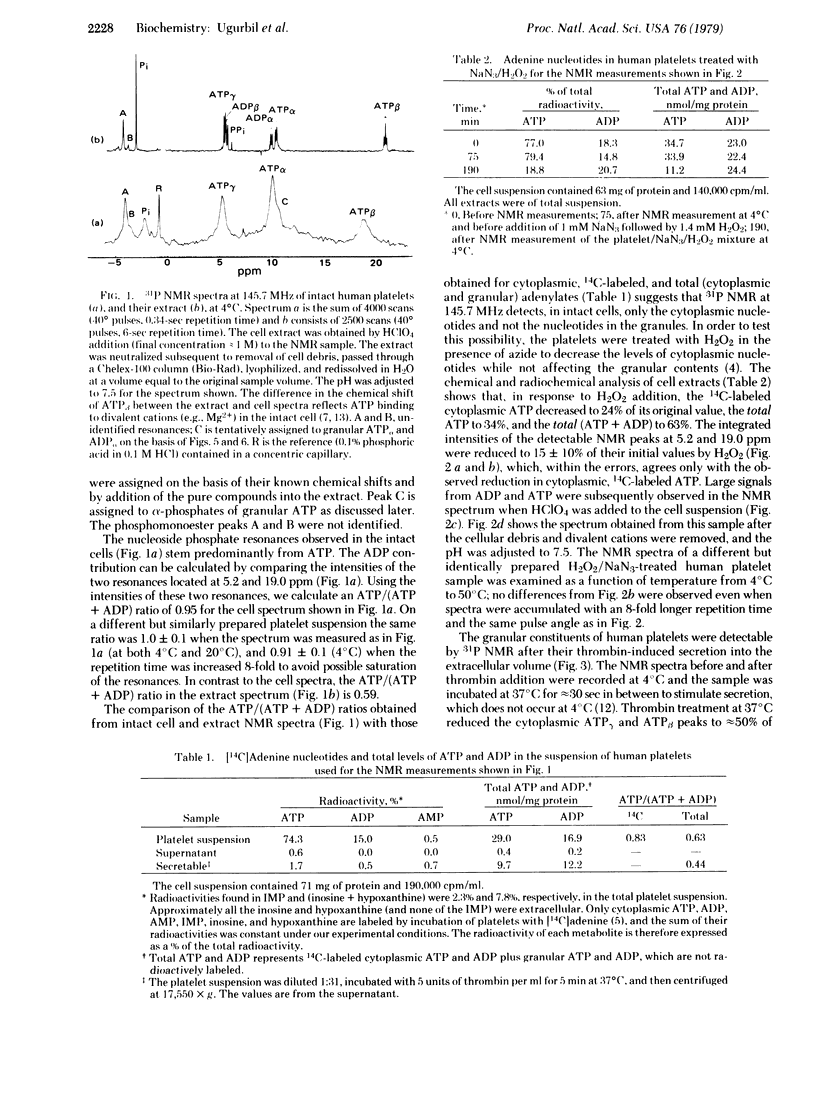
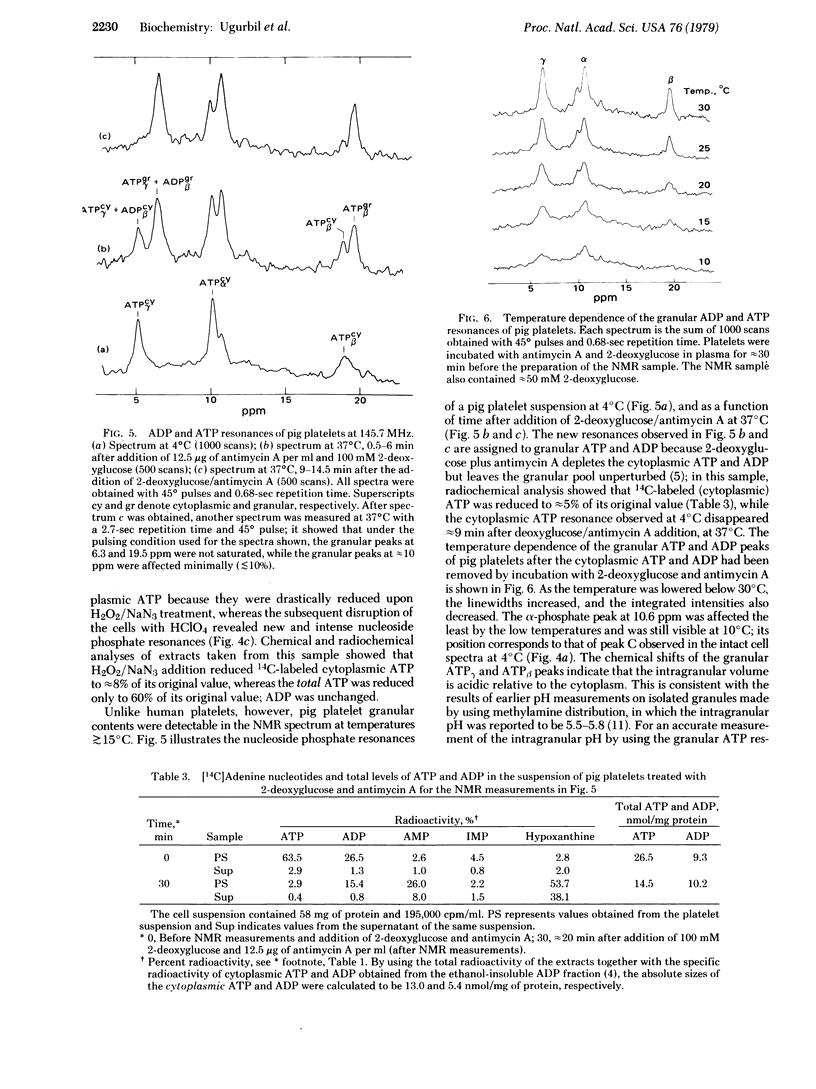
Suspensions of human and pig blood platelets have been studied by 31P NMR at 145.7 MHz and by chemical and radiochemical determination of nucleotide levels. In both types of platelets the cytoplasmic nucleotide pool, which was prelabeled by incubation with [14C]adenine, was selectively reduced by addition of H2O2/NaN3 or 2-deoxyglucose/antimycin A. After the reduction of cytoplasmic ATP in human platelets, the 31P NMR spectra showed an almost complete loss of the nucleoside di- and triphosphate resonances at temperatures examined (4--50 degrees C), indicating that only the cytoplasmic nucleotides had been observed, with no detectable contributions from the granular ATP, ADP, and pyrophosphate. Slow tumbling of the granular nucleotides, possibly due to aggregation, is the probable explanation of their undetectability at 145.7 MHz. Similar experiments showed that in pig platelets, granular ATP and ADP were not detected by 31P NMR at 4 degrees C but were observed at higher temperatures, indicating that aggregation may be occurring at the lower temperatures. Upon thrombin stimulation of human platelets, the NMR spectra and the chemical and radioactivity analyses showed that the granular adenylates and pyrophosphate were secreted, and that cytoplasmic ATP levels were appreciably reduced.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berneis K. H., Da Prada M., Pletscher A. Metal-dependent aggregation of nucleotides with formation of biphasic liquid systems. Biochim Biophys Acta. 1970 Sep 22;215(3):547–549. doi: 10.1016/0304-4165(70)90106-6. [DOI] [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- Cohen S. M., Ogawa S., Rottenberg H., Glynn P., Yamane T., Brown T. R., Shulman R. G. P nuclear magnetic resonance studies of isolated rat liver cells. Nature. 1978 Jun 15;273(5663):554–556. doi: 10.1038/273554a0. [DOI] [PubMed] [Google Scholar]
- Costa J. L., Dobson C. M., Kirk K. L., Poulsen F. M., Valeri C. R., Vecchione J. J. Studies of human platelets by 19F and 31P NMR. FEBS Lett. 1979 Mar 1;99(1):141–146. doi: 10.1016/0014-5793(79)80266-5. [DOI] [PubMed] [Google Scholar]
- French P. C., Wachowicz B. Investigation of the binding component of the ethanol-insoluble metabolic ADP of human platelets and its relation to the release reaction. Haemostasis. 1974;3(5-6):271–281. doi: 10.1159/000214065. [DOI] [PubMed] [Google Scholar]
- Fukami M. H., Salganicoff L. Human platelet storage organelles. A review. Thromb Haemost. 1977 Dec 15;38(4):963–970. [PubMed] [Google Scholar]
- Guéron M., Shulman R. G. 31P magnetic resonance of tRNA. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3482–3485. doi: 10.1073/pnas.72.9.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Robkin L. Hydrogen peroxide lowers ATP levels in platelets without altering adenyalte energy charge and platelet function. J Biol Chem. 1977 Mar 10;252(5):1752–1757. [PubMed] [Google Scholar]
- Holmsen H., Setkowsky C. A., Day H. J. Effects of antimycin and 2-deoxyglucose on adenine nucleotides in human platelets. Role of metabolic adenosine triphosphate in primary aggregation, secondary aggregation and shape change of platetets. Biochem J. 1974 Nov;144(2):385–396. doi: 10.1042/bj1440385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlough-Rathbone R. L., Chahil A., Mustard J. F. Effect of external calcium and magnesium on thrombin-induced changes in calcium and magnesium of pig platelets. Am J Physiol. 1973 Apr;224(4):941–945. doi: 10.1152/ajplegacy.1973.224.4.941. [DOI] [PubMed] [Google Scholar]
- Mürer E. H. Factors influencing the initiation and the extrusion phase of the platelet release reaction. Biochim Biophys Acta. 1971 Feb 28;261(2):435–443. doi: 10.1016/0304-4165(72)90068-2. [DOI] [PubMed] [Google Scholar]
- Reimers H. J., Packham M. A., Mustard J. F. Labeling of the releasable adenine nucleotides of washed human platelets. Blood. 1977 Jan;49(1):89–99. [PubMed] [Google Scholar]
- Salganicoff L., Fukami M. H. Energy metabolism of blood platelets. I. Isolation and properties of platelet mitochondria. Arch Biochem Biophys. 1972 Dec;153(2):726–735. doi: 10.1016/0003-9861(72)90391-8. [DOI] [PubMed] [Google Scholar]
- Son T. D., Roux M., Ellenberger M. Interaction of Mg2+ ions with nucleoside triphosphates by phosphorus magnetic resonance spectroscopy. Nucleic Acids Res. 1975 Jul;2(7):1101–1110. doi: 10.1093/nar/2.7.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K., Rottenberg H., Glynn P., Shulman R. G. 31P nuclear magnetic resonance studies of bioenergetics and glycolysis in anaerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2244–2248. doi: 10.1073/pnas.75.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]



